Introduction
The function of XAGE-1b, a member of the cancer
testis antigen (CTA) family, has been previously investigated, with
a focus on its expression profile and immunogenicity (1–3).
Overexpression of XAGE-1b in adenoid cystic carcinoma-M (ACC-M) and
ACC cell lines was observed in an earlier investigation (data not
yet published). Results of that study suggested that XAGE-1b is an
important gene that is relevant to the tumorigenesis and metastasis
of ACC. Additionally, XAGE-1b overexpression and RNA interference
confirmed that XAGE-1b promoted the cell growth and metastasis of
ACC in vivo and in vitro (unpublished data).
XAGE-1b is mainly expressed within the nucleus and
transcription activity domains such as GAL-4 are located at its C
terminal. Therefore, XAGE-1b potentially functions as a
transcription factor. Findings of a previous study showed that
GAGE-7, which belongs to the same family as CTAs, inhibited cell
apoptosis mediated by interferon or Fas receptor (4). At present, little is known about the
definite correlation between alterations of the cell cycle and
apoptosis and XAGE-1b over-expression in ACC.
To study the cell cycle, a eukaryotic vector with
transient XAGE-1b overexpression was constructed and transfected
into ACC-2 cells, and its anti-apoptotic effects were investigated.
The downstream signaling pathway and the interaction chaperone in
which XAGE-1b was involved were detected using the Mercury pathway
profiling system provided by Clontech (5). The cis-acting element of the molecule
associated with the cell signaling pathway highlighted the
reporting gene of the plasmid system, and the reporting gene
expression detected identified the direct or indirect interaction
between XAGE-1b protein and its gene enhancer. Results obtained in
the present study provide important evidence to elucidate the
mechanism for promoting tumor cell growth with XAGE-1b
overexpression.
Materials and methods
Plasmids and cell lines
The Mercury™ pathway profiling vector purchased from
Clontech (Mountain View, CA, USA) included the cis-enhancement
elements, TAL initiator and Luciferase reporter gene that belong to
the transcription factors of the signaling pathway. The vectors
included pAP1-Luc, pCRE-Luc, pGRE-Luc, pHSE-Luc, pNF-κB-Luc,
pSRE-Luc, pP53-Luc, pRB-Luc, pc-Myc-Luc, pE2F-Luc and pTAL-Luc
(control plasmid). The PCMV-Myc and the control pRL-SV40 plasmid
were purchased from Clontech and Promega (Madison, WI, USA),
respectively. The human salivary ACC-2 and 293T cells were obtained
from American Type Culture Collection (ATCC, Manassas, VA, USA).
ACC-2 cells cultured in RPMI-1640 were purchased from Gibco
(Langley, OK, USA) and supplemented with 10% fetal bovine serum
(FBS) from Sigma (St. Louis, MO, USA) at 37°C in a humidified
atmosphere of 5% CO2 in air. 293T cells were cultured in
DMEM obtained from Gibco and supplemented with 10% FBS. All
chemicals used for cell culture were purchased from Gibco.
Construction of the eukaryotic vector
with transient XAGE-1b overexpression
cDNA of XAGE-1b from ACC-2 cells was obtained and
amplified using XAGE-1b primers: PCMV-1B, F: 5′-CCGGAATTCGGATggAgAgCCCCAAAAAgAAgA-3′
and R: 5′-CCGGTCGAGTTGCGTTGTTTCAGCTTGTC-3′
with the restriction sites EcoRI and XhoI (underlined
base sequences) respectively. The fragments obtained were inserted
into the PCMV-Myc plasmid designated as PCMV-Myc-1b as the
eukaryotic transient overexpression vector.
Plasmid extraction
The E. coli DH5α containing PGL3-A1, PGL3-A2,
PGL3-A3, PGL3-B1, PGL3-B2, PGL3-B3, PGL3 and PRL plasmid were
cultured to its logarithm phase and the precipitations were
collected by centrifugation. Extraction was performed according to
the manufacturer’s instructions (Tiangen, Beijing, China). The
extracted expression plasmid comprised PCMV-Myc-1b, the control
negative PCMV-Myc, the double fluorescent control pRL-SV40 and the
Mercury™ pathway profiling vector plasmid, including pAP1-Luc,
pCRE-Luc, pGRE-Luc, pHSE-Luc, pNF-κB-Luc, pSRE-Luc, pP53-Luc,
pRB-Luc, pc-Myc-Luc, pE2F-Luc and pTAL-Luc.
