Introduction
Following food ingestion, carbohydrates are
converted into glucose by several enzymes. Among these,
α-glucosidase is produced by intestinal cells or tissues and
cleaves glycosidic bonds in oligosaccharides during the last step
of hydrolysis (1). Glucose is the
main component of blood sugar. Therefore, α-glucosidase may be an
ideal target for the treatment of type 2 diabetes mellitus
(2). To investigate antidiabetic
properties for nutraceutical purposes, this enzyme may also be used
as a marker for in vitro assays, since the α-glucosidase
inhibitor induces hypoglycemic symptoms less frequently compared to
other oral glucose-lowering agents (3).
Cudrania tricuspidata (CT) belongs to the
Moraceae family and is distributed throughout Korea, Japan and
China (4). Ethanol extracts of CT
contain numerous compounds, including butyrospermyl acetate,
glutinol, taraxerone, quercetin, kaempferol, isorhamnetin, orobol,
3′-O-methyorobol, 1,3,6,7-tetrahydroxyxanthone, taxifolin,
naringenin, steppogenin and 5,7-dihydroxy chromone (5). Various effects of CT, such as
tyrosinase inhibition (6),
anti-oxidative activity (7) and
anti-inflammatory activity (8) have
been investigated. Its active ingredients have also been isolated
and they include prenylated xanthones (mainly cudraxanthone) and
cudraflavone (9). Although isolated
CT compounds have already been proven to possess anti-diabetic
properties using α-glucosidase inhibitory assays (2), studies on the potential activity of CT
from various sources have not yet been performed.
Recently, the consumption of crude CT extract in
Korea was abruptly increased due to its potential benefits as a
traditional complementary therapy. However, information regarding
the functional activities of different plant components according
to harvesting time has not yet been obtained. Therefore, additional
studies are required to optimize the commercial preparation of CT
extracts. In the present study, CT samples were divided according
to plant component and harvesting period and extracts were
prepared. The antidiabetic activities of the extracts were then
analyzed using an α-glucosidase inhibitory assay.
Materials and methods
Reagents
α-glucosidase type 1 from baker's yeast (G5003;
Sigma-Aldrich, St. Louis, MO, USA), p-nitrophenyl
α-D-glucopyranoside (N1377, Sigma-Aldrich), sodium phosphate
monobasic (S3139, Sigma-Aldrich), sodium phosphate dibasic (S5136,
Sigma-Aldrich), filter paper (no. 1; Whatman Schleicher &
Schuell, Keene, NH, USA), xanthone (95502; Fluka, Buchs St. Gallen,
Switzerland) and acarbose (A8980, Sigma-Aldrich) were purchased for
the purposes of this study.
Preparation of test material
The CT samples were obtained in Hampyeong, Korea,
where the plants are collected on a monthly basis throughout the
year. The plants were separated into leaves, roots and stems prior
to being further subdivided into groups according to harvesting
time. The samples underwent aqueous extraction for 2.5 h, were
filtered with filter paper and lyophilized with a freeze dryer
(IlShin Biobase Co., Ltd., Dongducheon, Korea). The lyophilized
samples were dissolved in distilled water as 100 mg/ml stock and
diluted with distilled water prior to the experiment. Nine CT
samples were prepared: stems from plants harvested between late
April and early May, 2009 (CT 1); stems harvested in middle June
(CT 2); stems harvested between late July and early August (CT 3);
stems harvested in middle September (CT 4); stems harvested in
middle January (CT 5); roots and stems harvested in middle January
(CT 6); leaves harvested in middle June (CT 7); leaves harvested
between late July and early August (CT 8); and leaves harvested in
middle September (CT 9).
Concentration of α-glucosidase and
substrate
Sodium phosphate buffer (0.1 M) was adjusted by 0.1
N HCl to pH 7.0 with a pH meter (Thermo Fisher Scientific Inc.,
Waltham, MA, USA). p-Nitrophenyl α-D-glucopyranoside (10 mM)
and α-glucosidase solutions (1 U/ml) were solubilized in 0.1 M
sodium phosphate buffer (pH 7.0). All the reagents were
manufactured shortly before use and warmed to 37°C in a water
bath.
