Introduction
Forkhead box m1 (Foxm1) is involved in various cell
proliferations and it disappears in differentiated cells (1–4).
Studies have focused on the function of Foxm1 in relation to lung
development in species such as mice and rats. Kalin et
al(5) reported that
conditionally Foxm1 deleted mice had no changes in lung growth,
branching morphogenesis, or epithelial proliferation in lung
maturation of mice, but it caused a respiratory failure after
birth. Foxm1 deficiency resulted in the reduction of the size of
peripheral saccules, the number of type I pulmonary epithelial
cells and mRNA expression of T1-α and aquaporin 5 in quantitative
real-time reverse transcription-polymerase chain reaction (qRT-PCR)
analysis in mice (5). Foxm1 was
found to regulate the expression of surfactant protein (SP)-A, -B,
-C and -D in lung maturation (5).
Although the relationship between Foxm1 and lung
maturation in mice was previously reported, no study has focused on
the functions of Foxm1 in lung maturation of human or rabbit. Since
rabbit lungs are known to have coterminous characteristics in the
functional and structural aspects to those of humans (6,7), the
effects of Foxm1 on the lung development of humans could be studies
more closely by using rabbit models.
This study was conducted to identify the Foxm1
expression of preterm rabbits and to compare the expression levels
of SP-A, -B, -C and Foxm1 mRNA between the lung tissues from
preterm and term rabbits with different lung developmental
stages.
Materials and methods
Animal protocol
The Animal Experimentation Committee in Kyung Hee
University at Gangdong approved the protocol (KHNMC-IACUC-10-03).
Eleven pregnant New Zealand white rabbits delivered their newborn
rabbits by cesarean section according to gestational age on
25.8±0.7 days, which corresponded to the canalicular stage of lung
development (preterm group) and 30.2±0.4 days of gestation, which
represented the alveolar stage (term group). The weights at birth
of the two groups were 32.9±7 and 50.3±9.6 g. The lung tissues of
other newborn rabbits were dissected and frozen quickly in liquid
nitrogen for total RNA extraction (n=18 of preterm group and n=18
of term group).
RNA isolation
Total RNA was extracted from the rabbits’ fetal lung
tissues of the preterm and term groups using the TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s instructions.
Reverse transcription-polymerase chain
reaction
For mRNA analysis, RT-PCR for Foxm1 was performed
according to the manufacturer’s instructions. PCR was carried out
using EconoTaq® PLUS GREEN 2X Master mix (Lucigen
Corporation, WI, USA) with Bioer (GeneQ) PCR machine (Bioer
Technology Co., Ltd., Hangzhou, China). All applications were run
according to the manufacturer’s instructions. PCR products were
applied to electrophoresis on an agarose gel (1.5%) and stained
with ethidium bromide. PCR products were then quantified using the
software program ‘ImageJ’, which measures gel density and
normalized against the expression of housekeeping gene (18S)
mRNA.
Quantitative real-time reverse
transcription-polymerase chain reaction
qRT-PCR analysis was performed for the
quantification of mRNA expression of SP-A, -B, -C and Foxm1 of
rabbit lungs using a Chromo4 real-time PCR (Applied Bio-Rad, Foster
City, CA, USA) according to the manufacturer’s instructions.
Samples were amplified with AccuPower® 2X Greenstar qPCR
Master mix (Bioneer, Daejeon, Korea) and combined with inventoried
SYBR®-Green I gene expression assay. Reactions were
analyzed in triplicate and mRNA expression levels were normalized
to 18S. Relative quantification of mRNA expression was performed
using the 2−ΔΔCT method.
The primer design of Foxm1 gene of
rabbits
Foxm1 primers were designed due to the lack of
sequences of Foxm1 of rabbits. Sequence alignments of primers were
generated by comparing human Foxm1 sequences (GenBank; accession
no. NM_202002.1) to whole sequences of chromosome no. 8 of rabbit
(GenBank; accession no. NW_003159267.1) using the software program
of primer 3, version 3.0 site (http://biotools.umassed.edu/bioapps/primer3_www.cgi;
accessed October 11, 2011). The size of the PCR product was 327 bp
and the annealing temperature was 50.5°C.