Cell cycle alterations with XAGE-1b
overexpression
ACC-2 cells at a density of 2×105/ml were
seeded in 6-well plates and cultured for 18–24 h, and grown until
70–80% confluence at 37°C in an atmosphere of 5% CO2
prior to transfection. PCMV-Myc-1b (2 μg) sequencing and the
control negative PCMV-Myc were transfected with 4 μl of
Lipofectamine 2000, respectively, and with 3 wells/transfected
cells. The cells were continuously cultured for 36 h at 37°C, and
the collected cells (1–5×105) were centrifuged at
55.5–111 × g for 5 min and washed with 3 ml PBS. The precipitations
were fixed in cold 70% alcohol at 4°C overnight. Subsequent to
centrifugation, the sediment was suspended with 3 ml PBS and then
centrifuged at 55.5–111 × g for 5 min. The precipations were
stained with 1 ml PI (10 μg/ml, Sigma) containing Rnase A
(20 mg/l) and 1.5% Triton X-100 at 4°C for 30 min in the dark.
After washing with PBS, the cell cycle was analyzed with a
FACSCalibur flow cytometer according to the manufacturer’s
instructions (Becton-Dickinson and Company, Franklin Lakes, NJ,
USA).
Anti-apoptotic effects of XAGE-1b
ACC-2 cells (8×104) were seeded in
24-well plates and cultured for 18–24 h, and grown to 70–80%
confluence at 37°C in an atmosphere of 5% CO2.
PCMV-Myc-1b (1 μg) and the control negative PCMV-Myc were
transfected with 2 μl Lipofectamine 2000 and with 24
wells/transfected cells. Cells were continuously cultured for 24 h
at 37°C. Apoptosis was subsequently induced by tumor necrosis
factor-α (TNF-α) (Xinbainuo, Shanghai, China) and serum
deprivation, respectively. The cell content representing the number
of necrotic and apoptotic cells at the Sub-G1 phase was
detected and analyzed with a FACSCalibur flow cyto-meter following
48- and 72-h induction, respectively.
Regulatory effects on the main signaling
pathway transcripts with XAGE-1b overexpression
293T cells (1×104) were inoculated in
96-well plates and cultured for 24 h, and grown to 70–80%
confluence at 37°C in an atmosphere of 5% CO2. The
plasmids were simultaneously transfected by Lipofectamine 2000. The
amount of PCMV-Myc-1b and PCMV-Myc empty plasmid (control) as well
as the amount of the reporter plasmid containing cis-acting
elements were 150 ng/well, while the amount of pRL-SV40 as an inner
referencing plasmid representing the transfection efficiency was 20
ng/well. During the concentration-gradient experiment, the amount
of Mercury Pathway Profiling system plasmid and the pRL-SV40
plasmid remained unchanged. However, the content of PCMV-Myc-1b was
arranged according to the gradients 300, 150, 100, 75, 50 and 0
ng/well, while the amount in the controls reached 300 ng by
PCMV-Myc empty plasmid. Cells were lysed after 36 h culture, and
the luciferase activity was detected using the Dual-Luciferase kit
(Promega, Madison, WI, USA). Fluorescence intensity was measured by
Lumat LB9507 luminometer (Berthold Technologies GmbH & Co. KG,
Bad Wildbad, Germany). The relative value of fluorescence intensity
was recorded, and the ratio of M1/M2 was calculated.
Statistical analysis
Data were presented as the mean values and standard
deviation of the sample. Statistical analysis was performed using
the two-tailed Student’s t-test. P<0.05 was considered
statistically significant.
Results
Cell cycle alterations of ACC-2
cells
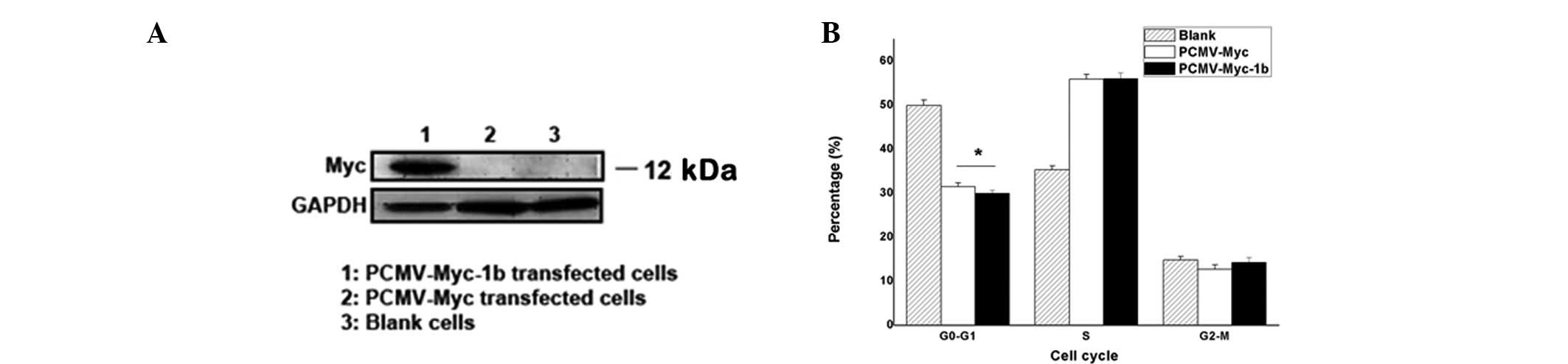
XAGE-1b overexpression transfected with PCMV-Myc-1b
plasmid in ACC-2 cells was observed. Results of the western blot
analysis are shown in Fig. 1A.
