Introduction
Cancer of the bladder is one of the most common
types of cancer, while urothelial cancer is the most common
histological type of transitional cell carcinoma (TCC), accounting
for approximately 90% of cases and it has a poor prognosis
(1).
According to the guidelines on bladder cancer, the
treatment options are: surgery, radiation treatment, chemotherapy
and immunotherapy, depending on the staging and histological type
(2). One of the therapeutic
approaches with promising results in clinical trials is the
non-specific immunostimulant, keyhole limpet hemocyanin (KLH)
Megathura crenulata (3).
Hemocyanins (Hcs) are copper-containing respiratory proteins found
in arthropods and mollusks (4,5). Due
to their high molecular weight, structural heterogeneity and
xenogeneic nature, they are known as some of the strongest
antigens. The mechanism of action is immune response activation due
to the presence of cross-reacting epitopes, such as the
Thomson-Friederich antigen [Gal(b1-3) N-acetyl epitope]
cross-reactive with an equivalent epitope on the bladder tumor cell
surface (6) and a carbohydrate
epitope on the surface of Schistosoma mansoni larval
schistosomes (7). In vivo it
induces a protective antibody production against this carbohydrate
sequence along with a cytotoxic T-cell response. Subsequent to KLH
immunization, patients generate IgG antibodies against KLH
(8). Apart from KLH, the antitumor
activities of other Hcs have also been observed. A previous study
on the therapeutic properties of Hcs, isolated from the garden
snail Helix lucorum (HlH) and the marine snail Rapana
venosa (RvH), has shown their activity against Guerin ascites
tumor (9).
We hypothesized that HlH and RvH may also have a
therapeutic effect on bladder cancer. Therefore, the effect of
these Hcs on CAL-29 and T-24 bladder cancer cell lines was examined
in vitro, in comparison with KLH, doxorubicin hydrochloride
(DOX) and mitomycin-C (MIT-C) (used for bladder cancer
chemotherapy).
Materials and methods
Materials and assays
Two bladder cancer cell lines were used in this
study: T-24 (TCC, grade III) and CAL-29 (grade IV, stage T2)
obtained from the Interfaculty Institute for Cell Biology,
Department of Immunology, University of Tübingen, Tübingen,
Germany.
The antibiotics, MIT-C and DOX, KLH and Bradford
reagent were purchased from Sigma-Aldrich Chemie Gmbh
(Eschenstrasse, Germany). The WST-1 cell proliferation assay kit
was purchased from Roche Diagnostics Deutschland GmbH (Mannheim,
Germany), while the Limulus amebocyte lisate (LAL) assay from
(Lonza Verviers Sprl, Verviers, Belgium).
Cell culture
The CAL-29 and T-24 cells were cultured as a
monolayer in Dulbecco’s modified Eagle medium (DMEM, Lonza)
supplemented with 10% fetal calf serum (FCS) and 1%
penicillin-streptomycin (P/S) (Gibco Invitrogen GmbH, Karlsruhe,
Germany) at 37°C in a humidified atmosphere with 5% CO2
until 80% confluent. Cells were harvested using trypsin/EDTA
(Lonza) and counted using a hemocytometer.
Test substance preparation
In the current experiments whole molecules of HlH
and RvH and 2 structural subunits, RvH1 and RvHI2, were used. Hcs
were isolated from the hemolymph of the garden snail HlH and the
marine snail RvH as described by Dolashka et al (10) and Velkova et al (11). The 2 structural subunits, RvH1 and
RvH2, were purified after dissociation of the RvH.
The tested substances were filtered using a
bacterial filter with a pore size of 0,2 μm
(Corning® Incorporated Life Sciences, St. Lowell, MA,
USA) under sterile conditions. The concentration of the Hc
solutions was determined spectrophotometricaly with Bradford
reagent. KLH was used for standard curve preparation, (C=5, 1
mg/ml; Sigma-Aldrich). Optical density (OD) was read on an ELISA
reader (SpectraMax 340), λ=595 nm.
