Introduction
T-type calcium channels are divided into three
subtypes, Cav3.1, Cav3.2 and Cav3.3, coded by α1G, α1H and α1I,
respectively. They are a class of low voltage-dependent calcium
channels that may be activated following minor depolarizations of
the cell membrane. Furthermore, there is a window current in the
T-type calcium channel, which refers to the voltage overlap between
the activation and steady-state inactivation at low or resting
membrane potentials (1–3). As a result, extracellular calcium ions
are able to enter the intra-cellular compartment through a small
proportion of channels that remain open under the window current.
These electro-physiological characteristics are responsible for the
T-type calcium channels being a key element in the regulation of
neuron excitability and neurotransmitter release (4–6).
The functions of the T-type calcium channels have
yet to be fully elucidated, which may be the reason for the lack of
specific antagonists for this type of channel. The existing T-type
calcium channel inhibitors exhibit poor specificity and may block
the high voltage-dependent calcium channels, such as the L- and
N-type channels (7). Furthermore,
there is no selectivity to the subtype of the T-type calcium
channel. Therefore, the development of a specific T-type calcium
channel inhibitor may contribute to the elucidation of the
functions and characteristics of this type of calcium channel.
In our previous study, all three subtypes of the
T-type calcium channel, Cav3.1, Cav3.2 and Cav3.3, were detected in
SH-SY5Y cells (8). However, there
was some diversity in the expression levels. Cav3.1 was the
dominant subtype in SH-SY5Y cells, whereas the expression of Cav3.2
and Cav3.3 was significantly lower. The aim of the present study
was to silence Cav3.1 mRNA expression in SH-SY5Y cells via the RNA
interference (RNAi) method in order to construct
pshRNA-CACNA1G-SH-SY5Y cells and detect Cav3.1 mRNA and protein
expression by western blot analysis and reverse
transcription-polymerase chain reaction (RT-PCR) with the aim of
identifying the constructed cell line. Findings of the present
study may contribute to the elucidation of the functions of the
Cav3.1 T-type calcium channel in the SH-SY5Y cells.
Materials and methods
Materials
The SH-SY5Y cell line was purchased from the
Shanghai Institutes for Biological Sciences (Shanghai, China).
psPAX, pMD2.G lentiviral packaging system, 293FT packaging cells
and pSUPER-retro-puro plasmid were purchased from Laura Biotech
Co., Ltd. (Guangzhou, China). Goat polyclonal anti-Cav3.1 and
anti-β-actin antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The primer of Cav3.1 and
β-actin was synthesized by Shanghai Sangon Biotech Co., Ltd.
(Shanghai, China). Plasmid Maxiprep kits and DNA purification kits
were purchased from Tiangen Biotech Co., Ltd. (Beijing, China). DNA
polymerase, DNA ligase and PrimeSTAR HS DNA polymerase were
purchased from Takara Biotechnology Co., Ltd. (Dalian, China).
Restriction enzymes BglII and HindIII were purchased
from New England Biolabs (Beverly, MA, USA).
Cell culture
SH-SY5Y cells were cultured in DMEM/F12 medium with
15% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml
streptomycin in a humidified 5% CO2 incubator at 37°C.
The medium was renewed every 2 days (9).
shRNA sequence design
According to NM_018896 gene sequence, the human
Cav3.1 (α1G) gene, we investigated three interference targets and
designed three interference sequences and one negative sequence
(Table I) (http://www.genscript.com/ssl-bin/app/rnai and
https://rnaidesigner.invitrogen.com/rnaiexpress/).
The sequences were synthesized by Sangon Biotech. The interference
targets were as follows: 961: accaactgct cagcggggga gcacaacccc
ttcaagggcg ccatcaactt
tgacaacatt; 2281: ggcatcgaat accacgagca
gcccgaggag cttaccaacg
ccctagaaat cagcaacatc; and 3841: gtggtccttg tcatcatctt ccttaactgc
atcaccatcg ccatggagcg ccccaaaatt.
 | Table I.Primers of negative and interference
sequences. |
Table I.