Transient XAGE-1b overexpression in ACC-2 cells with PCMV-Myc-1b
plasmid transfection was identified, whereas no overexpression of
the target protein with control plasmid transfection or the blank
was observed. Therefore, the plasmid could be applied in the study
for its transient overexpression of XAGE-1b gene.
Cell cycle alterations are shown in Fig. 1B. G0-G1 phase
exhibited a marked decrease in the PCMV-Myc-1b and PCMV-Myc groups
compared with the blank. A statistical difference between
PCMV-Myc-1b and PCMV-Myc (P<0.05) was observed. S phase
increased more evidently than the blank, although no statistical
difference was noted. A decrease was observed in the cell cycle of
the G2-M phase compared with the blank, while
G2-M phase of PCMV-Myc-1b was increased compared with
PCMV-Myc. There was no statistical difference (P>0.05).
Anti-apoptotic effects of XAGE-1b
overexpression
Cytokine TNF-α and serum deprivation were applied to
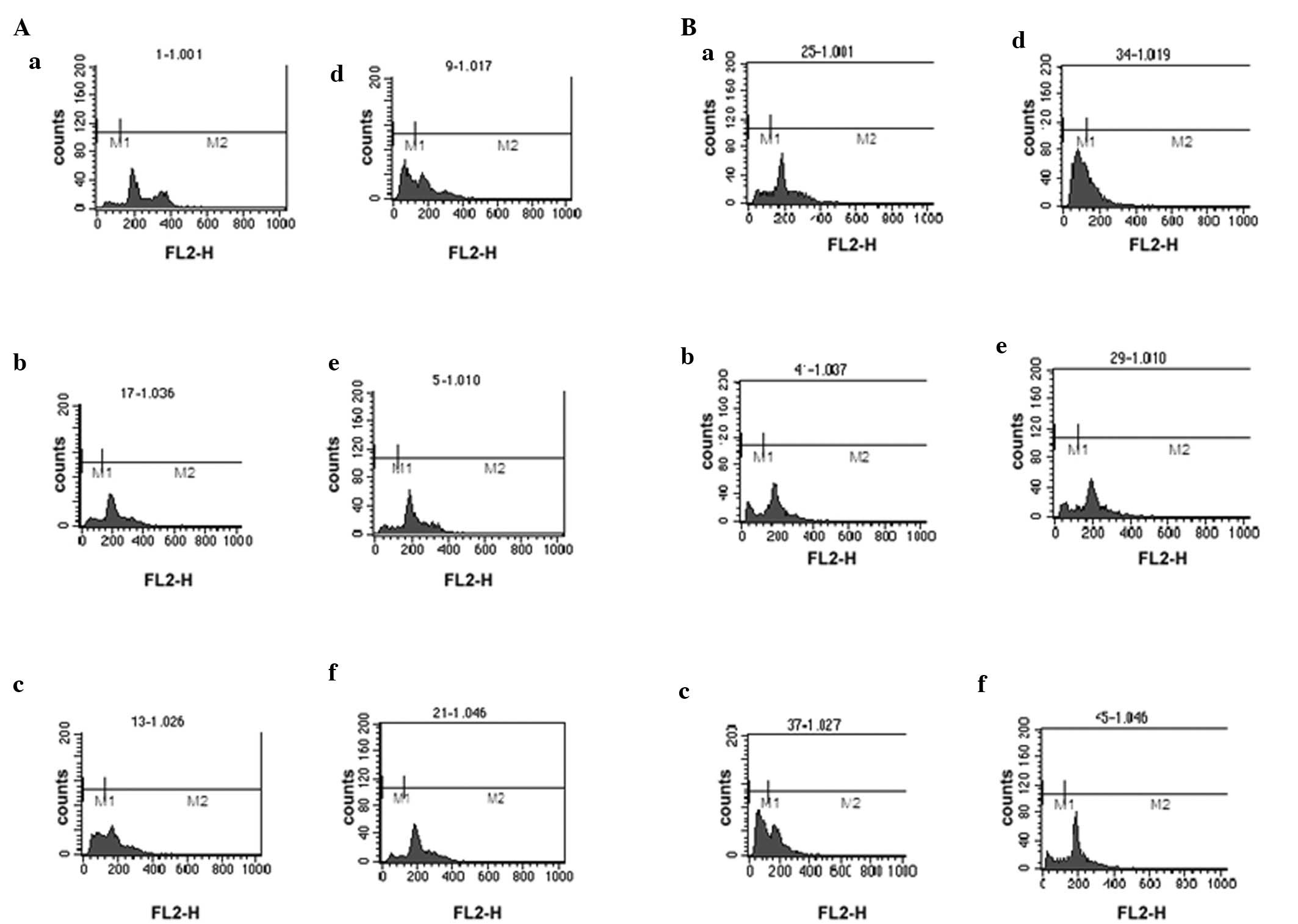
induce the apoptosis and necrosis of ACC-2 cells (Table I). Fig.
