Introduction
A large proportion of patients with thoracic
carcinomas receive thoracic radiotherapy (TRT) as part of their
treatment. Some of these patients are likely to have esophageal
toxicity such as acute radiation-induced esophagitis (ARIE) and
radiation-induced fibrosis (RIF). The occurrence of these
toxicities results in unplanned treatment delays or interruption of
treatment. In addition, tumor control and survival rates as well as
patient quality of life may also be reduced.
ARIE, which is the primary dose-dependent
complication for radiotherapy, is fairly common. ARIE has been
reported in 5–50% of the patients treated with TRT at different
volumes of thoracic irradiation, and this rate was further
increased by concurrent chemotherapy (1,2).
Dysphagia, odynophagia and substernal burning sensation are the
major clinical features of ARIE. Inflammatory cell infiltration in
esophageal tissues is a prominent histopathological change that
occurs in ARIE. These inflammatory cells including mast cells,
macrophages and lymphocytes may secrete pro-inflammatory cytokines
and growth factors that are important in the inflammatory processes
(3,4). Of those factors, epidermal growth
factor (EGF) is crucial in the growth and proliferation ability of
various cells including the epithelium, endothelium and fibroblasts
(5,6). Similarly, transforming growth factor
β1 (TGF-β1) is a type of substantial growth factor involved in the
start and termination of tissue repair. Furthermore, TGF-β1
downregulates the peroxides and nitric oxide generated by
inflammatory cells to reduce the extent of inflammation (7).
Following the inflammatory response induced by
irradiation RIF, a late sequela of radiation therapy, is mainly
characterized by an increased production of extracellular matrix
(ECM) components and mesenchymal cell proliferation, migration and
accumulation. RIF is an occasional irreversible damage that is
unavoidable and may continue for years after TRT (8). In the fibrotic process, a number of
cytokines and growth factors have been shown to participate in this
process. TGF-β1, via the Smad proteins, is considered responsible
for the initiation, development and persistence of fibrosis, and to
be the main cytokine involved in the process of RIF in vivo.
TGF-β1 is also important in the synthesis and deposition of
collagen (8–10).
At present, treatments including adrenocorticotropic
hormone and certain antibiotics, such as mixture of lidocaine,
dexamethasone and gentamycin (mLDG), constitute the main drugs
used. However, these drugs have not proven to be efficacious in a
wide range of patients (4,11). Moreover, adverse effects of these
drugs such as the increased risk of osteoporosis and resistance to
antibiotics negatively impact the therapeutic ratio. Drugs of
herbal origin with few side-effects are of great interest as
alternatives and the traditional Chinese herbal medicine (tChm) may
provide a novel therapy that may relieve clinical symptoms and
improve general functions such as eating, sleeping and immune
function (12,13).
Compound of White Peony Root Oral Liquid (cWPROL) is
a prescription formula independently developed by our
investigators. Previous experimental studies revealed a certain
therapeutic effect of cWPROL on ARIE (14). The present study was designed to
evaluate the therapeutic effect of cWPROL in an animal model of
radiation-induced toxicity as well as to elucidate the molecular
mechanisms underlying this therapeutic effect.
Materials and methods
Experimental animals
A total of 64 adult male Wistar rats with an average
weight of 180–220 g were used in the present study. Animals were
housed with 12-h light/dark cycle and had access to food and water
ad libitum. The experimental animal techniques and animal
handling procedures were approved by the Institutional Animal Care
and Use Committee of the Hebei Medical University, and were
consistent with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (certification no.
DK0512053).
Grouping and irradiation
Animals were divided into four groups: the cWPROL
treatment group (ctg) where rats were administered cWPROL at a dose
of 0.475 g/ml by intra-esophageal perfusion after irradiation; the
mLDG treatment group (mtg) in which rats were treated with mLDG
using the same route of administration after irradiation; the
radiation group (rg) in which rats were not administered any
treatment following irradiation; and the non-intervention group
(nig) where rats did not receive irradiation or administration of
drugs. Animals received administrations at a volume of 2 ml each
time, three times a day starting on the seventh day following
irradiation and continuing for 7 days. Rats were deprived of food
and water for 30 min following drug administration.
Following arousal of rats, single irradiation on
chest with a total dose of 43 Gy was performed with a
60Co therapy apparatus at a dose rate of 0.111 Gy/min.
The irradiation field was 3×30 cm with a centre dose point on the
back of rats 1 cm under the body surface and an irradiation range
of 3 cm on the upper esophagus, while other parts were covered.
Rats in nig were not irradiated, but otherwise treated as the
irradiated ones.
Staining
Rats were anesthetized with 2% pentobarbital sodium
administered by intraperitoneal injection (45 mg/kg). Esophageal
samples were fixed with 4% paraformaldehyde for 24 h, embedded in
paraffin and sectioned at 4 μm.
Histological staining
Paraffin sections were stained with hematoxylin and
eosin as usual following deparaffinization and rehydration. Light
and electron microscopes were used to observe the histopathologic
and ultrastructural changes of esophageal tissue. The extent of
pathological changes comprised tissue damage and infiltration of
phagocytes.
Masson staining
Tissue sections were deparaffinized and hydrated,
stained in hematoxylin for 3 min and differentiated in 1%
hydrochloric acid alcohol for 3–5 sec. The sections were then
treated with ponceau for 3 min, differentiated in 1% phosphoric
acid molybdenum for 1 min, counterstained in aniline blue for 1 min
and dehydrated rapidly through 95% alcohol, followed by two changes
of 100% alcohol, until the collagen was green.