In vitro cytotoxicity assay
Cell viability was determined using a standard WST-1
cell proliferation assay. Briefly, the cell lines mentioned above
were seeded in 96-well plates (20,000 cells/well). Different
concentrations of Hcs ranging from 0.8 to 500 μg/ml were
added to the solution after 12–18 h. KLH (Sigma-Aldrich) was used
as the positive control at the same concentration as Hcs, while DOX
and MIT-C were used at concentrations of 10 μg/ml and 1
μM, respectively. Medium alone (cells without treatment) was
used as the negative control. After incubation for 24, 48 and 72 h,
20 μl ready-to-use WST-1 reagent was added to each well and
cultured for another 2 h and the cell viability was determined.
LAL assay
The working procedures were completed under sterile
conditions, with pyrogen-free material and the solutions that came
into contact with the cells were assayed at <200 EU/ml endotoxin
using the LAL test.
Statistical analysis
The data are presented as the means with standard
deviation (SD). Significance testing was performed using one-way
analysis of variance (ANOVA), followed by Bonferroni’s post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference (shown as *P<0.05,
**P<0.01 and ***P<0.001 in the
figures). The experiments were performed in triplicate and at least
3 times. Most of the experiments were performed 5 times.
Results
Based on the published information regarding the
antitumor effect of Hcs, the antitumor properties of 2 molluscan
Hcs extracted from the Bulgarian species, RvH and HlH, were
examined in comparison with KLH. The structure of HlH and RvH has
been studied thoroughly and their organization was found to differ
markedly from the structure of KLH. RvH, HlH and KLH are composed
of several oligosaccharide residues exhibiting various tertiary
constitutions. In RvH and KLH 2 structural subunits have been
identified, whereas 3 isoforms have been isolated from HlH.
To date, many different protocols have been used to
study the antitumor effect of potential pharmacological substances
on cancer cell lines (12,13). Two main steps were applied to
analyze the antitumor properties of KLH, HlH and RvH Hcs and their
isoforms. The first step was to determine the appropriate (working)