Primers of negative and interference
sequences.
| Primer name | Primer sequence |
|---|
| NC | |
| Up |
5′-gatccccgccagcttagcactgactcttcaagagagagtcagtgctaagctggcttttta-3′ |
| Down |
5′-agcttaaaaagcgccttccgtcttgggaatctcttgaattcccaagacggaaggcgcggg-3′ |
| shRNA1 (RNAi1) | |
| Up |
5′-gatccccgccatcaactttgacaacattttcaagagaaatgttgtcaaagttgatggcttttta-3′ |
| Down |
5′-agcttaaaaagccatcaactttgacaacatttctcttgaaaatgttgtcaaagttgatggcggg-3′ |
| shRNA2 (RNAi2) | |
| Up |
5′-gatcccccgcttaccaacgccctagaaatttcaagagaatttctagggcgttggtaagcgttttta-3′ |
| Down |
5′-agcttaaaaacgcttaccaacgccctagaaattctcttgaaatttctagggcgttggtaagcgggg-3′ |
| shRNA3 (RNAi3) | |
| Up |
5′-gatcccccccttgtcatcatcttccttaattcaagagattaaggaagatgatgacaagggttttta-3′ |
| Down |
5′-agcttaaaaacccttgtcatcatcttccttaatctcttgaattaaggaagatgatgacaaggggg-3′ |
Construction of
pshRNA-pSUPER-retro-puro
Following annealing, the synthetic nucleotide
described above formed a double chain and was combined with the
enzymatically digested pSUPER-retro-puro to construct pNC-puro
(NC), pshRNA-puro-CACNA1G-RNAi1 (RNAi1), pshRNA-puro-CACNA1G-RNAi2
(RNAi2) and pshRNA-puro-CACNA1G-RNAi3 (RNAi3). The reaction system
of the enzymatic digestion of pSUPER-retro-puro plasmid was as
follows: 4 μl NEB 10 buffer, 1 μl BglII (10 units), 1 μl
HindIII (10 units), 20 μl pSUPER-retro-puro plasmid (2 μg)
and 14 μl DDH2O. The reaction system of the connection
of shRNA with the pSUPER-retro-puro plasmid was as follows: 5 μl
enzymatically digested pSUPER-retro-puro plasmid, 4.5 μl
double-chain shRNA, 2 μl connection buffer, 1 μl T4 DNA ligase and
7.5 μl ddH2O. The products were transferred into
competent cells and the recombinant plasmids were measured with a
DNA sequencing system (Sangon Biotech).
Construction of pshRNA-CACNA1G-SH-SY5Y
cells
The calcium phosphate mixture was prepared as
follows: 30 μl CaCl2(2M), 15 μg pSPAX2, 5 μg pMD2.G, 20
μg pNC-puro (NC) or pshRNA-puro-CACNA1G-RNAi1 (RNAi1) or
pshRNA-puro-CACNA1G-RNAi2 (RNAi2) or pshRNA-puro-CACNA1G-RNAi3
(RNAi3) and 30 μl ddH2O. The ratio of recombinant
plasmid:pSPAX2:pMD2.G was 4:3:1. Six hours after the mixture was
prepared at room temperature, 293T cells were cultured with this
mixture for 36–48 h. The liquid supernatant of the culture solution
was collected 3–4 times. The collected liquid supernatant was added
to the SH-SY5Y cell culture solution and incubated at 37°C for 3 h.
The liquid supernatant was then changed and the process was
repeated twice. The transfected SH-SY5Y cells were conserved at
−80°C.
Cav3.1 mRNA detected by RT-PCR
Total RNA from the transfected SH-SY5Y cells was
extracted using TRIzol reagent (Takara Biotechnology Co., Ltd.).
The isolated RNA was subsequently treated with RNase-free DNase to
remove genomic DNA contamination. Conventional gene expression
analysis was performed according to previously published protocols
(10). Briefly, isolated RNA was
reverse-transcribed and amplified with a commercial kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer’s
protocol. Oligonucleotide primers (Table II) were designed using Oligo 6
Primer Analysis software (Molecular Biology Insights Inc., Cascade,
CO, USA). Total RNA (2 μg) was reverse-transcribed for 60 min at
42°C followed by PCR amplification. The PCR products were separated
on 1.5% agarose gels and visualized by ethidium bromide staining.
The intensity of the bands was measured by densitometry and the
relative value of Cav3.1 to the β-actin band was calculated in each
sample.
 | Table II.Primer sequences of β-actin and
Cav3.1. |
Table II.