Immunohistochemistry
Tissue sections were deparaffinized with xylene, and
rehydrated through an ethanol series and Tris-buffered saline
(TBS), and then immersed in 3% formaldehyde hydrogen peroxide
liquid to block endogenous peroxidase. Antigen retrieval was
performed by microwave treatment in the presence of antigen
retrieval solution. The sections were incubated with primary
antibody at 4°C overnight and treated with biotin-labeled secondary
antibody at 37°C for 20 min, followed by the addition of
streptavidin peroxidase-conjugated antibody at 37°C for 20 min.
Antibodies for EGF (1:50) and TGF-β1 (1:100) served as the primary
antibodies. The sections were counterstained with hematoxylin,
dehydrated, transparentized and then sealed with neutral gum. Black
control and replacing control were treated with phosphate-buffered
saline (PBS) and normal rabbit serum. The appearance of brown
particles in the stained sections was regarded as the positive
judgment standards. Five successive visual fields centering on the
lesion area of each section under the microscope (magnification,
×400) were obtained, and the average of their integral optical
density was regarded as the representative value.
Statistical analysis
Experimental results were analyzed for statistical
significance using the SPSS13.0 software package. Groups were
compared by one-way ANOVA. The Student-Newman-Keuls test was used
when the variance was equal, while the Kruskal-Wallis H test was
used when the variance was unequal. Results were presented as the
mean ± standard deviation (SD). P<0.05 was considered to
indicate a statistically significant difference.
Results
Light and electron microscope
analysis
Inflammation caused by direct exposure to radiation
results in epithelium apoptosis or necrosis, and mast cells and
leukocytes are then recruited to the site of the damage. The
pathological criteria of injury centers around two main aspects:
the extent of damaged mucous epithelium, and the extent and depth