2A and B shows the peak for Sub-G1. The results of
overexpression and negative control group were compared with those
of the induction-free group, and the ratio was used to evaluate the
effects of apoptosis-induction.
 | Table IGroups of antagonizing
apoptosis-induced by TNF-α and serum deprivation with XAGE-1b. |
Table I
Groups of antagonizing
apoptosis-induced by TNF-α and serum deprivation with XAGE-1b.
| Time point | PCMV-Myc-1b
| PCMV-Myc
|
|---|
| TNF-α (1,250
μ/ml) | Serum
deprivation | Normal culture | TNF-α (1,250
μ/ml) | Serum
deprivation | Normal culture |
|---|
| 48 h | 2A a | 2A b | 2A c | 2A d | 2A e | 2A f |
| 72 h | 2B a | 2B b | 2B c | 2B d | 2B e | 2B f |
After a 48-h induction by TNF-α, the ratio of
XAGE-1b (0.85±0.23) was lower than that of the negative control
(0.98±0.07), although the difference was not significant. However,
the ratio of XAGE-1b (0.88±0.08) was significantly lower than that
of the negative control (1.15±0.05) (P<0.01) after 72 h of
induction. These results suggest an inherent tolerance of
antagonizing apoptotic induction by TNF-α in ACC-2 cells.
Similarly, after a 48-h induction by serum deprivation, no
difference was observed between the ratio of XAGE-1b (2.56±0.60)
and the negative control (2.36±0.87). By contrast, the ratio of
XAGE-1b (2.93±0.17) was significant lower than that of the negative
control (3.50±0.18) (P<0.01) after a 72-h induction. Thus, the
results suggest an inherent tolerance in ACC-2 cells. In general,
the results showed anti-apoptotic effect after 72-h induction with
XAGE-1b overexpression in ACC-2 cells.
Regulation of the transcripts of the
downstream signaling pathway
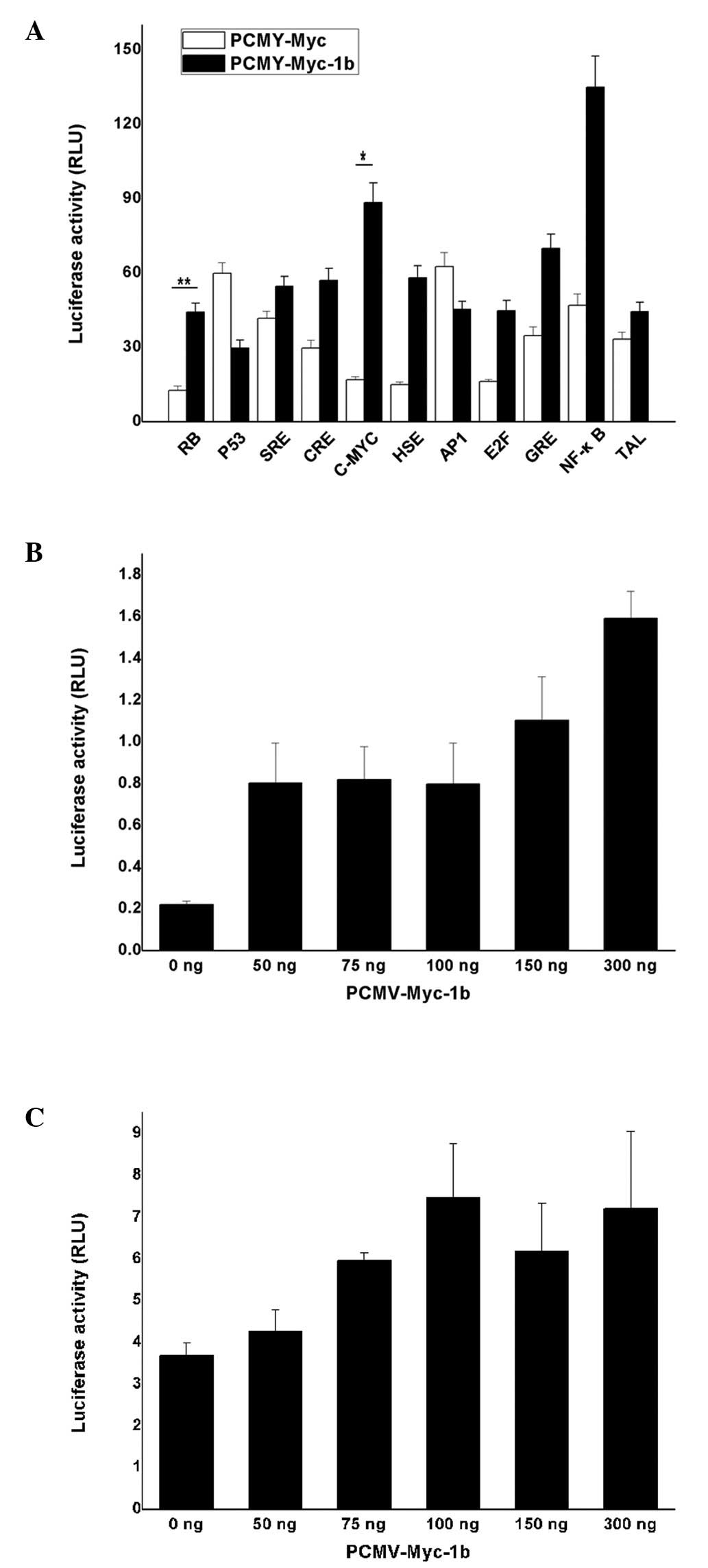
The elements of the signaling pathway included
RB, E2F, c-Myc, p53, CRE,
GRE, HSE, SRE, AP-1 and NF-κB.
293T cells were co-transfected with the vectors containing
PCMV-Myc-1b, and the transcription factor containing an element and
reporter gene. The ratio of M1/M2 was then calculated. The
increased ratio was observed in the groups that included
retinoblastoma (RB), CRE, c-Myc, HSE, E2F, GRE and NF-κB when