Primer sequences of β-actin and
Cav3.1.
| Gene | Primer sequence | Product size
(bp) |
|---|
| β-actin | | |
| Forward |
5′-TGGCACCCAGCACAATGAA-3′ | 186 |
| Reverse |
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ | |
| Cav3.1 | | |
| Forward |
5′-GCCATCTTCCAGGTCATCAC-3′ | 140 |
| Reverse |
5′-ACCAGGCACAGGTTGATCAT-3′ | |
Cav3.1 protein detected by western blot
analysis
All the procedures were performed on ice to prevent
proteolysis of the calcium channel subunits. Culture flasks or
plates were quickly rinsed with chilled PBS. The cells were
collected using a plastic cell scraper, removed and lysed in lysis
buffer A [20.0 mmol/l Tris-HCl, 1.0 mmol/l
Na3VO4, 1.5 mmol/l MgCl2, 10
mmol/l KCl, 0.1 mmol/l ethylenediaminetetraacetic acid, 0.1 mmol/l
ethylene glycol tetraacetic acid, 0.5 mmol/l phenylmethylsulfonyl
fluoride and 0.02% protease inhibitor cocktail (pH 7.9)]. Protein
samples were dissolved in 4X sample buffer [250 mmol/l Tris-HCl,
200 mmol/l sucrose, 300 mmol/l dithiothreitol, 0.01% Coomassie
brilliant blue G and 8% SDS (pH 6.8)] and were subsequently
denatured at 95°C for 5 min. Equivalent amounts of protein were
separated by 7.5% sodium dodecylsulfate polyacrylamide gel
electrophoresis and were transferred onto nitrocellulose membranes.
The membranes were incubated overnight at 4°C with the following