α-glucosidase inhibition assay
Sodium phosphate buffer (0.1 M, 158 μl per well) was
added to a 96-well plate. α-Glucosidase (20 μl) and 2 μl of sample
were added to 20 μl of p-nitrophenyl α-D-glucopyranoside. In
the 200-μl final reaction volume (0.02 U/well, 0.1 U/ml) the
substrate concentration was adjusted to 10 mM. The background
signal due to the sample color was measured at 405 nm with the
PerkinElmer Wallac Victor3 spectrophotometer (PerkinElmer, Waltham,
MA, USA) prior to adding the enzyme. Immediately following
α-glucosidase addition, absorbance was measured at 405 nm 8 times
at 1-min intervals (10,11).
Creation of Lineweaver-Burk and Dixon
plots
To predict whether the extracts contained similar
patterns of compositions, a Lineweaver-Burk plot was created
according to the Michaelis-Menten equation (12) together with a Dixon plot. This was
performed instead of purifying, isolating and analyzing the active
compounds by high-pressure liquid chromatography or thin layer
chromatography. The protocol for obtaining data to create the
Lineweaver-Burk plot was identical to that used for the
α-glucosidase inhibitory assay. The α-glucosidase concentration was
0.1 U/ml, whereas the substrate was added at three concentrations:
0.1, 0.3, and 1.0 mM. The mode of inhibition was defined as
competitive, non-competitive or mixed-type non-competitive,
according to the Michaelis-Menten constants and the maximum
velocity on the Lineweaver-Burk plot (13).
Results and Discussion
In the course of screening active antidiabetic
agents, several medicinal plants were selected. The ability of CT