XAGE-1b was overexpressed (Fig.
3A). The t-test showed the evident activity effects on the
response element of RB (P<0.01) and c-Myc (P<0.05). However,
the ratio of p53 and AP1 decreased slightly, although no
statistical difference was observed.
The correlation between the concentration-gradient
of c-Myc (Fig. 3B) and RB (Fig. 3C) and XAGE-1b was investigated. The
results showed identical activity effects on the transcription
factor c-Myc with the XAGE-1b overexpression plasmid exhibiting an
increase of 50–100 ng. The final values were ∼3 to 4 times higher
than those of the PCMV-Myc-1b plasmid at a concentration of zero.
The activity of the transcription factor c-Myc was enhanced in
accordance with the increased concentration of the expressing
plasmid (150–300 ng) and it reached 4 and 7 times higher than that
of the expressing plasmid at the concentration of zero. Similarly,
the enhancement of the transcription factor RB with XAGE-1b
overexpression plasmid increasing from 50 to 100 ng, and the values
at a concentration of 100 ng were twice as high as the value at a
concentration of zero. This result indicated that the activity of
the transcription factor RB is potentially saturated at
concentrations of 150 and 300 ng.
Discussion
CTAs have an expression pattern that is
predominantly restricted to testis among normal tissues, but they
are expressed in various histological types of cancer. XAGE-1 is a
CTA that was demonstrated to be expressed at a significant
frequency and to be immunogenic in some solid tumors. Previous
findings (6) suggest that the
transcription of XAGE-1 gene is initiated from two distinct start
sites, resulting in the overlapping transcripts of XAGE-1a and
XAGE-1b. Additionally, XAGE-1a contains two in-frame ATG
translational start codons, whereas XAGE-1b is initiated downstream
of the first ATG start codon. XAGE-1b is potentially the dominant
transcript, and its translation is initiated with the second ATG
start codon (6). In the present
study, XAGE-1b gene overexpression promoted the growth and
metastasis of ACC cells in vivo and in vitro.