concentration of Hcs. RvH and HlH were then tested for their
effectiveness as antitumor agents on the 2 bladder cancer cell
lines. The second step may help us to determine whether Hcs and
their isoforms have the potential to be used as an alternative
treatment for TCC.
Determination of the appropriate
concentrations of Hcs
To determine the working concentration of the Hcs
(KLH, RvH and HlH and the isoforms, RvH1 and RvH2) 5 different
concentrations were used: 500, 100, 20, 4 and 0.8 μg/ml.
Each substance was added to the medium of the bladder cancer cell
lines, CAL-29 and T-24. Cell viability was analyzed using a
standard WST-1 cell proliferation assay after 24, 48 and 72 h of
incubation. The obtained results were compared with the positive
control KLH (500, 100, 20, 4 and 0.8 μg/ml), DOX (10
μg/ml) and MIT-C (1 μM) and the negative control
cells in medium without treatment. The effect of the tested
substances (HlH, RvH, RvH1 and RvH2) at 24 and 48 h was more
profound on the CAL-29compared to the T-24 cell line. The greatest
cytotoxic effect was observed at a concentration of 500
μg/ml of Hcs; therefore, it was chosen as a working
concentration to be used in further experiments. Given the higher
effect observed subsequent to the incubation of CAL-29 cells with a
lower concentration of RvH (100 μg/ml), additional
experiments were conducted. The effectiveness of the Hcs was
compared simultaneously at the concentrations of 100 and 500
μg/ml in both cell lines in order to determine the suitable
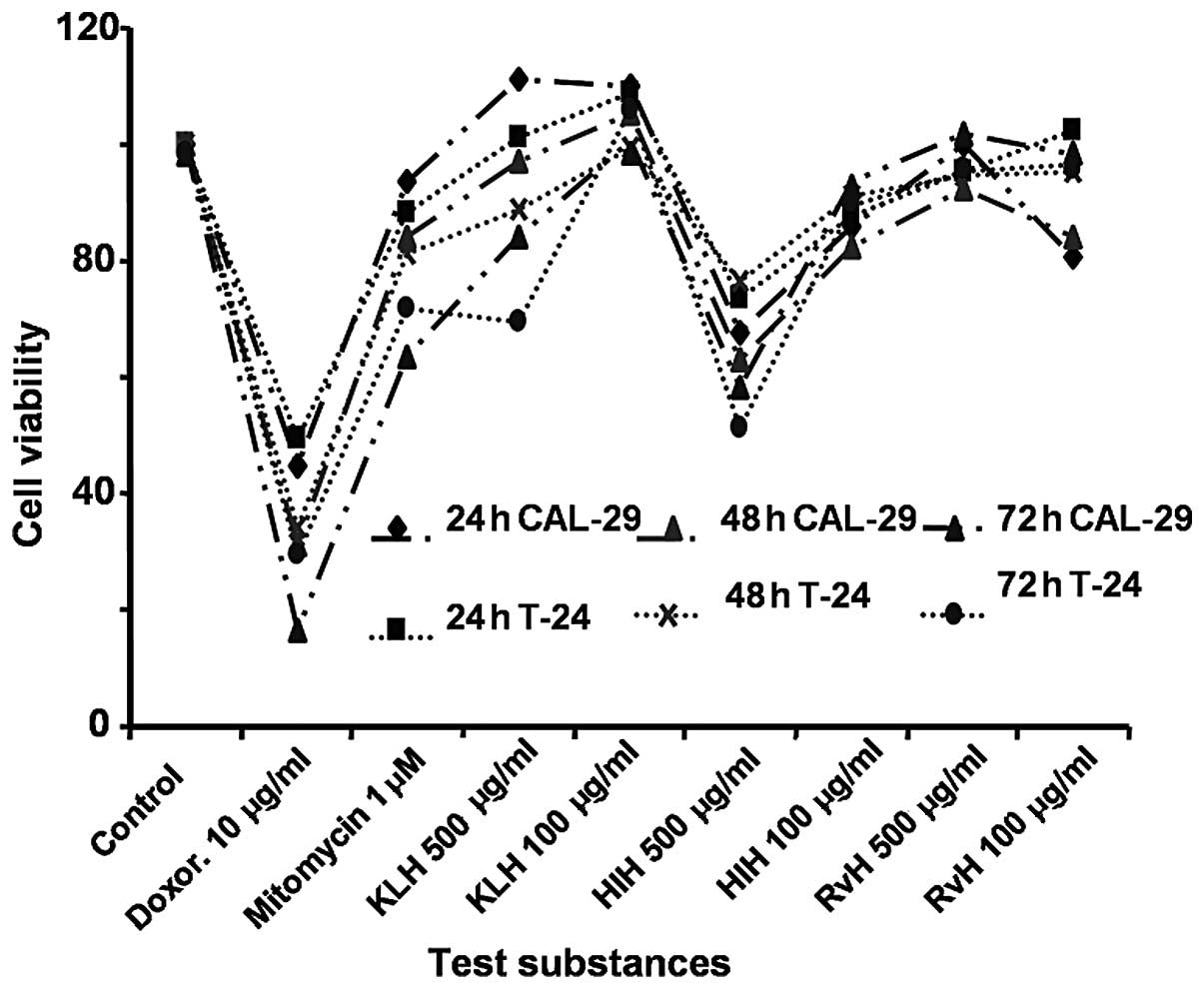
concentration. As shown in Fig. 1,
Hcs at a concentration equal to 500 μg/ml exhibited a
greater growth inhibitory effect. This concentration was determined
as the working concentration and was used in subsequent experiments
to further determine the concentration of Hcs with the best killing
effect on the CAL-29 and T-24 bladder cancer cell lines.
Determination of the test Hc with the
best cytotoxic effect
The direct in vitro effect of the tested Hcs
on the CAL-29 and T-24 bladder cancer cell lines was evaluated in a
number of experiments lasting for 24, 48 and 72 h. The Hc
concentration used in each experiment was the determined working
concentration mentioned above (500 μg/ml).