extracts derived from nine different plant parts to inhibite
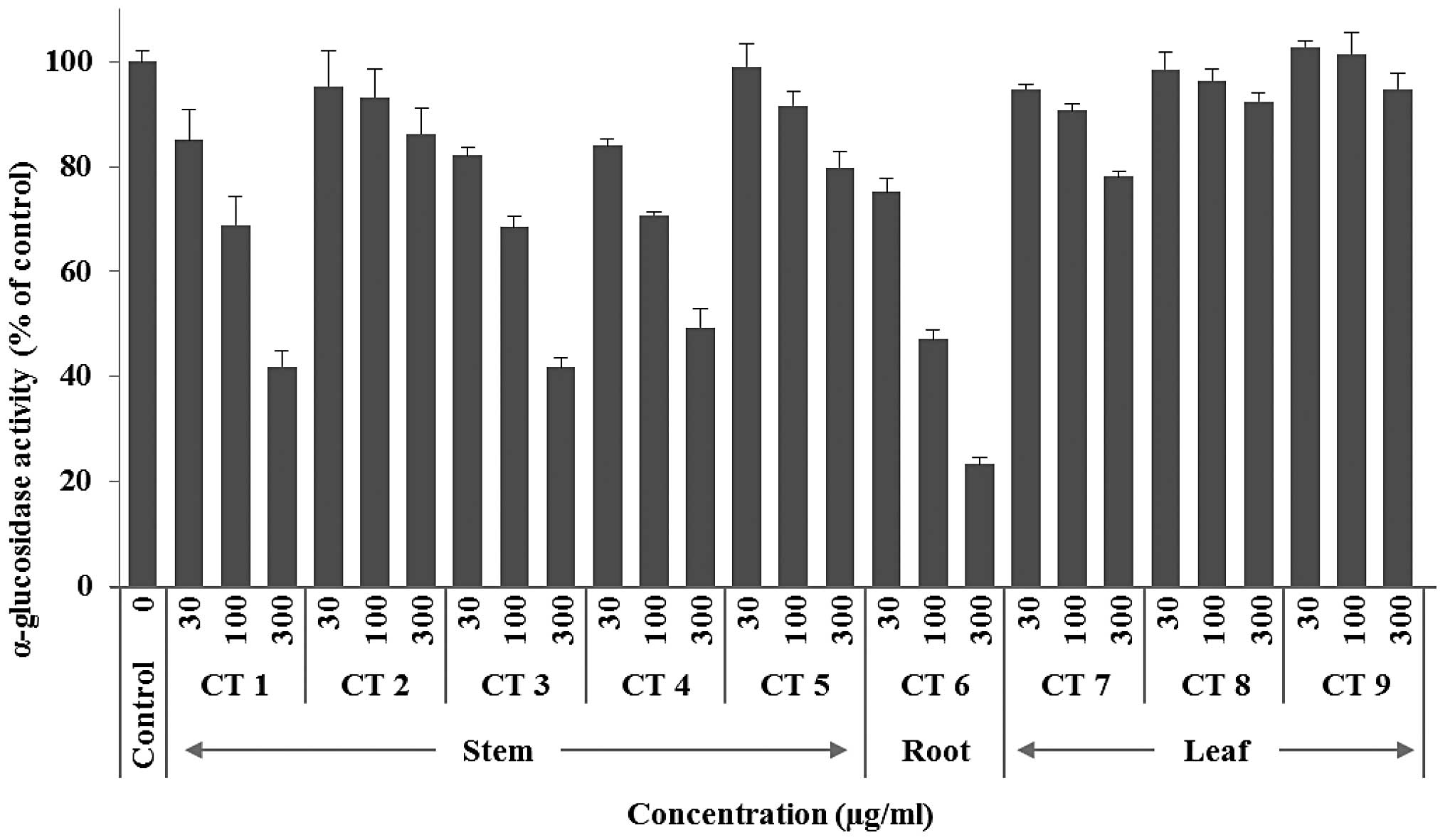
α-glucosidase activity was investigated. As shown in Fig. 1, CT 1, CT 3 and CT 6 exhibited a
significant inhibitory activity in a concentration-dependent
manner. A kinetic study was performed to assess the effects of
climate on the antidiabetic activity of the plant components, since
functional food manufacturers are interested in identifying the
active component concentrations during the growth of the leaves,
stem and bark of the CT plant. To compare the inhibitory activities
of the samples, acarbose was selected as positive control. With 1
mM of acarbose, the enzymatic velocity was decreased by ∼67%
(Fig. 1). Similar to acarbose,
almost all the extracts were associated with the same pattern of
enzymatic velocity decrement, which was concentration-dependent.
The most effective sample was demonstrated to be CT 6 (derived from
the root of plants collected in middle January). Specifically, 300
μg/ml of this sample inhibited the enzymatic velocity by 77%
(Fig. 2). By contrast, leaf
extracts (300 μg/ml) exhibited marginal to no inhibition of the
enzymatic activity (22% for CT 7, 8% for CT 8 and 0% for CT 9;
Fig. 2). In a recent study
(12), the majority of xanthone
derivatives were reported to have activities similar to that of
α-glucosidase. However, it was observed that xanthone, an organic
compound with the molecular formula
C13H8O2 that does not form
derivatives, did not exert any obvious effect on α-glucosidase
activity (data not shown).