of infiltrating inflammatory cells.
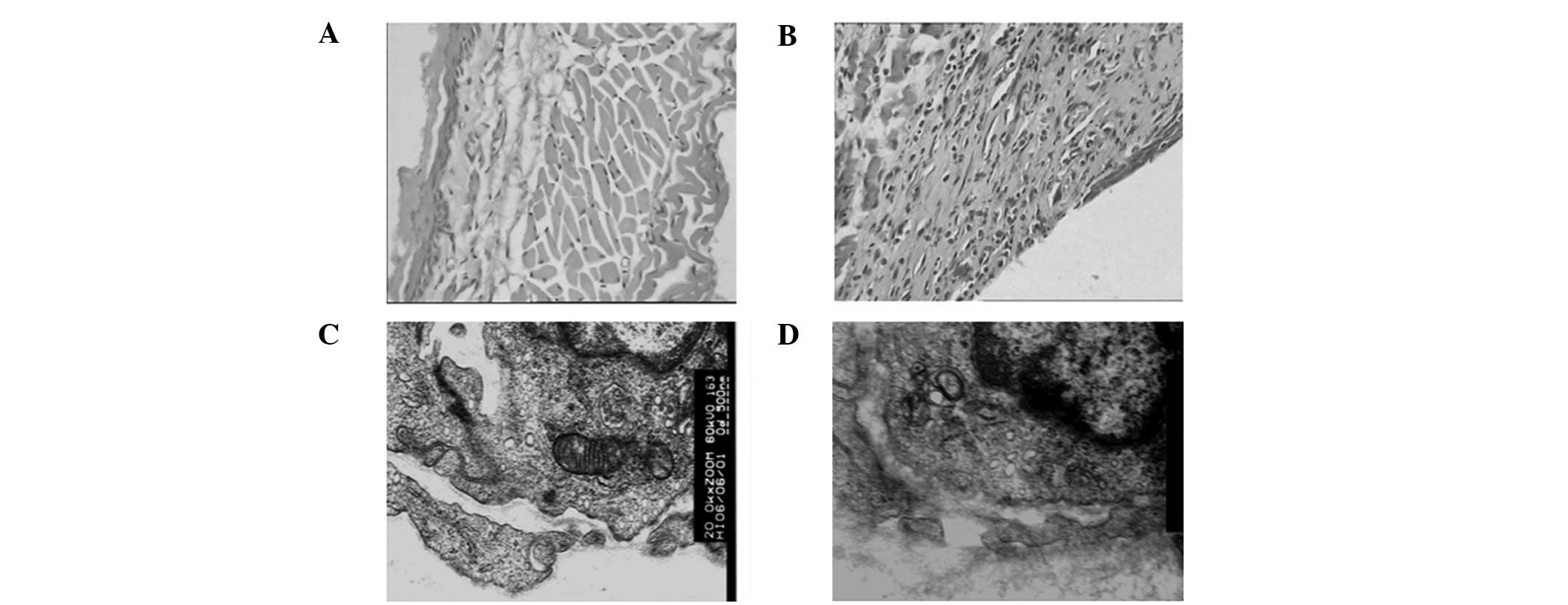
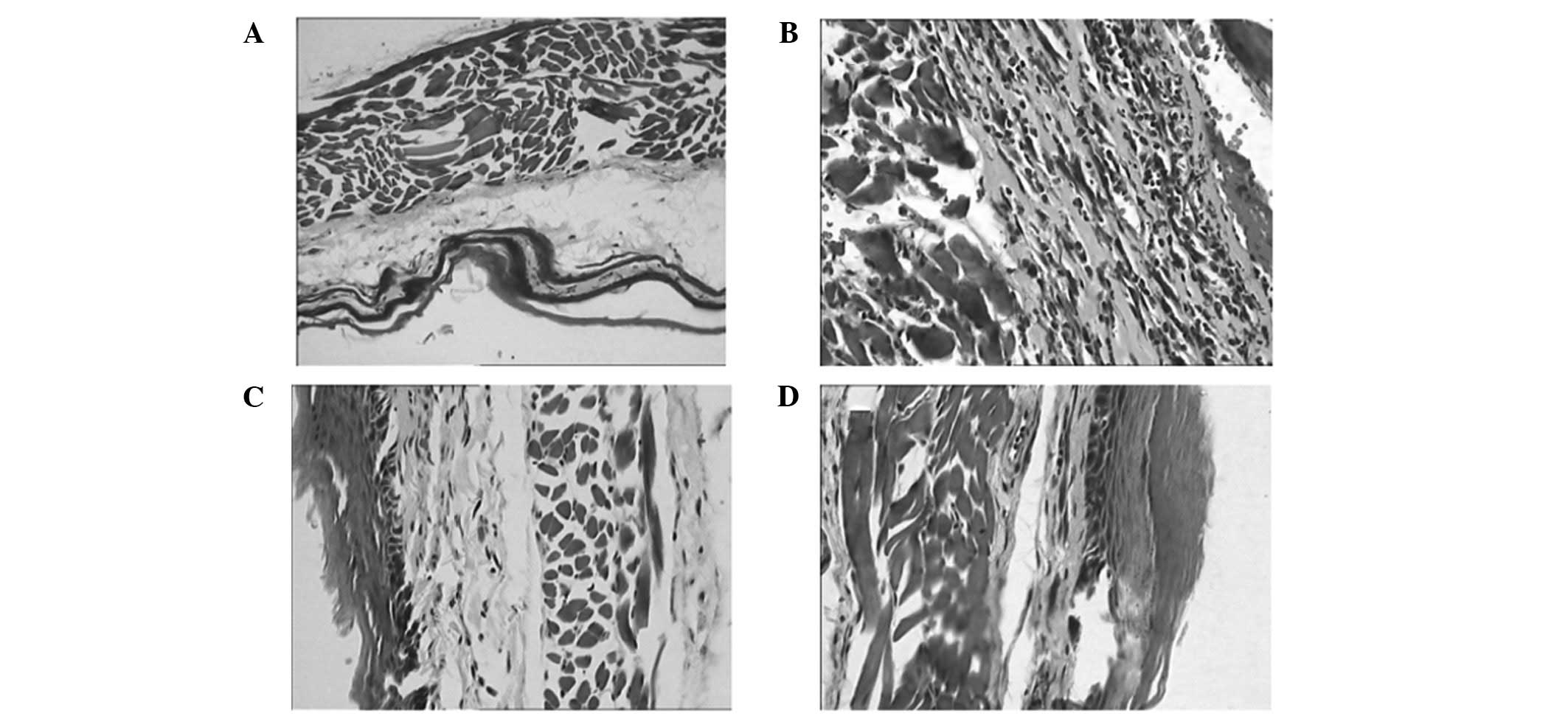
The normal structure of a Wistar rat esophagus
consists of a horny layer, a mucous membrane, a muscular layer and
the tunica adventitia. The mucous membrane is intact and
contiguous, and there are no inflammatory cells infiltrating under
the mucous membrane (Fig. 1A).
Following radiation, mucous erosion, telangiectasias, defluxion of
the epithelium and recruitment of inflammatory cells in the lamina
propia, the submucosal and muscular layers and the tunica
adventitia were observed through pathological examination (Fig. 1B).
The radiation-induced alterations of the subcellular
level organizations and functions play a significant role in the
development of acute radiation injury. Damage at this level in cell
organelles has been manifested. Structural changes of some
organelles were also observed in this study. The organelle
structure of capillary endothelium was normal in the nig group
(Fig. 1C). After radiation,
elongation and branching of the mitochondria as well as an increase
of their size and the development of giant forms, degranulation of
endoplasmic reticulum membranes as well as their dilatation and
vesicularization, and an increased number and volume fraction of
lysosomes were observed (Fig.
1D).
Rate of repair in each group
The determination of tissue repair was based on the
pathology score, according to two aspects: the histopathological
injury and inflammatory cell infiltration. The scores were divided
into different grades and amount of rats according to grade in each
group (Table I). The data showed
the repair rate to be 81% (13/16) in the ctg group, which was
higher compared with 69% (11/16) in the mtg group. However,
inflammatory cell infiltration showed a marked decrease in the ctg
compared with the mtg group.
 | Table IPathology score of rats in each
group. |
Table I
Pathology score of rats in each
group.
