Introduction
Mastoparan-7 (mas-7) is a basic tetradecapeptide
isolated from wasp venom, which activates guanine
nucleotide-binding regulatory proteins (G-proteins) by catalyzing
the guanosine 5′-diphosphate/guanosine 5′-triphosphate (GDP/GTP)
exchange. Thus, this compound mimics the action of activated
G-protein-coupled receptors. Mas-7 has been shown to stimulate
phospholipase C (PLC) in several cellular compartments, such as rat
mast cells, rat hepatocytes and HL-60 human leukaemia cells. By
contrast, the inhibition of PLC by mastoparan has been demonstrated
in SH-SY5Y human neuroblastoma cells and in human astrocytoma cells
(1). Recent studies suggested the
possibility of programmed cell death stimulation in different types
of cells (2–4).
The pertussis toxin (PT) catalyzes the adenosine
5′-diphosphate-ribosylation of the α subunits of the heterotrimeric
Gi, Go and Gt proteins. This
prevents the G-protein heterotrimers from interacting with their
receptors, thus blocking their coupling and activation. Since the
Gα subunits remain in their GDP-bound, inactive state,
they are unable to inactivate adenylyl cyclase or open
K+ channels (5). PT is
commonly used in several models of signaling pathways.
Calcium (Ca2+) ions play a central role
in the life of the cell. Accordingly to pathological factors,
Ca2+ concentration changes occur in various cell
compartments, which may induce apoptosis. Prolonged Ca2+
ions concentration changes in the cytoplasm, nucleus or
mitochondria, may initiate the cascade of events that lead to cell
death. Following stimulation by pathological factors,
Ca2+ ions are released from the endoplasmic reticulum
and bind to several molecules, such as calpain or calcineurin.
Calpain belongs to the cysteine protease family, which activates
BH3 interacting-domain death agonist and Bcl-2-associated X protein
and it promotes their transport to the mitochondria. In addition,
Ca2+ excess in the mitochondria leads to release of
proapoptotic proteins located in the intracellular space, such as
the second mitochondria-derived activator of caspases/Diablo and
cytochrome c(6,7).
The main aim of this study was to evaluate the
physiological effect of the direct stimulation of the G-proteins in
comparison to the typical stimulation of α-adrenergic receptors and
vasopressin receptor type 1 in vascular smooth muscle cells
(VSMCs).
Materials and methods
Animals
The experiments were performed on the isolated and
perfused tail artery of Wistar rats (weight, 250–270 g). The
animals were housed under a 12-h light/dark cycle and had unlimited
access to food and water. The rats were narcotized by
intraperitoneal injection of 120 mg urethane per 1 kg body weight
and were sacrificed by stunning and cervical dislocation. The study
protocol was approved by the local Ethics Committee. All the
studies were performed in accordance with the United States NIH
guidelines [Guide for the Care and Use of Laboratory Animals
(1985), DHEW Publication No. (NIH) 85-23: Office of Science and
Health Reports, DRR/NIH, Bethesda, MD, USA].
Drugs and solutions
Mas-7 was used as a G-protein activator and
mastoparan-17 (mas-17) was used as negative control. The Krebs
solution consisted of NaCl (71.8 mM/l), KCl (4.7 mM/l),
CaCl2 (1.7 mM/l) NaHCO3 (28.4 mM/l),
MgSO4 (2.4 mM/l), KH2PO4 (1.2
mM/l) and glucose (11.1 mM/l). All the reagents were purchased from
Sigma-Aldrich Chemical Co. (Poznań, Poland). Study design and
conduction. Following dissection from the surrounding tissues, a
2–3-cm long segment of a rat tail artery was cannulated and
connected to a perfusion device. The distal part was weighted with
a 500-mg weight and the tail was placed in a 20-ml container filled
with oxygenated Krebs solution at 37°C (pH 7.4). The perfusion
pressure was continuously measured. The perfusion solution flow was
gradually increased using a peristaltic pump to 1 ml/min, until the
optimum perfusion pressure of 2–4 kPa (8,9).
Data analysis and statistical
procedures
The investigations were performed on the TSZ-04
system (Experimetria, Ltd., Balatonfüred, Hungary). The perfusion
pressure was measured on BPR-01 and BPR-02 devices and the vascular
smooth muscle tension was measured on an FSG-01 transducer. All the
transducers used in our experiments were provided by Experimetria,
Ltd. The peristaltic pump was provided by Zalimp, Warsaw,
Poland.
The concentration response curves (CRCs) were
calculated according to the van Rossum method. The maximum response
of the tissue (Emax) was calculated as the percentage of
the maximal response for phenylephrine. The half maximal effective
concentration (EC50) was estimated using classical
pharmacological methods with pD2, the negative logarithm of the
EC50. We used CRC and Emax in all the
calculations estimating statistical significance. Mas-17 was used
as negative control.
The results are presented as mean values ± standard
deviation. The statistical analysis was performed using the
analysis of variance for multiple comparisons of the means.
P<0.05 was considered to indicate a statistically significant
difference.