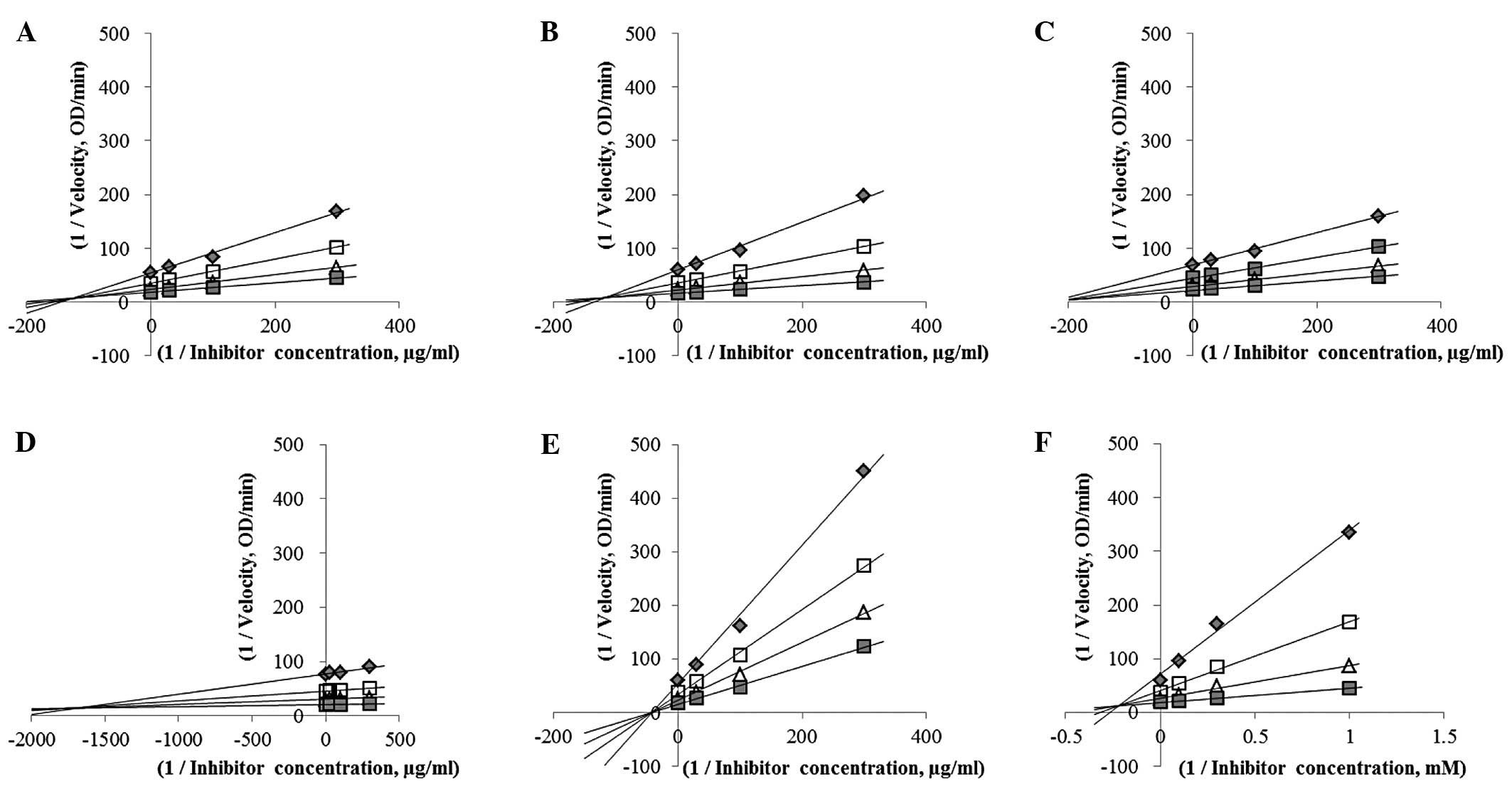
 | Figure 2.Comparison of α-glucosidase inhibition
according to a Lineweaver-Burk plot. Plots were generated based on
the Michaelis-Menten equation. (A) Cudrania tricuspidata
(CT) 1, (B) CT 3, (C) CT 4, (D) CT 5, (E) CT 6, (F) acarbose.
Concentrations of A–E (μg/ml): ■, 300; △, 100; □, 30; ◆, 0.