primary antibodies: rabbit anti-human Cav3.1 or β-actin (1:500;
Santa Cruz Biotechnology, Inc.). The membranes were washed
thoroughly with Tris-buffered saline/Tween-20 and incubated for 2 h
in peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(1:500, Santa Cruz Biotechnology, Inc.) at room temperature. The
immune complexes were detected by enhanced chemiluminescence.
Membranes were then exposed to X-ray film. Quantification of the
protein bands was conducted by scanning the films and importing the
images into Adobe Photoshop software (Adobe, San Jose, CA, USA).
Scanning densitometry was used for semi-quantitative analysis of
data. The Cav3.1 protein was normalized to the corresponding
β-actin product.
Statistical analysis
Data are expressed as means ± standard error of the
mean and were analyzed using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). The comparisons of protein and mRNA expression
between the three T-type calcium channel subtypes in cultured
SH-SY5Y cells were performed using repeated measures analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
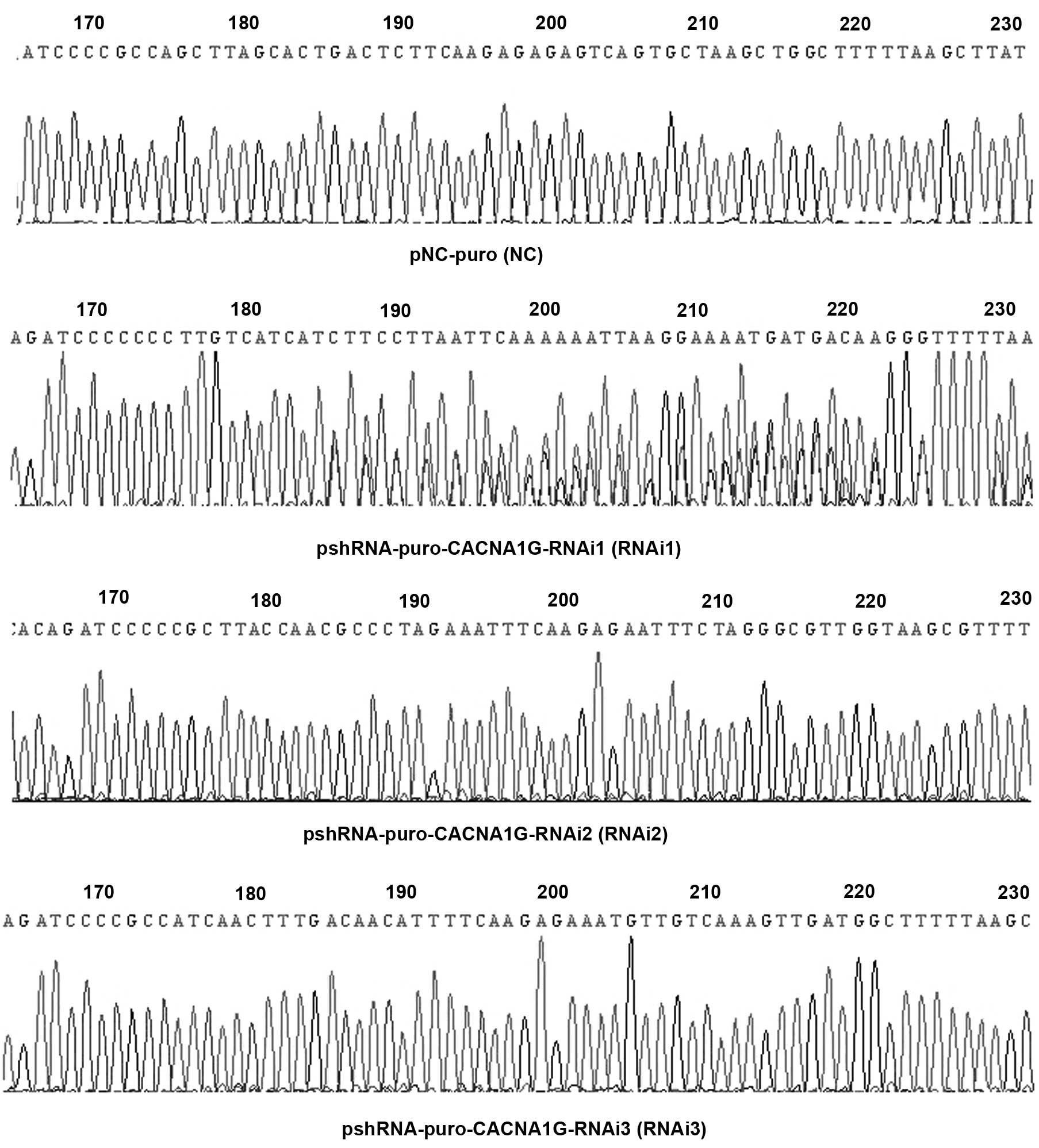
DNA sequence analysis of
pshRNA-pSUPER-retro-puro
The DNA sequences of pshRNA-pSUPER-retro-puro vector
were detected and were in complete accordance with the three
designed interference sequences and the negative sequence (Fig. 1).