XAGE-1b-positive expression in the nucleus of ACC cells obtained
from the patients was also detected. We hypothesized that this
expression affects cell growth and apoptosis by regulating
transcription. In the present study, we investigated the manner in
which XAGE-1b overexpression affects cell growth and apoptosis,
promotes tumorigenesis.
Apoptosis is regarded as a carefully regulated
energy-dependent process, characterized by specific morphological
and biochemical features and it elicits a range of non-phlogistic
homeostatic mechanisms that regulate the microenvironments of
normal and diseased tissues (7,8).
Tumorigenesis is thought to be involved in the pathological process
with abnormal apoptosis of numerous cells, including the processes
of signaling pathway, replication and transcription (9). However, little is known regarding the
correlation between the XAGE-1b gene and apoptosis of salivary ACC.
In this study, we applied TNF-α and serum deprivation as the
inducer of apoptosis to examine the correlation between XAGE-1b
overexpression and apoptosis in order to confirm the mechanism of
anti-apoptotic effects in the signaling pathway. Apoptosis of
salivary ACC induced by TNF-α and the related gene expression of
apoptosis has been previously studied with results suggesting that
apoptosis induced by TNF-α is capable of increasing the expression
of bax and Bcl-2 (10). Neural cell
adhesion molecular (NCAM) is involved in the apoptosis of human
salivary gland tumor, and its effects mainly depend on NCAM
expression through a transcriptional activator of NF-κB (11). Our results suggest that XAGE-1b
overexpression reduced G0-G1 phase and
increased the G2-M phase as compared with the control.
The G0-G1 phase was significantly reduced and
S phase was increased compared with the blank. Additionally,
XAGE-1b overexpression may promote ACC-2 cells to exit the
G0-G1 phase immediately, and enter the S or
G2-M phase rapidly. However, the results demonstrated no
significant difference between PCMV-Myc-1b- and
PCMV-Myc-transfected cells, which may be associated with the lower
transfection efficiency of ACC-2 cells due to lack of selection and
comparison of the positive cell lines. Cell cycle alterations
suggested this association may affect the cell cycle regulators
with XAGE-1b overexpression.
The effects on apoptosis with XAGE-1b overexpression
could be regarded as a breakthrough for the exploration and study
of the regulatory effects on the cell cycle. Subsequently, serum
deprivation and TNF-α were applied as apoptosis inducers in the
present study. The mechanisms of apoptosis are extremely complex
and sophisticated. The extrinsic or death receptor pathway and the
intrinsic or mitochondrial pathway, are connected and have an
impact on each other (12). TNF-α
is an extrinsic pathway protein that affects a wide range of
biological activities, including cell proliferation and apoptosis.
In the present study, TNF-α was applied as an apoptosis inducer at
concentrations of 2,500 μ/ml and 1,250 μ/ml, and
after a 48- and 72-h induction, the anti-apoptotic effects with
XAGE-1b overexpression were observed. The higher concentration of
2,500 μ/ml was applied prior to that of 1,250 μ/ml
due to the higher mortality rate observed after 72 h of induction.
Anti-apoptosis in ACC-2 cell lines was observed at the
concentration of 2,500 μ/ml as compared to that of 1,250
μ/ml, and the degree of cell death in the XAGE-1b
overexpression group was less than that of the control after 72 h
induction. Similarly, when apoptosis induction occurred via serum
deprivation, anti-apoptosis was identified, and the degree of cell
death in XAGE-1b overexpression group was less than that of the
control after 72 h induction. The results demonstrate the
dose-effect relationship between the effects of anti-apoptosis and
the amounts of XAGE-1b overexpression.
Tumor necrosis factor-α activated the
multi-signaling transcription pathway by recruiting the
extracellular ligands and activating the downstream pathway of
apoptosis. TNF-α also promoted cell proliferation and
differentiation, and contributed to the immune and inflammatory
response via transcription factors, such as NF-κB and JHK (13). NF-κB as the key transcription factor
is crucial in the anti-apoptotic effects through its involvement in
initiating the expression of survival genes, including Bfl-1/A1,
Bcl-2 and Bcl-Xl, with the anti-apoptosis induced by TNF (14–16).