| Pathology injury of
esophagus
| Infiltration of
inflammatory cells
|
|---|
| Group | 0 | I | II | III | 0 | I | II | III |
|---|
| nig | 16 | 0 | 0 | 0 | 16 | 0 | 0 | 0 |
| rg | 0 | 2 | 6 | 8 | 0 | 2 | 4 | 10 |
| ctg | 13 | 1 | 2 | 0 | 3 | 8 | 3 | 2 |
| mtg | 11 | 1 | 4 | 0 | 3 | 5 | 6 | 2 |
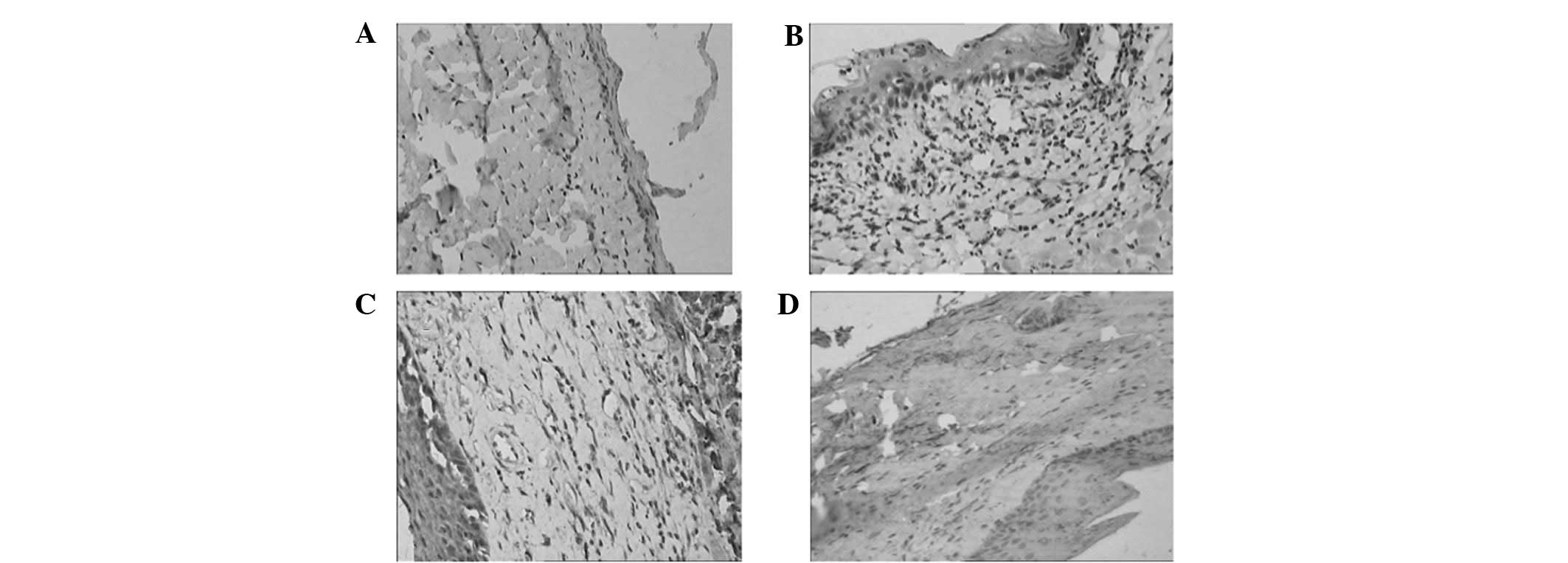
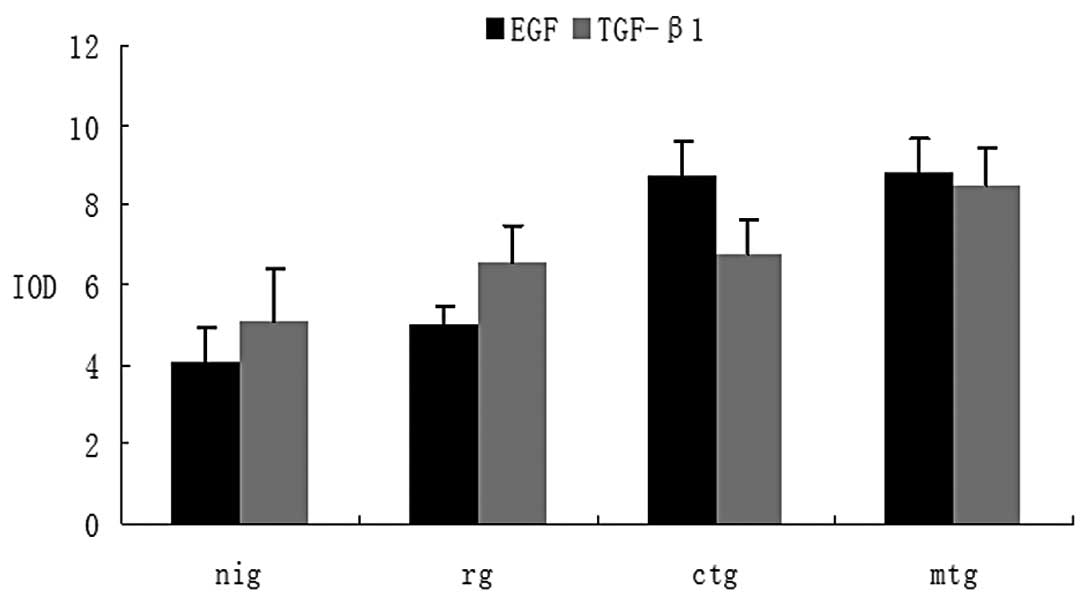
The variation of EGF and TGF-β1
expression
Figs. 3 and 4 show the variations of EGF and TGF-β1
expression by immuno histochemistry, respectively. In the normal
epithelium of esophageal mucosa, a weak expression of EGF and
TGF-β1 was observed, as well as a slight EGF expression in basilar
membrane cells and a slight TGF-β1 expression in the fibroblasts of
the lamina propria (Figs. 2A and
3A). The level of EGF expression
was significantly upregulated following radiation compared with the
nig group, which was mainly distributed in the epithelium
surrounding ulcers, fibroblasts and vascular endothelium in
inflammatory tissues (Fig. 2B). No
significant difference in EGF expression was detected between the
ctg and rg groups (P=0.071), although a significant difference was
observed compared with the nig group (P=0.027) (Fig. 2C). In the mtg group, EGF expression
was higher compared with that in the rg group (P=0.001) and
significantly higher compared with that in the nig group
(P<0.001) (Fig. 2D). The
comparison of EGF between the ctg and mtg groups elucidated a
difference between the two groups, although this difference was not
statistically significant (P=0.927) (Fig. 4).