Cloning and sequencing for Foxm1 mRNA of
rabbit
The PCR products of Foxm1 of rabbit lung tissues
were purified with a gel extraction kit (LaboPass™ Gel; Cosmo
Genetech, Seoul, Korea) according to the manufacturer’s
instructions. Cloning was carried out using a TA
Cloning® kit (Invitrogen Life Technologies) without
restrictive enzymes, according to the manufacturer’s instructions.
Nucleotide sequences were aligned using BigDye®
Terminator v3.1 Cycle Sequence kit (Applied Biosystems, Inc.,
Foster City, CA, USA).
Immunohistochemical staining
Lung tissues of obtained preterm and term rabbits
were fixed overnight with 10% buffered formalin and then embedded
into paraffin blocks. The tissue blocks were sectioned into 3–4 μm
sections. Slides were incubated with primary antibody against Foxm1
(ab55006, mouse monoclonal antibody; Abcam, Cambridge, MA, USA) at
a dilution of 1:400. The slides were stained with liquid
diaminobenzidine tetrahydrochloride (DAB+), a high-sensitivity
substrate-chromogen system (K3468; DakoCytomation, Glostrup,
Denmark). Counterstaining was performed with Meyer’s haematoxylin.
The images on the slides were visualized using an Olympus BX40
light microscope (Olympus Optical Co., Ltd., Tokyo, Japan).
Statistical analysis
Data analyses were performed using Statistical
Package for Social Sciences (SPSS, version 17). Comparisons of mRNA
expression levels between preterm and term groups were performed by
the Student’s t-test. The Pearson’s correlation coefficient was
used to confirm statistical correlations of the mRNA expression.
Data are presented as means ± SD and P<0.05 was considered to
indicate a statistically significant difference.
Results
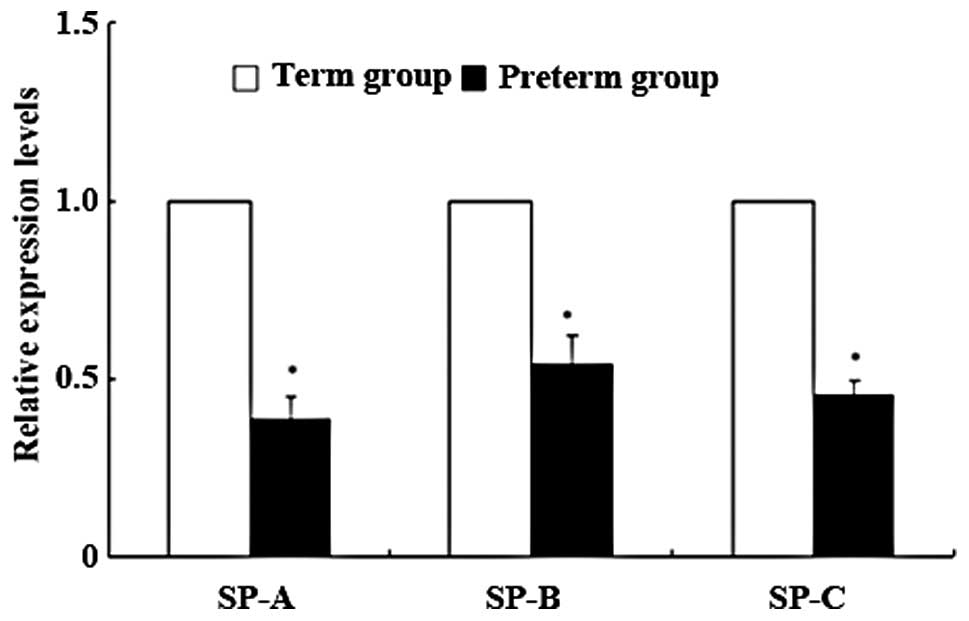
Expression levels of SP-A, -B and -C mRNA
using qRT-PCR
When the relative ratio of the mRNA expression
levels of SP-A, -B and -C in the term group was fixed at 1, the
expression levels were clearly decreased to 0.386, 0.539 and 0.450,
respectively, in the preterm group (P<0.01, Fig. 1). There were no differences in the
mRNA expression levels of SP-A, -B and -C in relation to birth
order or locations of lung tissues of fetal rabbits in the same
mother.