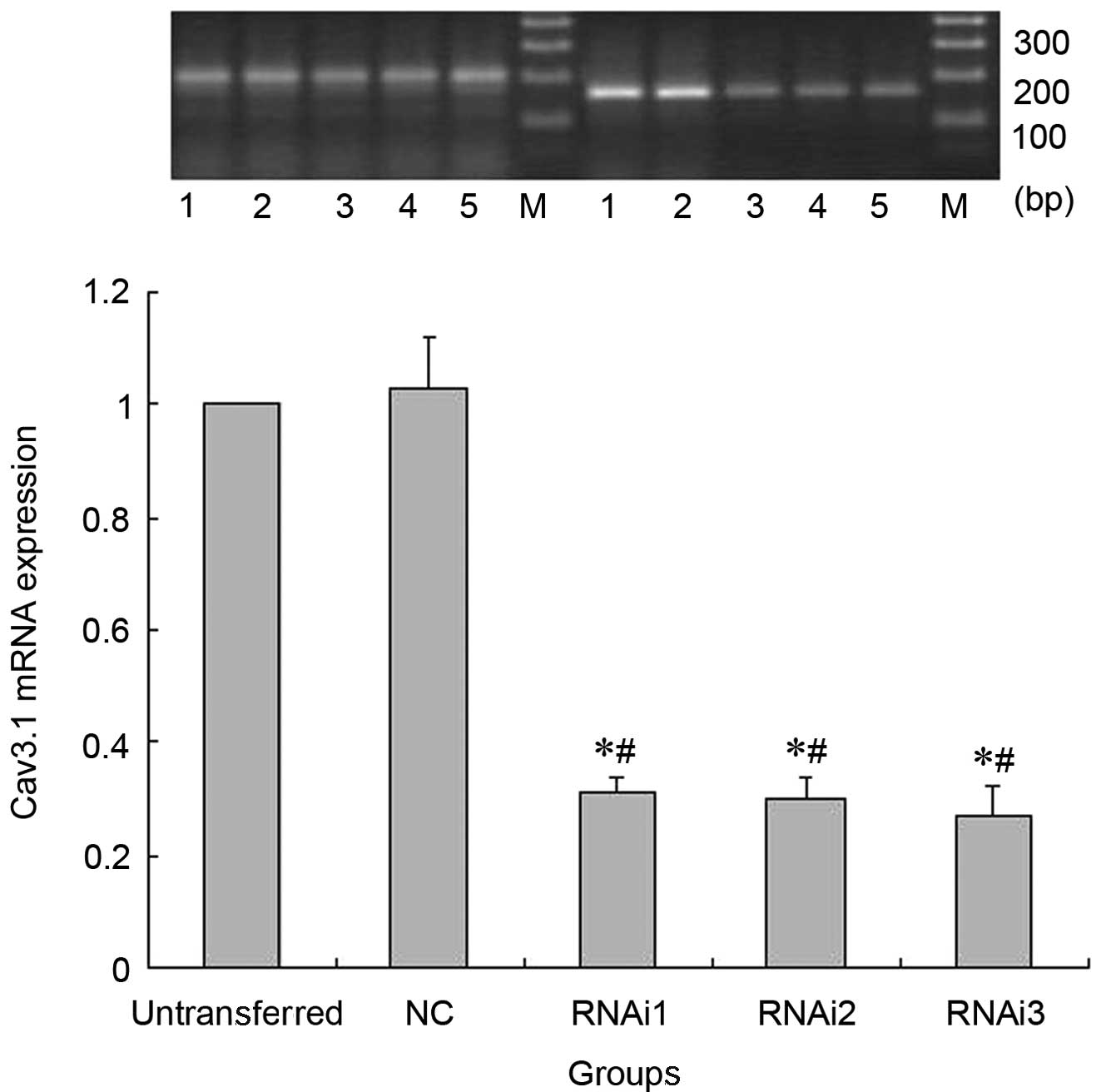
Cav3.1 mRNA expression detection by
RT-PCR
Compared to the untransferred SH-SY5Y cells and
pNC-puro cells, Cav3.1 mRNA expression in the cells of the RNAi1,
RNAi2 and RNAi3 groups was distinctly decreased. However, there
were no significant differences in the Cav3.1 mRNA expression
between the cells in the untransferred and NC groups (Fig. 2).
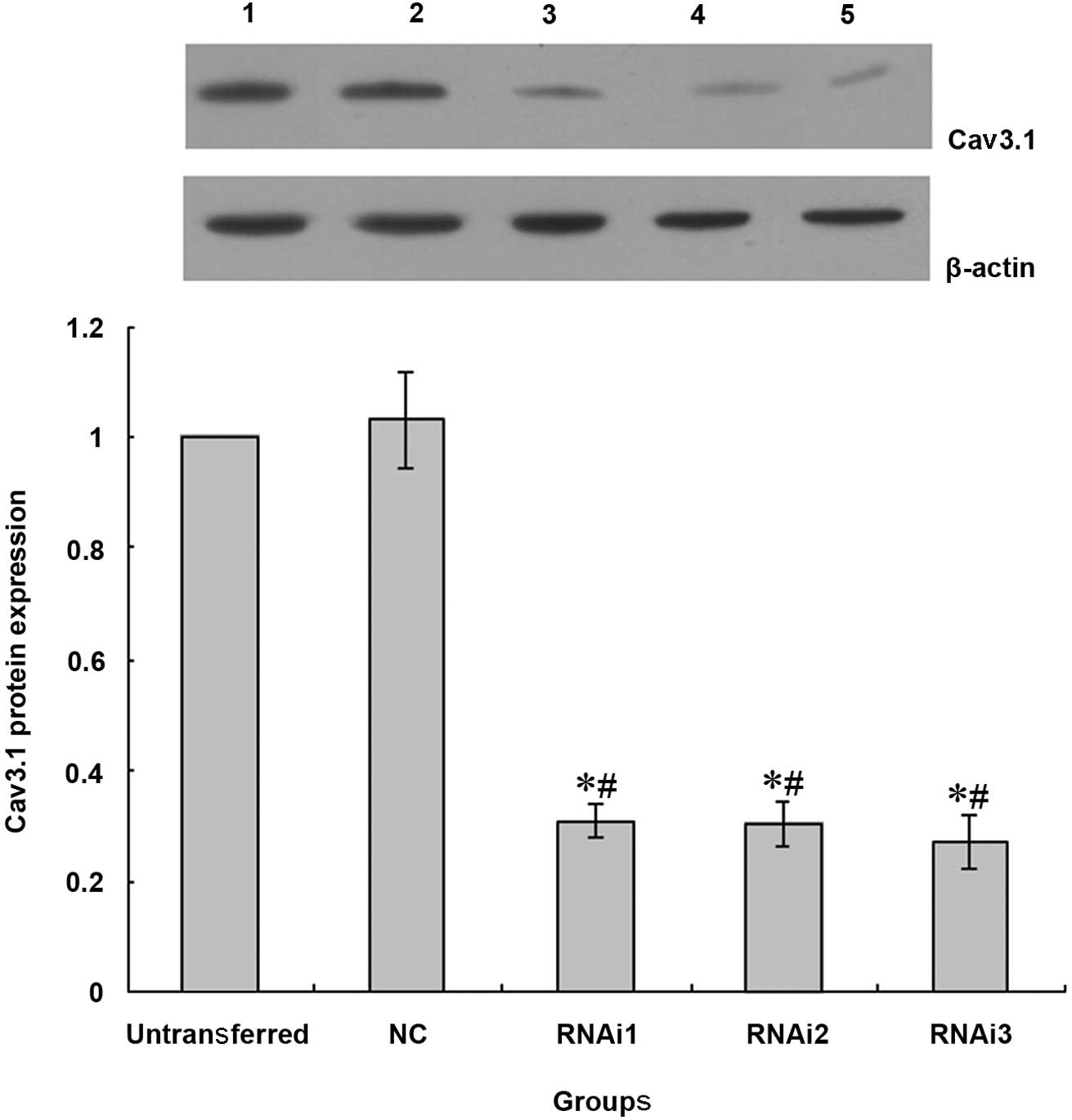
Cav3.1 protein expression detection by
western blot analysis
The Cav3.1 protein and mRNA expression were similar.
There were no significant differences in the Cav3.1 protein
expression between the cells in untransferred and NC groups.
However, the Cav3.1 protein expression in the cells of the RNAi1,
RNAi2 and RNAi3 groups was clearly decreased (Fig. 3).
Discussion
With the development of biomedical technology,
genetic engineering has become an important method in the clinical
setting and scientific research (11–14).
The small double-stranded RNA molecules may silence gene
expression, which is considered a sequence-specific gene
inactivation system. RNAi is a phenomenon of homologous specificity
mRNA degradation induced by the highly conservative double-stranded
RNA. RNAi technology may specifically eliminate or shut down
specific gene expressions. The RNAi method exhibits the following
characteristics: i) functions at the transcriptional level of the
gene silencing mechanism; ii) is of high specificity, with
degradation of only the corresponding single endogenous gene mRNA;
iii) is highly efficient and a relatively small amount of
double-stranded RNA (dsRNA) molecular may fully suppress the
corresponding gene expression via catalytic amplification; iv) the
inhibitory effect of RNAi on specific gene expressions may be
transmitted intercellularly for a long distance; and v) the dsRNA
usually is ≥21-bp long and dsRNAs >30 bp are not able to induce
specific RNAi in mammals.
Several methods are adapted to RNAi. First, the
chemical synthesis method is in common use (15–17).
The small interfering RNA molecules (siRNAs) consisting of 21 bases
with two free nucleotides in the 3′ terminal are synthesized in
vitro. Compared to the other synthesized RNA molecules, siRNAs
are able to degrade the target gene with the highest efficiency.
Second, siRNAs are connected to the vector. Plasmids or viruses are
often used as vectors. The synthesis of the sense or antisense
strand is regulated by the U6 snRNA and T7 RNA polymerase.
Following annealing, the sense and antisense strands form the dsRNA
that connects to the vector and interferes with gene expression.
The construction of the dsRNA vector is also often used in lower
organisms.