With regard to the levels of TGF-β1, the results
were similar to those of EGF. The expression of TGF-β1 in normal
esophageal tissues was weak positive, and was mainly distributed in
the cytoplasm of the epithelial cells of the esophageal mucosa and
cells in the muscular layer (Fig.
3A). Following radiation, the level increased but without any
significance as compared to the nig group (P=0.101). The expression
of TGF-β1 presented in the sections with ulcers, mainly located in
the cytoplasm of the epithelial cells of the esophageal mucosa and
the fibroblast around the inflammation, as well as vascular
endothelial cells in the ulcer and cells in the muscular layer
(Fig. 3B). The expression of TGF-β1
in the ctg and mtg groups was significantly induced compared with
that in the nig group (P=0.013 and 0.016, respectively), while the
difference was not statistically significant compared with that in
the rg group (P= 0.082 and 0.184, respectively) (Fig. 3C and D). The level of TGF-β1 in the
mtg group was higher compared with that in the ctg group, although
the difference was not statistically significant (P=0.246)
(Fig. 4).
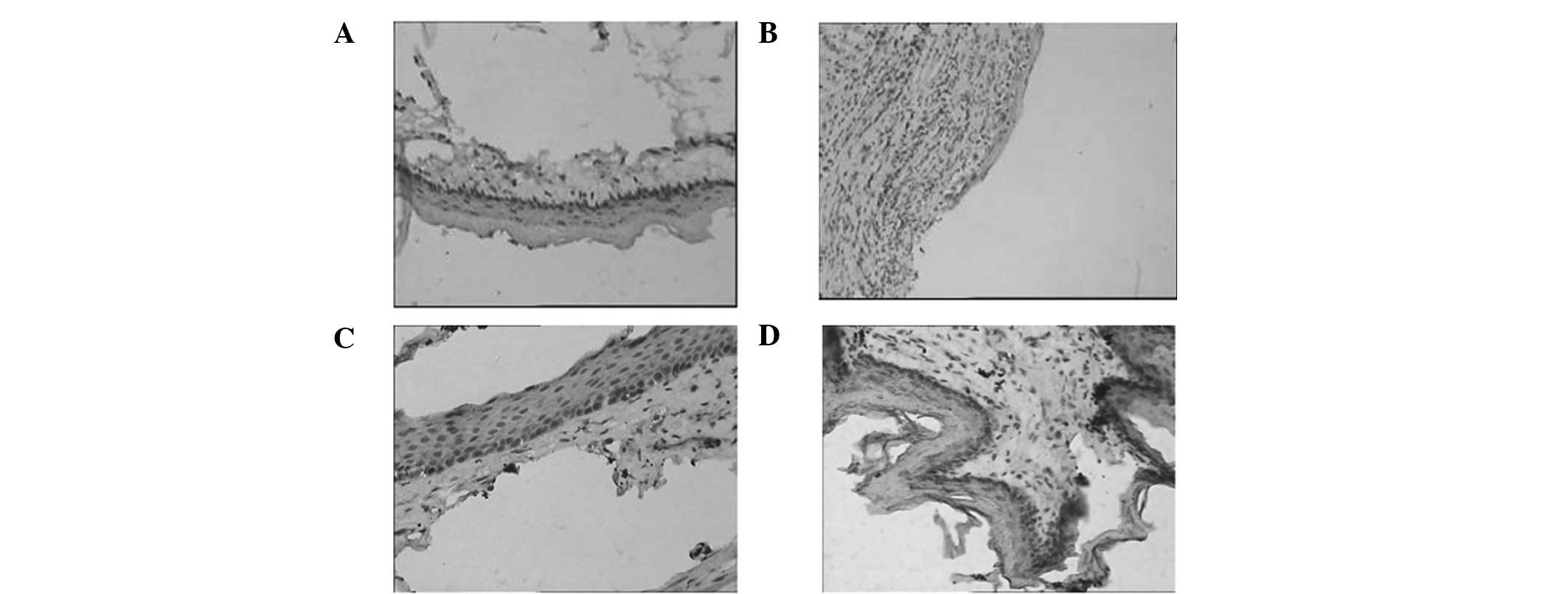
Comparison of collagen fibers in each
group
There were a few sparse collagen fibers in the
lamina propria of the esophagus of the rat in the nig group
(Fig. 5A). A number of exudate and
inflammatory cell infiltrations were present in the inflammatory
regions in the rg group, and the proliferation of collagen fibers
was distributed in several inflammatory cells (Fig. 5B). In the ctg group, mature
granulation tissues were evident in the lamina propria and the
collagen fibers exhibited a slight increase compared with those in
the nig group (Fig. 5C) In the mtg
group, the collagen fibers in the lamina propria of the esophagus
exhibited mild proliferation (Fig.