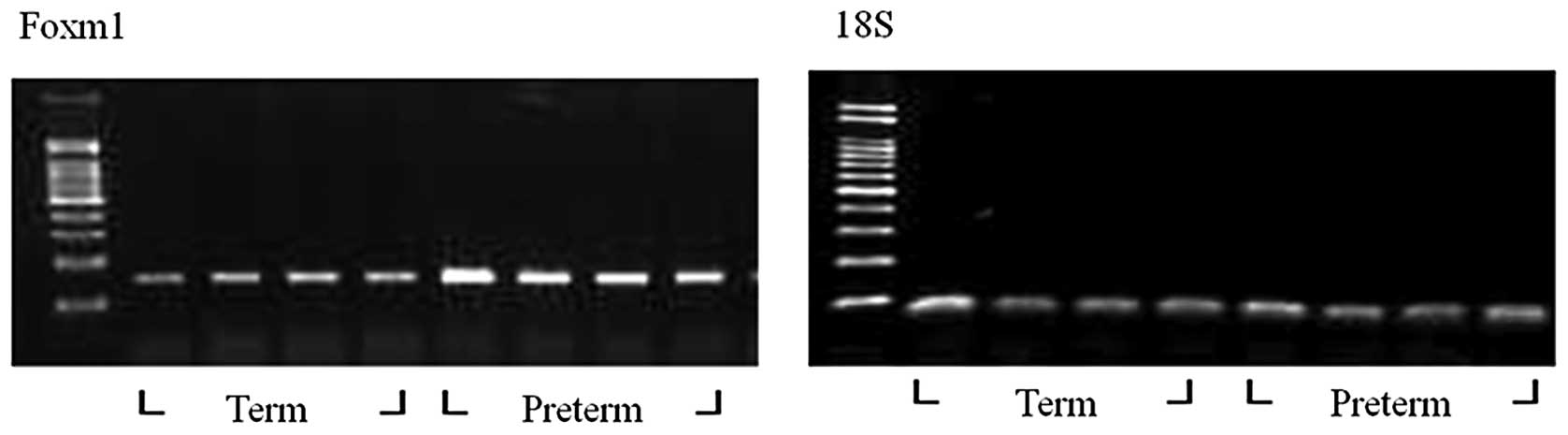
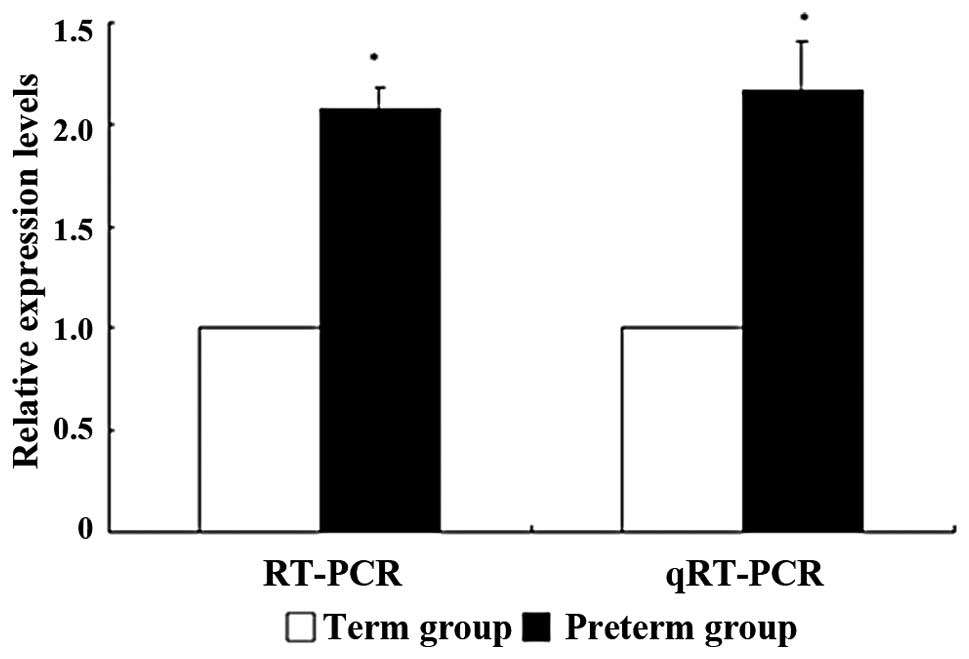
Comparisons of Foxm1 expression levels of
preterm and term rabbit lung models using RT-PCR and qRT-PCR
Gel density of RT-PCR products was measured to
quantify the expression level of Foxm1 in each group (Fig. 2). Using RT-PCR, when the ratio of
the term to preterm group was 1:2.08, the Foxm1 mRNA expression
level of the preterm group was increased to 2.1 times more than
that of the term group. The ratio of multiple changes in the
threshold cycle (CT) of the two groups was 1:2.166 in qRT-PCR.
Therefore, the Foxm1 mRNA expression level was increased by more
than double in the preterm group (Fig.
3) and this indicated that both qRT-PCR and RT-PCR had similar
results.
After performing RT-PCR, the products were examined
by gel refinement, cloning and gene sequencing analysis (Cosmo
Genetech, Co., Ltd., Seoul, Korea). Based on that result,
homological confirmation and gene sequence analysis were performed
using the BLAST program. Our reported sequence matched 99% from
that of another study for Foxm1 gene of rabbits and we ascertained
that those gene sequences matched 89% with the human Foxm1 gene
sequence. The GenBank accession no. of our Foxm1 sequence of
rabbits is JN817622.
We confirmed that there were statistical
correlations of mRNA expression between SP-A, -B, -C and Foxm1
through Pearson’s correlation coefficient. Statistically, a strong
positive correlation was determined among SP-A, -B and -C, but
Foxm1 had a negative correlation with SP-A regardless of the group.
However, when the results were separated into preterm and term
groups, the results were different. There were no correlations
between Foxm1 and SP-A, -B and -C mRNA expression levels in the
term group, while there were negative correlations between Foxm1
and SP-A and -B statistically in the preterm group (Table I).
 | Table IPearson’s correlation coefficient
between surfactant protein (SP)-A, -B, -C and forkhead box m1
(Foxm1) mRNA expression in real-time reverse
transcription-polymerase chain reaction. |
Table I
Pearson’s correlation coefficient
between surfactant protein (SP)-A, -B, -C and forkhead box m1
(Foxm1) mRNA expression in real-time reverse
transcription-polymerase chain reaction.