The effects of native Hc molecules of KLH, molluscan
RvH and HlH and 2 structural subunits, RvH1 and RvH2, on the CAL-29
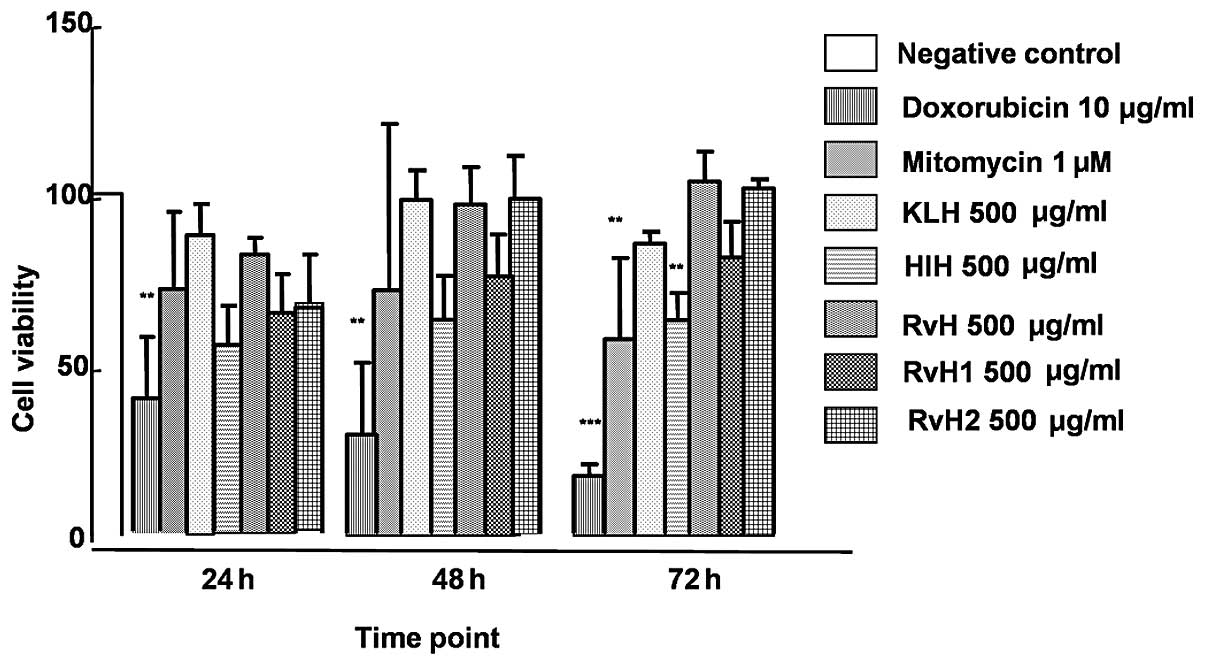
bladder cancer cell line are presented in Fig. 2. The viability measured at 24, 48
and 72 h subsequent to the incubation with the native molecule of
KLH was 111.58, 98.14 and 85.23%, respectively. At the same
time-points, the cell viability of CAL-29 cells measured subsequent
to RvH treatment was 103.64, 96.81 and 103.23%, respectively. The
lowest viability achieved with HlH was 69.99, 63.06 and 62.73%,
respectively. As shown in Fig. 2
only HlH of the native Hcs molecules showed a cytotoxic effect
above 30% (% cytotoxicity = 100% − % viability) after 24 h of
incubation. A slight inhibitory effect was observed after 24 h of
treatment of the CAL-29 cells with the subunits, RvH1 and RvH2
(18.01 and 15.53%, respectively). On the contrary, no cytotoxic
effects, and stimulation were observed with the native molecule of
RvH and KLH. The cell viability of the CAL-29 cell line after 72 h
of incubation with the native molecule of HlH was the lowest
(62.73%) and a growth inhibition of 37.3% was achieved (at 72
h).