To determine the exact anti-apoptotic mechanism of XAGE-1b,
induction of apoptosis by TNF-α and serum deprivation should
initially be conducted. Thus, the mechanism of apoptosis may be
crucial for examining the anti-apoptotic mechanism. While exami
ning TNF-α-mediated apoptosis, reactive oxygen species (ROS)
produced by TNF-α was found to have an important function in cell
death by activating c-Jun N-terminal kinase (17). XAGE-1b overexpression promoted
anti-apoptosis induced by TNF-α, the effects of which may involve
the increased transcriptional activities of NF-κB and promotion of
survival gene expression, or potential interference with other gene
expressions associated with the death signaling pathway. The
abovementioned hypotheses remain to be confirmed. Serum
deprivation-induced cell death, a characteristic of apoptosis,
results in a possible increase of death receptor activation and
oxygen pressure, DNA breakage, caspase-3 and -9 activation,
cytochrome c release, bax expression increase, Bcl-2
expression decrease, as well as the reduction of combining
activites of NF-κB and lower glutathione content in vivo
(18–20). XAGE-1b overexpression increased
anti-apoptosis induced by serum deprivation, and its mechanism of
action and its correlation with the mechanism of anti-apoptosis
induced by TNF-α should be investigated.
The regulation of different signaling pathways may
explain the results regarding the cell cycle and apoptosis. Results
of the present study have shown the different activity of
transcriptional factors, including RB, CRE, c-Myc, HSE, E2F, GRE
and NF-κB, with XAGE-1b gene overexpression, but inhibitory
activity to p53 and AP1. No significant differences were observed
among the factors, with the exception of RB and c-Myc. The role of
the Myc gene family in the biology of normal and cancer cells has
been intensively studied since the early 1980s. Myc gene expression
is known to cause tumors and is one of the oncogenes found to be
altered in human cancers. Myc is a multifunctional protein that is
able to regulate cell cycle, cell growth, differentiation,
apoptosis, transformation, genomic instability, and angiogenesis
(21,22). The Rb gene is one of the tumor
suppressor genes that affect cell proliferation and apoptosis.
Additional investigations revealed an improved linear association
between activation of the c-Myc response element and XAGE-1b
overexpression during the course of the c-Myc
concentration-gradient experiment, thereby proving there is a
direct or indirect activation of c-Myc element with XAGE-1b
overexpression. The c-Myc gene expresses the
nucleoprotein-regulating gene function in alteration of the cell
cycle, cell growth and metabolism, gene instability, stimulation of
angiogenesis, cell malignancy transformation, differentiation and
apoptosis. Several target genes including Cdc25A, Cdk4 and cyclin
D2 and their expression were promoted, while growth inhibition
genes including gas1, p15, p21 and p27 suppressed gene expression
(23,24). The regulation of these genes led to
cell proliferation and eventually to malignant transformation.
However, it was demonstrated that the gradient activity of Rb gene
is weak, and the activity only increased twice compared with the
control when the saturated concentration was reached, suggesting
activation on the RB element is affected by XAGE-1b. The
transcription factor NF-κB plays an important role in the process
of anti-apoptosis. The value of NF-κB in the PCMV-Myc-1b group with
XAGE-1b overexpression greatly increased compared with PCMV-Myc,
although no statistical difference was identified. Therefore, we
hypothesized that the anti-apoptosis of XAGE-1b is associated with
NF-κB.
In conclusion, XAGE-1b is a potential anti-apoptotic
agent, with notable anti-apoptotic effects in ACC-2 cells. The
mechanism of its anti-apoptotic effects may be associated with
XAGE-1b overexpression in ACC-2 cell lines and regulation of the
transcription factor of the downstream signaling pathway. To the
best of our knowledge, this is the first study to confirm the
anti-apoptotic effects associated with the direct or indirect
activation of the c-Myc element and the indirect activation of the
RB element. The general profile of the XAGE-1b gene, particularly
the exact effects associated with the cis-transcription elements in
promoting tumor cell growth remain to be investigated.
Acknowledgements
This study received financial support
from the Health Bureau of Zhejiang Province Foundation (no.
2009A055), the Natural Science Foundation of Zhejiang Province (no.
2090053) and was sponsored by the Chinese National Natural Science
Foundation (no. 30772591).
References
|
1
|
Nakagawa K, Noguchi Y, Uenaka A, Sato S,
Okumura H, Tanaka M, Shimono M, Ali Eldib AM, Ono T, Ohara N,
Yoshino T, Yamashita K, Tsunoda T, Aoe M, Shimizu N and Nakayama E:
XAGE-1 expression in non-small cell lung cancer and antibody
response in patients. Clin Cancer Res. 11:5496–5503. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji Y, Zhang W and Gu L: mRNA expression of
the XAGE-1b gene in human acute leukemia. Int J Hematol.