Concentrations of acarbose (mM): ◊, 3; ■, 0.1; △, 0.3; □, 0.1; ◆,
0. |
The type of bioactive compounds present in the CT
extracts and the composition changes in association with plant
growth through the year were then determined. Five samples were
selected and a Lineweaver-Burk plot was created based on the
reciprocals of four different concentrations and the corresponding
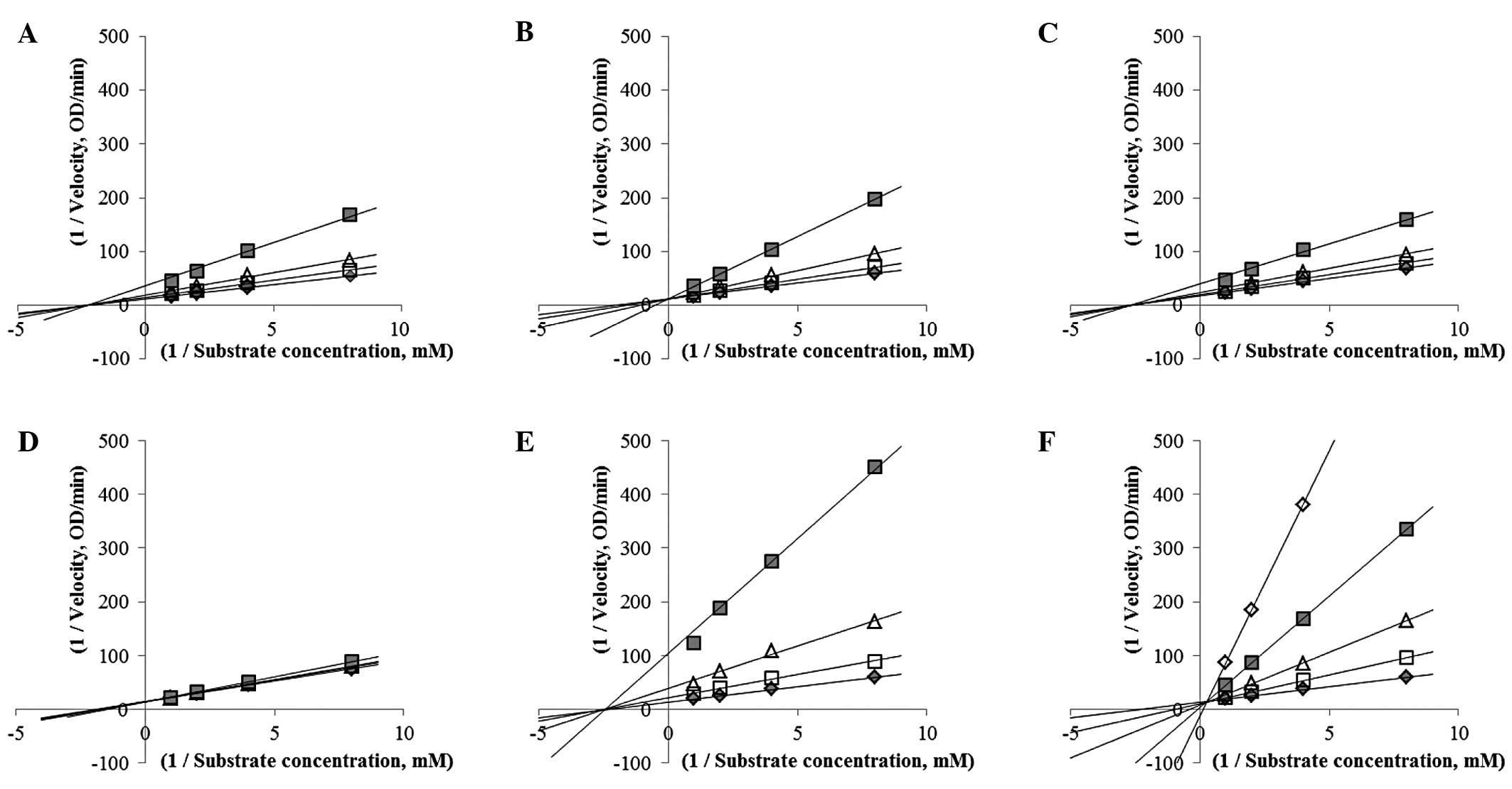
enzymatic velocities. As shown in Fig.
2, enzyme activities were reduced by the CT extracts in a
dose-dependent manner and increased with substrate in a
concentration-dependent manner. According to the Michaelis-Menten
equation (1), the samples were
classified according to the inhibition mode. The results were as
follows (Fig. 3): CT 1, CT 4 and CT
6 as non-competitive inhibitors, CT 3 and CT 5 as competitive
inhibitors and acarbose as a mixed-type non-competitive inhibitor.