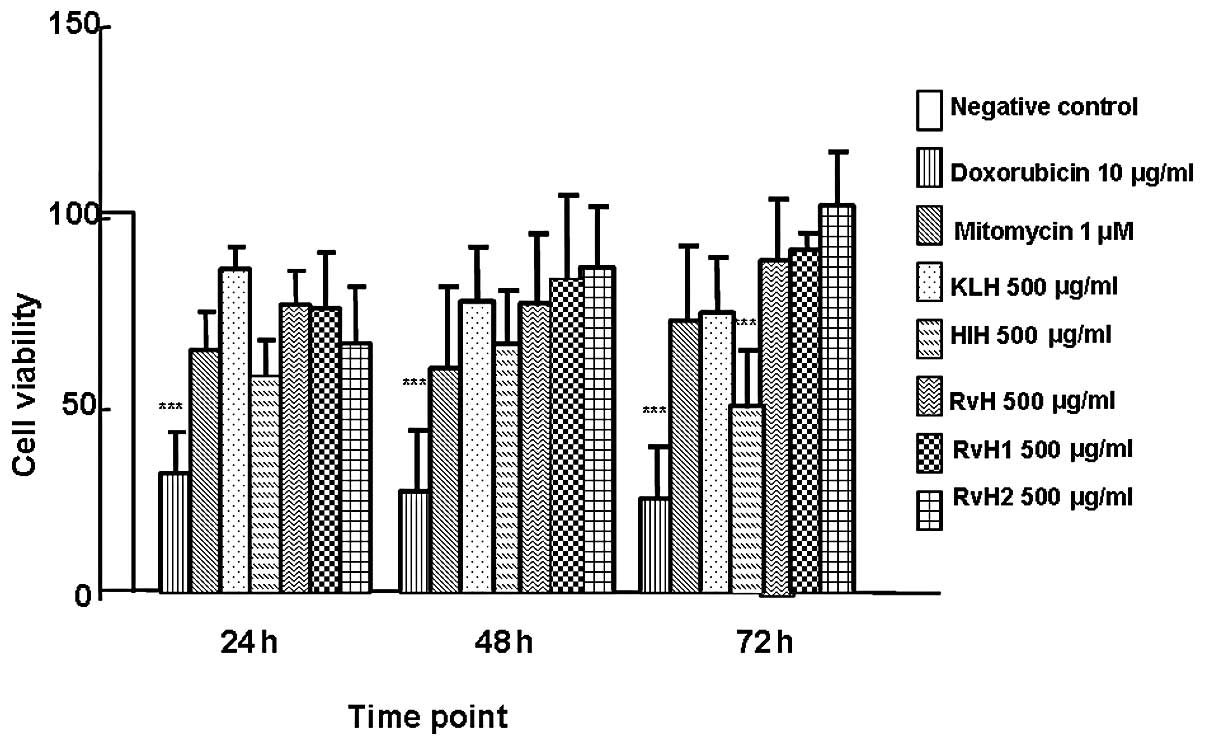
Similar effects were observed following the
treatment of the T-24 cell line with the native molecules, RvH,
KLH, HlH, and 2 structural subunits, RvH1 and RvH2. As shown in
Fig. 3, cell viability after
incubation with the native molecule of KLH at 24, 48, and 72 h was
107.01, 77.03 and 73.22%, respectively. Cell viability measured
subsequent to incubation with RvH for the same time period was
95.28, 76.53 and 87.52%, respectively. The cell viability of the
T-24 cells treated with HlH was determined at 71.70, 65.75 and
49.34% at 24, 48 and 72 h respectively. The cell viability of the
T-24 cells decreased from 71.7% after 24 h of incubation to 49.34%
after 72 h of HlH culturing. The highest growth inhibitory effect
(cytotoxic effect) among the Hcs was 50.66%, observed at 72 h of
incubation of the T-24 cells with HlH. An extremely low cytotoxic
effect was detected after 24 and 48 h of incubation with RvH2
(17.61 and 13.98%). At 72 h, a stimulation with RvH2 was observed.
The opposite tendency was observed subsequent to RvH1 treatment at
24, 48 and 72 h (4.72, 16.99 and 10.51% cytotoxicity,
respectively).
Discussion
Hcs are high molecular weight substances, with a
xenogenic nature, carbohydrate content and a complicated quaternary
structure. This explains their strong immunogenicity in mammals as
well as their adjuvanticity in vivo. Their structure,
biological function and potential usage in medicine have been
extensively studied. One of these Hcs is the molluscan, KLH, a
highly antigenic respiratory protein (5,9). It
has been used in phase II clinical trials as a drug against bladder
cancer (14–20). Moreover, a growth inhibitory in
vitro effect of KLH against multiple cancer cell lines,
including estrogen-dependent (MCF-7) and -independent breast
(ZR75-1), pancreatic (PANC-1, MIAPaCa), prostate (DU145) and
Barrett’s esophageal adenocarcinoma cancer cell lines, has also
been reported (14–16).
Recently, the effect of the application of RvH and
KLH on antibody-dependent cellular cytotoxicity (ADCC) and mitogen
activity of spleen lymphocytes in hamsters with progressing myeloid
Graffi tumors was found subsequent to tumor transplantation
(18). The antitumor properties of
both RvH and HlH as well as their immunological potential,
combining in vitro and in vivo methods, was also
analyzed (9,19). Strong activation of the immune
system of tumor-bearing animals following treatment with RvH was
demonstrated. Additionally, the immunostimulatant Hcs were found to
induce antitumor activity.