91:209–212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ali Eldib AM, Ono T, Shimono M, Kaneko M,
Nakagawa K, Tanaka R, Noguchi Y and Nakayama E: Immunoscreening of
a cDNA library from a lung cancer cell line using autologous
patient serum: identification of XAGE-1b as a dominant antigen and
its immunogenicity in lung adenocarcinoma. Int J Cancer.
108:558–563. 2004.PubMed/NCBI
|
|
4
|
Cilensek ZM, Yehiely F, Kular RK and Deiss
LP: A member of the GAGE family of tumor antigens is an
anti-apoptotic gene that confers resistance to Fas/CD95/APO-1,
interferon-gamma, taxol and gamma-cirradiation. Cancer Biol Ther.
1:380–387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu X, Zheng M, Fei X, Yang Z, Li F, Ji C,
Xie Y and Mao Y: ZNF435, a novel human SCAN-containing zinc finger
protein, inhibits AP-1-mediated transcriptional activation. Mol
Cells. 23:316–322. 2007.PubMed/NCBI
|
|
6
|
Egland KA, Kumar V, Duray P and Pastan I:
Characterization of overlapping XAGE-1 transcripts encoding a
cancer testis antigen expressed in lung, breast, and other types of
cancers. Mol Cancer Ther. 1:441–450. 2002.PubMed/NCBI
|
|
7
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gregory CD and Pound JD:
Microenvironmental influences of apoptosis in vivo and in vitro.
Apoptosis. 15:1029–1049. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
10
|
Wang J, Dong FS, Dong Q, Gu HT and Li HX:
The study of apoptosis of salivary adenoid cystic carcinoma in nude
mice. Zhonghua Kou Qiang Yi Xue Za Zhi. 38:358–360. 2003.(In
Chinese).
|
|
11
|
Fukuda M, Kusama K and Sakashita H:
Cimetidine inhibits salivary gland tumor cell adhesion to neural
cells and induces apoptosis by blocking NCAM expression. BMC
Cancer. 8:3762008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Igney FH and Krammer PH: Death and
anti-death: tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hehlgans T and Pfeffer K: The intriguing
biology of the tumour necrosis factor/tumour necrosis factor
receptor superfamily: players, rules and the games. Immunology.
115:1–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H and Lin X: Positive and negative
signaling components involved in TNF alpha-induced NF-kappaB
activation. Cytokine. 41:1–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Logan RM, Stringer AM, Bowen JM, Yeoh AS,
Gibson RJ, Sonis ST and Keefe DM: The role of pro-inflammatory
cytokines in cancer treatment-induced alimentary tract mucositis:
pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev.
33:448–460. 2007. View Article : Google Scholar
|
|
16
|
Ghali O, Chauveau C, Hardouin P, Broux O
and Devedjian JC: TNF-alpha’s effects on proliferation and
apoptosis in human mesenchymal stem cells depend on RUNX2
expression. J Bone Miner Res. 25:1616–1626. 2010.
|
|
17
|
Kim JJ, Lee SB, Park JK and Yoo YD:
TNF-alpha-induced ROS production triggering apoptosis is directly
linked to Romo1 and Bcl-X(L). Cell Death Differ. 17:1420–1434.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang XH, Kleeman LK, Jiang HH, Gordon G,
Goldman JE, Berry G, Herman B and Levine B: Protection against
fatal Sindbis virus encephalitis by beclin, a novel
Bcl-2-interacting protein. J Virol. 72:8586–8596. 1998.PubMed/NCBI
|
|
19
|
Zhuge J and Cederbaum AI: Serum
deprivation-induced HepG2 cell death is potentiated by CYP2E1. Free
Radic Biol Med. 40:63–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao F, Hu XY, Xie XJ, Xu QY, Wang YP, Liu
XB, Xiang MX, Sun Y and Wang JA: Heat shock protein 90 protects rat
mesenchymal stem cells against hypoxia and serum
deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways.
J Zhejiang Univ Sci B. 11:608–617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eilers M and Eisenman RN: Myc’s broad
reach. Genes Dev. 22:2755–2766. 2008.
|
|
22
|
Oster SK, Ho CS, Soucie EL and Penn LZ:
The myc oncogene: MarvelouslY Complex. Adv Cancer Res. 84:81–154.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mallette FA, Gaumont-Leclerc MF, Huot G
and Ferbeyre G: Myc down-regulation as a mechanism to activate the
Rb pathway in STAT5A-induced senescence. J Biol Chem.
282:34938–34944. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cappellen D, Schlange T, Bauer M, Maurer F
and Hynes NE: Novel c-MYC target genes mediate differential effects
on cell proliferation and migration. EMBO Rep. 8:70–76. 2007.
View Article : Google Scholar : PubMed/NCBI
|