| Item | SP-A total
(term/preterm) | SP-B total
(term/preterm) | SP-C total
(term/preterm) | Foxm1 total
(term/preterm) |
|---|
| SP-A | 1.00 (1.00/1.00) | - | - | - |
| SP-B | 0.89a (0.81a/0.76a) | 1.00 (1.00/1.00) | - | - |
| SP-C | 0.85a (0.77a/0.78a) | 0.87a (0.92a/0.55a) | 1.00 (1.00/1.00) | - |
| Foxm1 | −0.46a (−0.20/−0.48a) | −0.35
(−0.20/−0.44a) | −0.34
(0.16/−0.23) | 1.00 (1.00/1.00) |
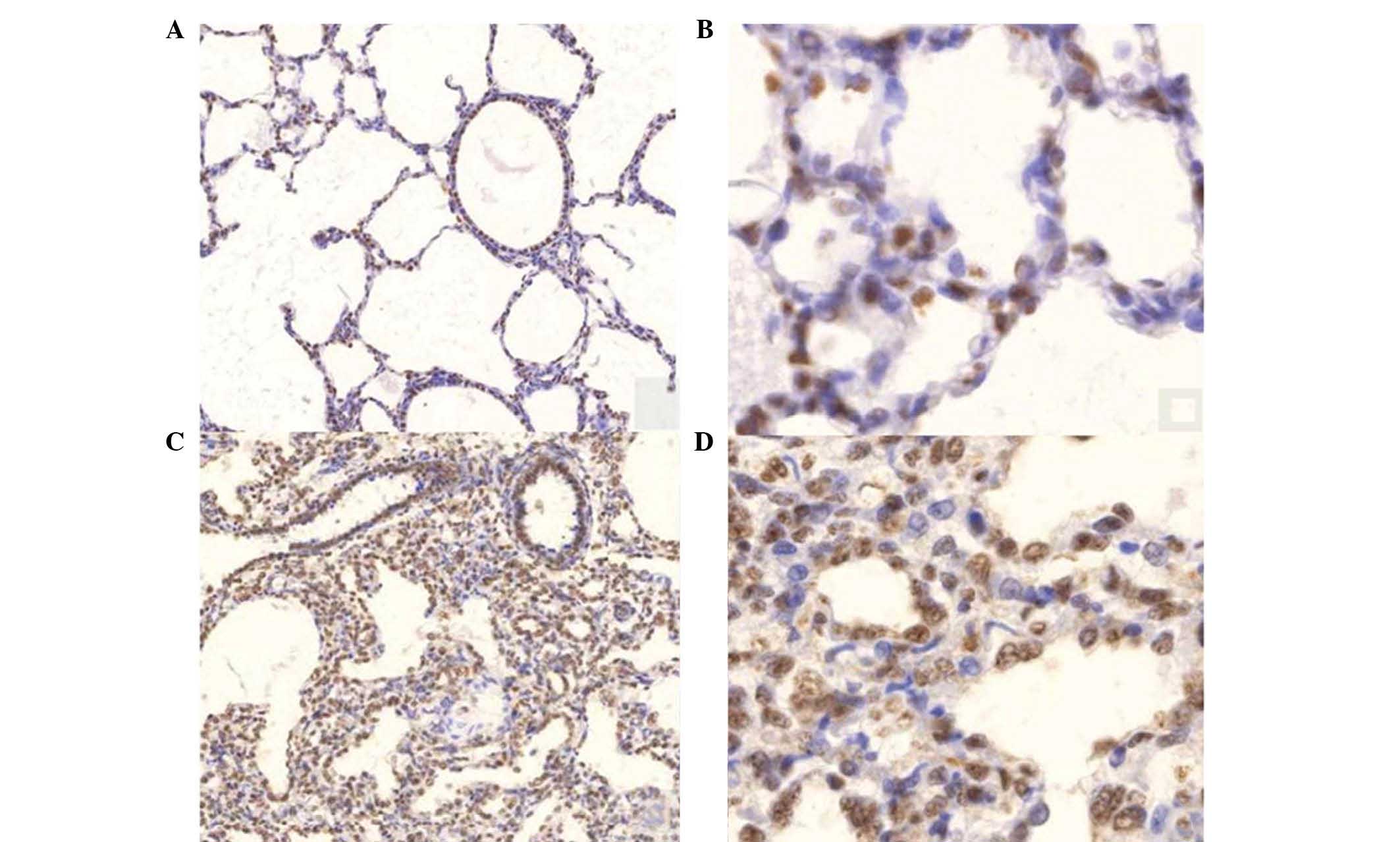
Immunohistochemical staining of Foxm1 of
lung tissue from rabbits
Decreased sacculation, aerations and mesenchymal
thickening were observed in the preterm rabbits as compared to the
term rabbit lungs. The cells, which were stained with Foxm1
antibody, were expressed in the most immature epithelial and
endothelial cells of the lung tissues of the preterm rabbits.