KLH, RvH and HlH and their isoforms differ markedly
in their structures. Their antitumor effects on 2 human bladder
cancer cell lines, CAL-29 and T-24 were studied, in comparison with
products used in clinical practice for chemotherapy, such as DOX
and MIT-C.
In the present study, we demonstrate the growth
inhibitory effect of Hcs in vitro. This effect was measured
in the presence of a negative and 2 positive controls. The growth
inhibitory effect at a range of concentrations between 0.8 and 500
μg/ml was shown in a series of experiments with 24, 48 and
72 h of incubation.
The growth inhibitory effect of common HlH was shown
to be superior to that shown by KLH; 80% cell was observed
viability following KLH incubation for 72 h, and approximately 55%
cell viability was observed following incubation with HlH (for the
CAL-29 and T-24 bladder cancer cell lines). The percentage
cytotoxicity was calculated according to the cell the viability (%
cytotoxicity = 100% − % viability). The KLH cytotoxicity in our
experiments (20%) differs from the one reported by Riggs et
al (16) (6–43%). The
difference may be due to the differences in the tumor cell lines
tested, the cell density per well and the method used for viability
detection. Further studies are required to compare the growth
inhibitory effect of structural units and functional subunits
isolated from HlH, which may possibly have an even more pronounced
effect. In the present study, the growth inhibitory effect of
structural subunits isolated from RvH was examined. The effect of
the whole molecule of RvH and the structural subunits (RvH1 and
RvH2) measured 72 h after incubation was found to be similar or
lower compared that of KLH.
These findings are consistent with findings from our
previous studies (9,11,18) on
the immuno-adjuvant properties of these Hcs, their derivatives and
conjugates. Those studies investigated the cell-mediated immunity
in experimental tumor-bearing animals with Guerin and Graffi
ascites tumor (19,20). It was suggested that Hcs
carbohydrate moieties are associated with the antitumor potential
of different Hcs. High-mannose type glycans, as observed in most
molluscan Hcs, were also identified in Hcs from RvH, HlH and
KLH.
In the present study on molluscan Hcs isolated from
the marine snail RvH and the garden snail HlH and the CAL-29 and
T-24 bladder cancer cell lines, a growth inhibitory effect of the
native molecule of HlH Hc and RvH1 subunit in vitro was
demonstrated. Moreover, HlH was much more effective compared to KLH
and RvH on the human bladder cancer cell lines.
In conclusion, of the Hcs tested only HlH showed a
higher efficacy compared to KLH investigated in clinical studies as
a potential therapy for bladder cancer. Therefore HlH may be
considered for further investigation, as an intravesical therapy
for bladder cancer.
Acknowledgements
This study was funded by a research
grant of the Bulgarian National Science Fund TK01-496/2009 and the
as well as project ДМУ03/48 from Bulgarian National Science Fund.
O.B. and P.D. would like to thank Erasmus and the German Academic
Exchange Service (DAAD) for financing this study.
References
|
1
|
Kochanek KD, Xu J, Murphy SL, Miniño AM
and Kung H: Deaths: preliminary data for 2009. Nat Vital Stat Rep.
59:2011.(Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_04.pdfuri).
|
|
2
|
Palou J: Patient risk profiles: prognostic
factors of recurrence and progression. Eur Urology. 41:105–112.
2002.
|
|
3
|
US National Institutes of Health: Keyhole
limpet hemocyanin compared with doxorubicin in treating patients
with bladder cancer. http://clinicaltrials.gov/ct2/show/NCT00006034uri.
Accessed March 3, 2011.