5D).
Discussion
Radiotherapy aims to deliver an effective dose to
the tumor, while maintaining an acceptable dose for the neighboring
normal tissues in order to maximize the therapeutic ratio. However,
the radiotherapy of thoracic neoplasms often exposes the esophagus
to high levels of ionizing radiation. Radiation-induced esophageal
toxicity triggered by various molecular responses induces acute and
chronic effects in the normal tissues following radiation therapy.
In the early stage, patients often complain of non-specific
symptoms such as dysphagia, odynophagia and substernal burning
sensation following radiotherapy. In the late stage, patients may
experience a serious degree of dysphagia and require endoscopic
dilation, caused by the fibroatropic process of the esophagus to
radiation (1). Table II defines the Radiation Therapy
Oncology Group (RTOG)/European Organization for Research and
Treatment of Cancer (EORTC) late esophageal toxicity grading.
Table II data indicate that the
grading criteria are mainly based on the clinical symptoms instead
of on histopathological evidences.
 | Table IIRTOG/EORTC late esophagitis morbidity
grading criteria. |
Table II
RTOG/EORTC late esophagitis morbidity
grading criteria.
| Grade | Description |
|---|
| 0 | No change over
baseline |
| 1 | Mild fibrosis,
slight difficulty in swallowing solids, no pain on swallowing |
| 2 | Unable to take
solid food normally, swallowing semisolid food, dilatation may be
indicated |
| 3 | Severe fibrosis,
able to swallow only liquids, may have pain on swallowing,
dilatation required |
| 4 |
Necrosis/perforation, fistula |
The pathological progression of radiation-induced
toxicity in normal esophageal tissues is apparently a consequence
of an early inflammatory phase followed by late stromal
alterations. Acute or early reactions are primarily characterized
by rapidly occurring changes, such as cell death as well as
inflammatory cell adhesion and infiltration (1,15).
Cell death caused by ionizing irradiation has been categorized into
two main classes, manifested as apoptosis and necrosis (16). The mitochondria, endoplasmic
reticule, Golgi-complex and the lysosome system have long been
considered to be direct intracellular targets of irradiation.
Consistent with our results, the necrosis process that ends in the
irreversible swelling and lysis of cells has the morphologic
hallmarks of mitochondrial swelling, dilatation and degranulation
of endoplasmic reticule and lysosomal rupture (17) (Fig.
1D). Apoptosis is suggested to be the main form of ionizing
radiation-induced cell death in several cell lines. However, the
dose of irradiation may also be important in determining the type
of cell death (18). The death and
defluxion of the mucous epithelium were observed in our study
outcomes (Fig. 1B). Following
treatment with cWPROL or mLDG, the injured esophageal tissues were
repaired via various biological activities were associated with
cell proliferation. In the present study, we identified that cWPROL
and mLDG promoted mucous epithelium proliferation by increasing the
expression of EGF. EGF is crucial in cell proliferation, migration
and locomotion. It is a monomeric peptide that promotes mitogenesis
in tissues of endodermal, mesodermal and ectodermal origin
(19,20). Following the binding of EGF to its
receptors, the modulatory effects exerted by EGF were associated
with the differentiation retardation and proliferation enhancement
via the cell cycle regulating genes (21).
Another feature of acute toxicity is inflammatory
cell infiltration. Inflammation caused by exposure to irritants
triggers a cascade of cytokines released that results in an
inflammatory response and tissue damage. Activated inflammatory and
immune cells, such as neutrophils, macrophages, monocytes and
natural killer cells, are recruited to the site of inflammation and
generate reactive oxygen species in the inflamed tissue, leading to
tissue injury (15,22). The present study demonstrated that
inflammatory cells mainly comprising neutrophils in the lamina
propia, the submucosal and muscular layer and the tunica adventitia
were observed in the rg group, while the amount of inflammatory
cells was reduced following administration in particular with
cWPROL (image not shown).
Late reactions following radiation exposure include
fibrosis, organ dysfunction and tissue necrosis (23). Of these reactions, fibrosis is a
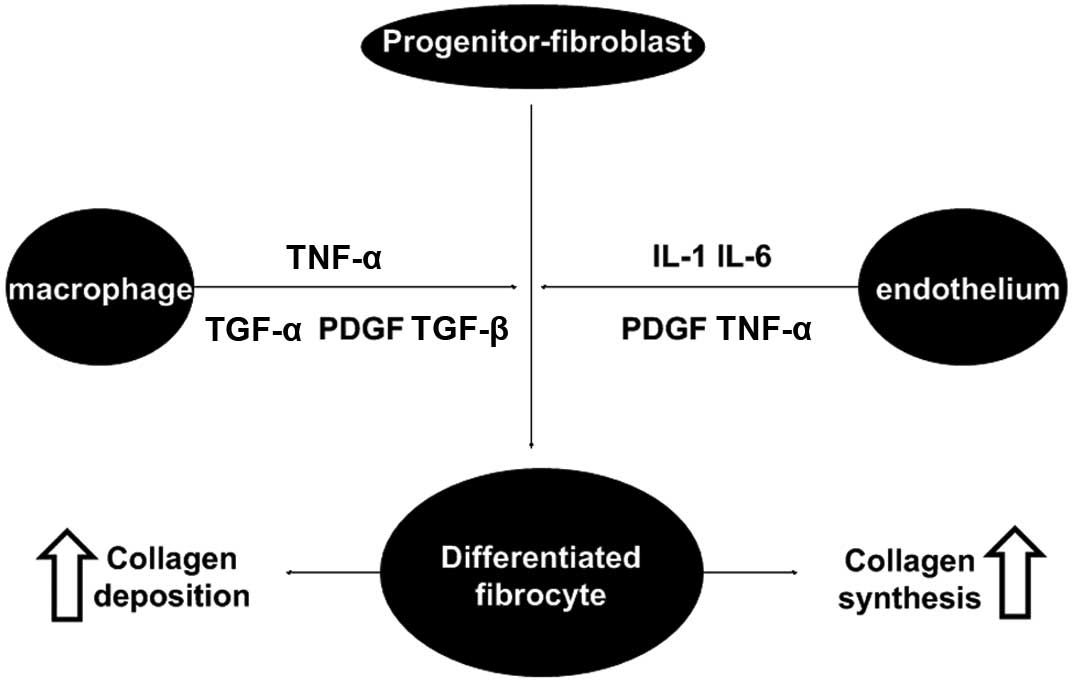
fundamental pathological process (8,10,23,24).