General expression of Foxm1 staining cells was decreased in the
term rabbit lung tissues (Fig.
4).
Discussion
Studying animal models to investigate the effect of
gene expressions in the lung maturation of humans is useful due to
their accessibility. However, each species of animal has different
lung maturation stages (8–10) and it is difficult to predict the
lung maturation of humans using animal models. Although rabbits
with Foxm1 are suspected to be associated with lung maturation, few
studies have been conducted regarding this association and most
studies have used rat or mouse models. Rabbits are commonly used to
study the human respiratory system and are a commonly used research
model. Moreover, alveolar maturation stages and SP production
processes of rabbits are known to be very similar to those of
humans (6,9). In addition, reciprocal chromosome
painting using human chromosomes showed complete homology in more
than three chromosomes (7). Based
on these observations, a rabbit seems to be a more appropriate
model compared with other classical animal models for studying
human lung development and various gene expressions during
intrauterine and early neonatal life (11–14).
To the best of our knowledge, this is the first
study to report the Foxm1 expression level of rabbit lung; the gene
sequence of Foxm1 of rabbits has also not been reported. For the
present study, we designed rabbit Foxm1 primers using human Foxm1
gene information to obtain mRNA products by RT-PCR and qRT-PCR from
rabbit lung tissues. Furthermore, partial cloning was conducted for
the first time using RT-PCR products and we reported the Foxm1
sequence. The Foxm1 sequence presented in this study is likely to
be useful for studies on the function of Foxm1 in lung
maturation.
In a study using a mouse model, Foxm1 expression was
found to be markedly decreased until birth and then to be slightly
recovered after birth (15). The
changes of Foxm1 expression level according to the gestational
period might be a result of cell proliferation and mitosis. In
undifferentiated respiratory cells, Foxm1 is involved in cell
proliferation by directly activating various gene expressions that
control the cell cycle and induce lung maturation as well (15). By deletion of Foxm1 gene, the lung
maturation was suppressed as a respiratory failure developed by
poor lung maturation, although morphologic property was not
affected (4). Similarly, we found
that the Foxm1 expression level of the preterm group was double
compared to that of the term group in RT-PCR and qRT-PCR, with
previous studies reporting similar findings (15). In the present study, lungs of the
preterm group were found to have respiratory cell proliferation and
SP secretion in correlation with Foxm1 expression until the lung
maturation was achieved.
The expression levels of SP-A, -B and -C of the lung
tissues of preterm groups were significantly different with term
groups which were measured by qRT-PCR and it agrees with some
previous studies. They reported that clearly increased mRNA
expression levels were found on 26th day of gestational age
compared with 28th or 30th day with mice models (16).
Furthermore, a significant statistical correlation
between SP-A, -B, -C and Foxm1 mRNA expression levels was found in
the preterm group. In this preterm group, the Foxm1 mRNA expression
level was found to be increased, whereas SP-A and -B mRNA
expression levels were found to be decreased, showing a negative
correlation between the two factors. SP genes are known to be
associated with lung maturation. However, Foxm1 might be associated
with lung maturation in a negative manner. By contrast, there was
no such correlation between the two variables for the term group,
suggesting Foxm1 mRNA expression was markedly reduced as the lung
was already mature in this group. Therefore, Foxm1 may be involved
in lung maturation in a negative manner.
However, several limitations exist in the present
study. First, further research on the serial changes of Foxm1
expression according to the gestational period is required to
obtain more detailed information on the roles of Foxm1 on lung
maturation. In addition, the exact time periods for when Foxm1
expression level stops increasing or when it starts decreasing
should be identified. Second, more detailed investigation on the
lung maturation is required using a numerical method despite the
expressions of SP genes being analyzed in the present study. Third,
measuring the protein expression levels of Foxm1 and SPs is
necessary to clarify the correlation between these molecules.