Our findings demonstrated that the stem extracts acted as
non-competitive inhibitors, although one of them (CT 5) was
classified as a competitive inhibitor. CT 6 exhibited the highest
level of activity and was found to be a non-competitive inhibitor.
Although the present data are not real activity values of CT, this
approach is unique in assessing whether the extracts possess
antidiabetic properties.
To analyze the mechanism underlying α-glucosidase
inhibition, the inhibitor constant Ki was determined
with a Dixon plot (Fig. 3). Based
on the Michaelis-Menten equation, the Michaelis constant
Km value also was calculated. The inhibitory types were
identified based on the Lineweaver-Burk plot. Inhibitor constants
were represented by intersections of the lines as substrate
condition on the Dixon plot (13).
As shown in Table I, competitive
inhibitors had only one Vmax value whereas
non-competitive inhibitors had one Km value. The
inhibitor constant of CT 6, the most effective inhibitor, was 41.6
μg/ml. The inhibitor constant of acarbose was 0.22 μM. A previous
study by Seo et al (2)
reported that xanthone derivatives isolated from CT exhibit potent
α-glucosidase inhibitory activity. Hwang et al (9) also obtained xanthone derivatives from
the root bark of CT. Those studies suggested that the potent
inhibitory effect of the CT root is attributed to the abundant
levels of xanthone derivatives. Acarbose was also reported to be a
competitive inhibitor of α-glucosidase activity (3,14).
However, the results of the present study demonstrated that
acarbose acted as a mixed-type non-competitive inhibitor.
 | Table I.Determination of inhibitor constants
for the Cudrania tricuspidata (CT) extracts. |
Table I.
Determination of inhibitor constants
for the Cudrania tricuspidata (CT) extracts.
| Materials tested | Km
(mM) | Km′
(mM) | Vmax | Vmax′ | Ki |
|---|
| CT 1 | 0.431 | - | 0.0805 | 0.0518 | 125.4±2.1 |
| CT 3 | 0.489 | 0.884 | 0.0898 | - | 115.1±1.1 |
| CT 4 | 0.376 | - | 0.0575 | 0.0416 | 221.3±4.7 |
| CT 5 | 0.548 | 0.588 | 0.0716 | - | 1,694.1±12.5 |
| CT 6 | 0.417 | - | 0.0717 | 0.0258 | 41.6±0.5 |
| Acarbose | 0.416 | 0.401 | 0.0716 | 0.1271 | 220.0±7.0 |
There were no differences observed in the inhibitory
activities of CT 1–4 or CT 7–9 according to harvesting time.
However, both stem and root extracts exerted potent inhibitory
effects on α-glucosidase activity. Xanthone derivatives were
previously isolated from the methanol fraction of the CT root and
were demonstrated to inhibit α-glucosidase (2). In the present study, the aqueous
fractions of the stem or root extracts were also found to possess
potential inhibitory activities (data not shown). Our findings
indicated that these fractions contain other compounds that are
effective against α-glucosidase. In addition, the distribution of
these compounds varied according to plant components and harvesting
time. A previous study by Sen and Mukherji (15) reported that the carotenoid content
of tomatoes undergoes seasonal variation. The results of their
investigation demonstrated that carotenoid contents are highest in
the winter and lowest during the rainy season. Therefore, it was
hypothesized that CT 2, CT 8 and CT 9, which exhibited the lowest
inhibitory activities among the stem and leaf samples, were
affected by climatic conditions (Fig.
1). Since CT extract production in Korea is currently
indiscriminate and in need of certification, our results may help
define optimum conditions for the production of CT-containing foods
or health beverages for antidiabetic purposes.
References
|
1
|
Li YQ, Zhou FC, Gao F, Bian JS and Shan F:
Comparative evaluation of quercetin, isoquercetin and rutin as
inhibitors of alpha-glucosidase. J Agric Food Chem. 57:11463–11468.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seo EJ, Curtis-Long MJ, Lee BW, Kim HY,
Ryu YB, Jeong TS, Lee WS and Park KH: Xanthones from Cudrania
tricuspidata displaying potent alpha-glucosidase inhibition.