The fibroblast cell system plays a predominant role on the fibrotic
process due to its secretary function, which produces the
components of ECM and ensures its renewal in a balance between
synthesis and degradation. Similar to other fibrotic responses, RIF
is a multi-cell process driven by intercellular communications via
cytokines and growth factors (22,23,25)
(Fig. 6). TGF-β1 stimulates
proliferation of early progenitor fibroblast and myofibroblasts,
which may be an initial step in the onset of fibrosis. With regard
to the process of tissue remodeling, TGF-β1 stimulates, through
TGF-β1 receptors and Smad signaling, the synthesis of most matrix
proteins, decreases the production of matrix degrading enzymes and
increases the production of the inhibitors of these enzymes
(26,27). Thus, TGF-β1 has a key role in the
development of fibrotic tissue alterations. Findings of the present
study have demonstrated that RIF may be reversed by administration
with cWPROL via a decrease in the expression of TGF-β1 following
repair of the injured tissue, which corresponds to collagen
depositions in the ctg and mtg groups.
For the preventive strategies of radiation-induced
esophageal toxicity, minimizing the amount of esophagus irradiated
is an effective means, however, reducing this amount is likely to
reduce the control of thoracic malignances. Investigators
previously studied the utility of sucralfate in preventing ARIE.
However, 58% of patients dropped out of that study due to nausea
and vomiting (28). Amifostine,
considered the best radioprotective compound screened by the U.S.
Army, was not shown to effectively reduce ARIE in a large clinical
trial (RTOG 9801) (29). With
regard to preventing and treating RIF, several drugs have also been
studied, including D-penicillamine, angiotensin II blocker,
interferon γ and anti-oxidant (29–32),
however, no clinical evidence has been found that supports the
hypothesis that these drugs may reverse RIF. Therefore, other
strategies to minimize radiation-induced esophageal toxicity need
to be investigated.
The present study focused on the use of cWPROL in
treating an animal model of ARIE and demonstrated that this
prescription formula was able to repair the damaged esophageal
tissues. Although it has been proven that tChm has the exact
function of improving clinical symptoms for the dysphagia in
particular, no report is currently available on the underlying
mechanisms of the effect. According to the findings of our study,
histopathological analysis allowed for the detection of the
decrease of collagen deposition in the ctg group, combined with a
significant reduction of TGF-β1 expression; cWPROL decreased the
TGF-β1 expression level following complete repair of the damaged
esophageal tissue. However, this change was not observed in the mtg
group. As mentioned above, TGF-β1 plays a critical role in the
fibrotic process in late stromal alterations, therefore, we
conclude that cWPROL likely promotes the repair of ARIE via an
increase in the expression of EGF and TGF-β1, and prevention of RIF
via the reduction of TGF-β1. Future studies are required to confirm
our conclusion in the RIF animal model via monitoring of the level
of TGF-β1 locally and in the blood circulation.
Acknowledgements
This study was supported by the
Foundation Science Research Program of the Hebei Province Science
and Technology Office (grant no. 04236101D-252004-2005).
References
|
1
|
Bradley J and Movsas B: Radiation
esophagitis: predictive factors and preventive strategies. Semin
Radiat Oncol. 14:280–286. 2004. View Article : Google Scholar
|
|
2
|
Byhardt RW, Scott C, Sause WT, et al:
Response, toxicity, failure patterns and survival in five RTOG
trails of sequential and/or concurrent chemotherapy and
radiotherapy for locally advanced non-small-cell carcinoma of the
lung. Int J Radiat Oncol Biol Phys. 42:469–478. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abdel-Latif MM, Duggan S, Reynolds JV and
Kelleher D: Inflammation and esophageal carcinogenesis. Curr Opin
Pharmacol. 9:396–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu YF, Yu HM, Zhang C, Cheng YF, Hu LK,
Meng XH and Zhao YX: Protective effects of berberine on
radiation-induced lung injury via intercellular adhesion
molecular-1 and transforming growth factor-beta-1 in patients with
lung cancer. Eur J Cancer. 44:2425–2432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goodlad RA and Wright NA: Epidermal growth
factor (EGF). Baillieres Clin Gastroenterology. 10:33–47. 1996.