In conclusion, in the present study, we designed a
partial sequence of Foxm1 gene of a rabbit and carefully defined
this gene as an important factor for the maturation of lung in
preterm rabbits. In addition, Foxm1 mRNA expression of preterm
rabbits was double that of term rabbits. Therefore, future studies
should be conducted to confirm Foxm1 is important in the prediction
of lung maturation in premature infants or prognosis of preterm
infants with respiratory distress syndrome.
Acknowledgements
We would like to thank Moon-Sook Park for technical
assistance and Jin-Sam Chang and Dr Hyun-Ju Cheung for helpful
comments about study design.
References
|
1
|
Korver W, Roose J and Clevers H: The
winged-helix transcription factor Trident is expressed in cycling
cells. Nucleic Acids Res. 25:1715–1719. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luscher-Firzlaff JM, Westendorf JM,
Zwicker J, et al: Interaction of the fork head domain transcription
factor MPP2 with the human papilloma virus 16 E7 protein:
enhancement of transformation and transactivation. Oncogene.
18:5620–5630. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yao KM, Sha M, Lu Z and Wong GG: Molecular
analysis of a novel winged helix protein, WIN. Expression pattern,
DNA binding property, and alternative splicing within the DNA
binding domain. J Biol Chem. 272:19827–19836. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye H, Kelly TF, Samadani U, et al:
Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in
proliferating epithelial and mesenchymal cells of embryonic and
adult tissues. Mol Cell Biol. 17:1626–1641. 1997.PubMed/NCBI
|
|
5
|
Kalin TV, Wang IC, Meliton L, et al:
Forkhead Box m1 transcription factor is required for perinatal lung
function. Proc Natl Acad Sci USA. 105:19330–19335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Durham PL, Nanthakumar EJ and Snyder JM:
Developmental regulation of surfactant-associated proteins in
rabbit fetal lung in vivo. Exp Lung Res. 18:775–793. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Korstanje R, O’Brien PC, Yang F, et al:
Complete homology maps of the rabbit (Oryctolagus cuniculus)
and human by reciprocal chromosome painting. Cytogenet Cell Genet.
86:317–322. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burri PH: The postnatal growth of the rat
lung. 3. Morphology Anat Rec. 180:77–98. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burri PH: Postnatal growth and maturation
of the lung. Chest. 67(Suppl 2): S2–S3. 1975. View Article : Google Scholar
|
|
10
|
Davies P, Reid L, Lister G and Pitt B:
Postnatal growth of the sheep lung: a morphometric study. Anat Rec.
220:281–286. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kovar J, Sly PD and Willet KE: Postnatal
alveolar development of the rabbit. J Appl Physiol. 93:629–635.
2002. View Article : Google Scholar
|
|
12
|
Gras-Le Guen C, Denis C, Franco-Montoya
ML, et al: Antenatal infection in the rabbit impairs post-natal
growth and lung alveolarisation. Eur Respir J. 32:1520–1528.
2008.PubMed/NCBI
|
|
13
|
Kaushal S, Ghosh S, Sharma N, Sanyal SN
and Majumdar S: Role of phospholipid transfer protein in rabbit
lung development. Cell Mol Life Sci. 58:2098–2107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roubliova XI, Van der Biest AM, Vaast P,
et al: Effect of maternal administration of betamethasone on
peripheral arterial development in fetal rabbit lungs. Neonatology.
93:64–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang IC, Zhang Y, Snyder J, et al:
Increased expression of FoxM1 transcription factor in respiratory
epithelium inhibits lung sacculation and causes Clara cell
hyperplasia. Dev Biol. 347:301–314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Margana RK and Boggaram V: Transcription
and mRNA stability regulate developmental and hormonal expression
of rabbit surfactant protein B gene. Am J Physiol. 268:L481–L490.
1995.PubMed/NCBI
|