Bioorg Med Chem Lett. 17:6421–6424. 2007.PubMed/NCBI
|
|
3
|
Osonoi T, Saito M, Mochizuki K, Fukaya N,
Muramatsu T, Inoue S, Fuchigami M and Goda T: The α-glucosidase
inhibitor miglitol decreases glucose fluctuations and inflammatory
cytokine gene expression in peripheral leukocytes of Japanese
patients with type 2 diabetes mellitus. Metabolism. 59:1816–1822.
2010.
|
|
4
|
Lee BW, Lee JH, Gal SW, Moon YH and Park
KH: Selective ABTS radical-scavenging activity of prenylated
flavonoids form Cudrania tricuspidata. Biosci Biotechnol
Biochem. 70:427–432. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guan Y, Yin Z, Guo L, Huang X, Ye W and
Shen W: Studies on chemical constituents from stems of Cudrania
tricuspidata. Zhongguo Zhong Yao Za Zhi. 34:1108–1110. 2009.(In
Chinese).
|
|
6
|
Lee HJ, Do JR, Kwon JH and Kim HK:
Physiological activities of extracts from different parts of
Cudrania tricuspidata. J Korean Soc Food Sci Nutr.
40:942–948. 2011. View Article : Google Scholar
|
|
7
|
Cha JY and Cho YS: Antioxidative activity
of extracts from fruit of Cudrania tricuspidata. J Korean
Soc Food Sci Nutr. 30:547–551. 2001.
|
|
8
|
Chang SH, Jung EJ, Lim DG, Oyungerel B,
Lim KI, Her E, Choi WS, Jun MH, Choi KD, Han DJ and Kim SC:
Anti-inflammatory action of Cudrania tricuspidata on spleen
cell and T lymphocyte proliferation. J Pharm Pharmacol.
60:1221–1226. 2008.
|
|
9
|
Hwang JH, Hong SS, Han XH, Hwang JS, Lee
D, Lee H, Yun YP, Kim Y, Ro JS and Hwang BY: Prenylated xanthones
from the root bark of Cudrania tricuspidata. J Nat Prod.
70:1207–1209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi CW, Choi YH, Cha MR, Yoo DS, Kim YS,
Yon GH, Hong KS, Kim YH and Ryu SY: Yeast α-glucosidase inhibition
by isoflavones from plants of Leguminosae as an in vitro
alternative to acarbose. J Agric Food Chem. 58:9988–9993. 2010.
|
|
11
|
Nishio T, Hakamata W, Kimura A, Chiba S,
Takatsuki A, Kawachi R and Oku T: Glycon specificity profiling of
alpha-glucosidases using monodeoxy and mono-O-methyl derivatives of
p-nitrophenyl alpha-D-glucopyranoside. Carbohydr Res. 124:629–634.
2002. View Article : Google Scholar
|
|
12
|
Li GL, He JY, Zhang A, Wan Y, Wang B and
Chen WH: Toward potent α-glucosidase inhibitors based on xanthones:
a closer look into the structure-activity correlations. Eur J Med
Chem. 46:4050–4055. 2011.
|
|
13
|
Dixon M: The determination of enzyme
inhibitor constants. Biochem J. 55:170–171. 1953.
|
|
14
|
Kim MJ, Lee SB, Lee HS, Lee SY, Baek JS,
Kim D, Moon TW, Robyt JF and Park KH: Comparative study of the
inhibition of alpha-glucosidase, alpha-amylase, and
cyclomaltodextrin glucanosyltransferase by acarbose, isoacarbose,
and acarviosine-glucose. Arch Biochem Biophys. 371:277–283. 1999.
View Article : Google Scholar
|
|
15
|
Sen S and Mukherji S: Season-controlled
changes in biochemical constituents and oxidase enzyme activities
in tomato (Lycopersicon esculentum Mill.). J Environ Biol.
30:479–483. 2009.PubMed/NCBI
|