View Article : Google Scholar
|
|
6
|
Fatimah SS, Tan GC, Chua KH, Tan AE and
Hayati AR: Effects of epidermal growth factor on the proliferation
and cell cycle regulation of cultured human amnion epithelial
cells. J Biosci Bioeng. 114:220–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pohlers D, Brenmoehl J, Löffler I, et al:
TGF-beta and fibrosis in different organs - molecular pathway
imprints. Biochim Biophys Acta. 1792:746–756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Delanian S and Lefaix JL: The
radiation-induced fibroatrophic process: therapeutic perspective
via the antioxidant pathway. Radiother Oncol. 73:119–131. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barcellos-Hoff MH: How do tissues respond
to damage at the cellular level? The role of cytokines in
irradiated tissues. Radiat Res. 150:109–120. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin M, Lefaix JL and Delanian S:
TGF-beta1 and radiation fibrosis: a master switch and a specific
therapeutic target? Int J Radiat Oncol Biol Phys. 47:277–290. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kosaka Y, Mitsumori M, Araki N, et al:
Avascular necrosis of bilateral femoral head as a result of
long-term steroid administration for radiation pneumonitis after
tangential irradiation of the breast. Int J Clin Oncol. 11:482–486.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou W and Zhou YM: Function of traditional
chinese medicine in cancer radiotherapy and its prospect. Mode
Tradit Chin Med Mater Med. 11:742–746. 2009.
|
|
13
|
Zhang P and Hu PL: TCM VVM Therapy’s
influence on tumor patients’ survival. Chin J Oncol.
25:3022003.
|
|
14
|
Shen L, Shan BE, Zhang L, et al: The
experimental research of compound of White Pony Root Oral Liquid on
radiation-induced esophagitis. J Radiol Prot. 27:219–227. 2007.
|
|
15
|
Hallahan DE: Radiation-mediated gene
expression in the pathogenesis of the clinical radiation response.
Semin Radiat Oncol. 6:250–267. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Somosy Z: Radiation response of cell
organelles. Micron. 31:165–181. 2000. View Article : Google Scholar
|
|
17
|
Falcieri E, Gobbi P, Zamai L and Vitale M:
Ultrastructural features of apoptosis. Scan Microsc. 8:653–666.
2000.
|
|
18
|
Payne CM, Bjore CG and Schultz DA: Change
in the frequency of apoptosis after low- and high-dose
X-irradiation of human lymphocytes. J Leukoc Biol. 52:433–440.
1992.PubMed/NCBI
|
|
19
|
Barnard JA, Beauchamp RD, Russell WE, et
al: Epidermal growth factor-related peptides and their relevance to
gastrointestinal pathophysiology. Gastroenterology. 108:564–580.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garner A: Therapeutic potential of growth
factors and their antagonists. Yale J Biol Med. 65:715–723.
1992.PubMed/NCBI
|
|
21
|
Gibbs S, Silva Pinto AN, Murli S, Huber M,
Hohl D and Ponec M: Epidermal growth factor and keratinocyte growth
factor differentially regulate epidermal migration, growth, and
differentiation. Wound Repair Regen. 8:192–203. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal B: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radical Bio Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodemann HP and Marcel AB: Responses of
normal cells to ionizing radiation. Semin Radiat Oncol. 17:81–88.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fournier C, Scholz M, Kraft-Weyrather W,
et al: Changes of fibrosis-related parameters after high- and
low-LET irradiation of fibroblasts. Int J Radiat Biol. 77:713–722.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rodemann HP and Bamberg M: Cellular basis
of radiation-induced fibrosis. Radiother Oncol. 35:83–90. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Border WA and Noble NA: Transforming
growth factor beta in tissue fibrosis. N Engl J Med. 331:1286–1292.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schultze-Mosgau S, Blaese MA, Grabenbauer
G, Wehrhan F, et al: Smad-3 and Smad-7 expression following
antitransforming growth factor beta 1 (TGFbeta1)-treatment in
irradiated rat tissue. Radiother Oncol. 70:249–259. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McGinnis WL, Loprinzi CL, Buskirk SJ, et
al: Placebo-controlled trail of sucralfate for inhibiting
radiation-induced esophagitis. J Clin Oncol. 15:1239–1243.
1997.PubMed/NCBI
|
|
29
|
Movsas B, Scott C, Langer C, et al: Phase
III study of amifostine in patients with locally advanced non-small
cell lung cancer (NSCLC) receiving chemotherapy and
hyperfractionated radiation (Chemo/HFxRT): Radiation Therapy
Oncology Group (RTOG) 98-01 (abstract 2559). Proc Am Soc Clin
Oncol. 22:6362003.
|
|
30
|
Steen VD, Medsger TA Jr and Rodnan GP:
D-penicillamine therapy in progressive systemic sclerosis. Ann
Intern Med. 97:652–659. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsushima H, Kawata S, Tanura S, et al:
Reduced plasma transforming growth factor-beta1 levels in patients
with chronic hepatitis C after interferon alpha therapy:
association with regression of hepatic fibrosis. J Hepatol. 30:1–7.
1999. View Article : Google Scholar
|
|
32
|
Vozenin-Brotons MC, Sivan V, Gault N, et
al: Anti-fibrotic action of Cu/Zn SOD is mediated by TGF-beta1
repression and phenotypic reversion of myofibroblasts. Free Radic
Biol Med. 30:30–42. 2001. View Article : Google Scholar : PubMed/NCBI
|