|
|
4
|
Dolashka-Angelova P, Dolashki A, Savvides
SN, Hristova R, Beeumen JV, Voelter W, Devreese B, Weser U, Di Muro
P, Salvato B and Stevanović S: Structure of hemocyanin subunit
CaeSS2 of the crustacean Mediterranean crab Carcinus
aestuarii. J Biochem. 138:303–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wirguin I, L.Suturkova M, Briani C and
Latov N: Keyhole limpet hemocyanin contains Gal(beta 1-3)-GalNAc
determinants that are cross-reactive with the T antigen. Cancer
Immunol Immunother. 40:307–310. 1995.PubMed/NCBI
|
|
6
|
Li Y, Rabello ALT, Simpson AJG and Katz N:
The serological differentiation of acute and chronic Schistosoma
japonicum infection by ELISA using keyhole limpet haemocyanin as
antigen. Trans R Soc Trop Med Hyg. 88:249–251. 1994. View Article : Google Scholar
|
|
7
|
Burke GP, Smith KA, Stocking RIG, Ferm M
and McIntyre OR: Anti-keyhole limpet hemocyanin antibody in normal
unsensitized individuals. J Allergy Clin Immunol. 59:309–313. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hortobagyi GN, Smith TL, Swenerton KD,
Legha SS, Buzdar AU, Blumenschein GR, Gutterman JU and Hersh EM:
Prognostic value of prechemotherapy skin tests in patients with
metastatic breast carcinoma. Cancer. 47:1369–1376. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dolashka P, Velkova L, Iliev I, Beck A,
Dolashki A, Yossifova L, Toshkova R, Voelter W and Zacharieva S:
Antitumor activity of glycosylated molluscan hemocyanins via Guerin
ascites tumor. Immunol Investig. 40:130–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dolashka P, Genov N, Pervanova K, Voelter
W, Geiger M and Stoeva S: Rapana thomasiana grosse
(gastropoda) haemocyanin: spectroscopic studies of the structure in
solution and the conformational stability of the native protein and
its structural subunits. Biochem J. 315:139–144. 1996.
|
|
11
|
Velkova L, Dimitrov I, Schwarz H,
Stevanović S, Voelter W, Salvato B and Dolashka-Angelova P:
Structure of hemocyanin from garden snail Helix vulgaris.
Comp Biochem Physiol Mol Biol. 157:16–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rudner J, Ruiner C-E, Handrick R, Eibl
H-J, Belka C and Jendrossek V: The Akt-inhibitor Erufosine induces
apoptotic cell death in prostate cancer cells and increases the
short term effects of ionizing radiation. Radiat Oncol. 16:1082010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamat A and Lamm D: Antitumor activity of
common antibiotics against superficial bladder cancer. Urology.
63:457–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lamm DL: Laboratory and clinical
experience with keyhole limpet hemocyanin (Immucothel) in
superficial bladder sancer. J Urol Urogynäkol. 18–21. 2003.
|
|
15
|
Jurincic-Winkler CD, Metz KA, Beuth J and
Klippel KF: Keyhole limpet hemocyanin for sarcinoma in situ
of the bladder: a long-term follow-up study. Eur Urol. 37:45–49.
2000.
|
|
16
|
Riggs DR, Jackson BJ, Vona-Davis L, Nigam
A and McFadden DW: In vitro effects of keyhole limpet
hemocyanin in breast and pancreatic cancer in regards to cell
growth, cytokine production, and apoptosis. Am J of Surg.
189:680–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moltedo B, Faunes F, Haussmann D, De
Ioannes P, De Ioannes AE, Puente J and Becker MI: Immunotherapeutic
effect of concholepas hemocyanin in the murine bladder cancer
model: evidence for conserved antitumor properties among
hemocyanins. J Urol. 176:2690–2695. 2006. View Article : Google Scholar
|
|
18
|
Toshkova R, Ivanova E, Hristova R, Voelter
W and Dolashka-Angelova P: Effect of Rapana venosa
hemocyanin on antibody-dependent sell sytotoxicicity (ADCC) and
mitogen responsibility of lymphocytes from hamsters with
progressing myeloid tumors. World J Med Sci. 4:135–142. 2009.
|
|
19
|
Dolashka-Angelova P, Stefanova T, Livaniou
E, Velkova L, Klimentzou P, Stevanović S, Salvato B, Neychev H and
Voelter W: Immunological potential of Helix vulgaris and
Rapana venosa hemocyanins. Immunol Invest. 37:822–840.
2008.
|
|
20
|
Graffi A: Chloroleukemia of mice. Ann NY
Acad Sci. 68:540–558. 1957. View Article : Google Scholar
|
|
21
|
Yakimov M, Mladenov Z, Konstantinov A and
Yanchev I: Transplantable myeloid tumor in hamsters induced by
virus of Graffi. General and Comparative Pathology. 6:24–30.
1979.
|