In this study, three RNAi sequence were designed
according to the CACNA1G gene sequence NM_018896 and the RNAi
design principle. Following annealing, the sense and antisense
sequences formed dsRNA molecules and were connected to the
pSUPER-retro-puro plasmid vector. The sequences of the connected to
pSUPER-retro-puro plasmid vector were same as those designed by DNA
sequencing. The dsRNA molecules were packaged with the lentiviral
vectors and 293FT cells. The supernatant liquor of the virus was
collected following centrifugation. The SH-SY5Y cells were infected
with the collected supernatant liquor to construct
pshRNA-CACNA1G-SH-SY5Y cells and Cav3.1 protein or mRNA were
detected with western blot analysis or RT-PCR. The results
demonstrated that Cav3.1 protein and mRNA expression significantly
decreased following infection of the SH-SY5Y cells by the
supernatant liquors. These data suggest that the
pshRNA-CACNA1G-SH-SY5Y cells were successfully constructed.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (no.
81100831) and the Medical Research Foundation of Guangdong (no.
B2011303).
References
|
1
|
McRory JE, Santi CM, Hamming KS, et al:
Molecular and functional characterization of a family of rat brain
T-type calcium channels. J Biol Chem. 276:3999–4011. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee S, Han TH, Sonner PM, et al: Molecular
characterization of T-type Ca2+ channels responsible for
low threshold spikes in hypothalamic paraventricular nucleus
neurons. Neuroscience. 155:1195–1203. 2008.PubMed/NCBI
|
|
3
|
Williams ME, Washburn MS, Hans M, et al:
Structure and functional characterization of a novel human
low-voltage activated calcium channel. J Neurochem. 72:791–799.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Isope P, Hildebrand ME and Snutch TP:
Contributions of T-type voltage-gated calcium channels to
postsynaptic calcium signaling within Purkinje neurons. Cerebellum.
11:651–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mahapatra S, Calorio C, Vandael DH, et al:
Calcium channel types contributing to chromaffin cell excitability,
exocytosis and endocytosis. Cell Calcium. 51:321–330. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kitchens SA, Burch J and Creazzo TL:
T-type Ca2+current contribution to
Ca2+-induced Ca2+release in developing
myocardium. J Mol Cell Cardiol. 35:515–523. 2003.
|
|
7
|
Leuranguer V, Mangoni ME, Nargeot J and
Richard S: Inhibition of T-type and L-type calcium channels by
mibefradil: physiologic and pharmacologic bases of cardiovascular
effects. J Cardiovasc Pharmacol. 37:649–661. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen XJ, Xu SY, Wang LL, et al: T-type
calcium channel expression in cultured human neuroblastoma cells.
Neural Regen Res. 6:2410–2413. 2011.
|
|
9
|
Lu J, Xu SY, Zhang QG, et al: Bupivacaine
induces apoptosis via mitochondria and p38 MAPK dependent pathways.
Eur J Pharmacol. 657:51–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wen XJ, Xu SY, Chen ZX, et al: The roles
of T-type calcium channel in the development of neuropathic pain
following Chronic Compression of rat dorsal root ganglia.
Pharmacology. 85:295–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gentner B and Naldini L: Exploiting
microRNA regulation for genetic engineering. Tissue Antigens.
80:393–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang H, Huang L, Cao J, et al: Regulation
of mammalian gene expression by exogenous microRNAs. Wiley
Interdiscip Rev RNA. 3:733–742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lambeth LS and Smith CA: Short hairpin
RNA-mediated gene silencing. Methods Mol Biol. 942:205–232. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gavrilov K and Saltzman WM: Therapeutic
siRNA: principles, challenges, and strategies. Yale J Biol Med.
85:187–200. 2012.PubMed/NCBI
|
|
15
|
Lakatos L, Csorba T, Pantaleo V, et al:
Small RNA binding is a common strategy to suppress RNA silencing by
several viral suppressors. EMBO J. 25:2768–2780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J and Li LC: Small RNA and its
application in andrology and urology. Transl Androl Urol. 1:33–43.
2012.PubMed/NCBI
|
|
17
|
Huang Q and Hartung JS: Construction of
infectious clones of double-stranded DNA viruses of plants using
citrus yellow mosaic virus as an example. Methods Mol Biol.
451:525–533. 2008. View Article : Google Scholar : PubMed/NCBI
|