Results
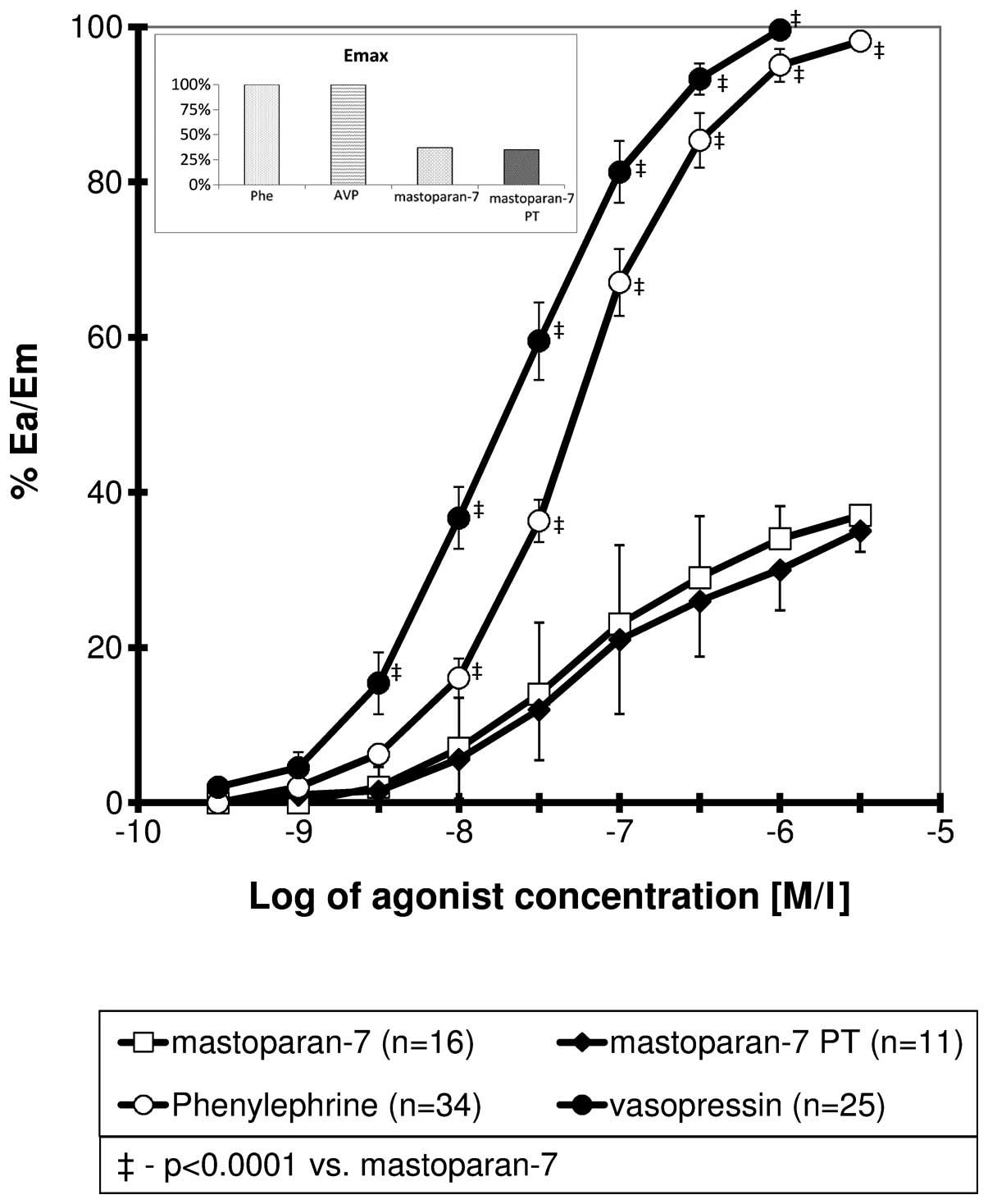
Mas-7 CRCs
The CRCs obtained for mas-7 were sigmoidal. In
comparison to the curves for phenylephrine and vasopressin, the
mas-7 curve was shifted to the right with a significant reduction
in maximal response (Fig. 1). For
all the points for a relative effect of ≥20%, the differences were
statistically significant. The curve obtained for mas-7 in the
presence of PT did not differ significantly from the control. The
calculated Emax, EC50 and pD2 values are
presented in Table I.
 | Table IHalf maximal effect concentration
(EC50), maximal tissue response (ECmax) and
relative potency (RP) for mas-7 for controls and in the presence of
PT. |
Table I
Half maximal effect concentration
(EC50), maximal tissue response (ECmax) and
relative potency (RP) for mas-7 for controls and in the presence of
PT.
| Compound | na |
%Emaxb | EC50
(M/l) |
pD2e | RPc | P-valued |
|---|
| Mas-7 | 16 | 37±4 | 4.41 (±2.33)
×10−8 | 7.40±0.20 | 1.000 | - |
| Mas-7+PT | 11 | 35±4 | 6.12 (±3.40)
×10−8 | 7.21±0.22 | 0.721 | 0.1593 |
| Phenylephrine | 34 | 100 | 7,51 (±0,97)
×10−8 | 7,13±0,06 | - | <0.0001 |
| Vasopressin | 25 | 100 | 1,82 (±0,61)
×10−8 | 7,76±0,14 | - | <0.0001 |
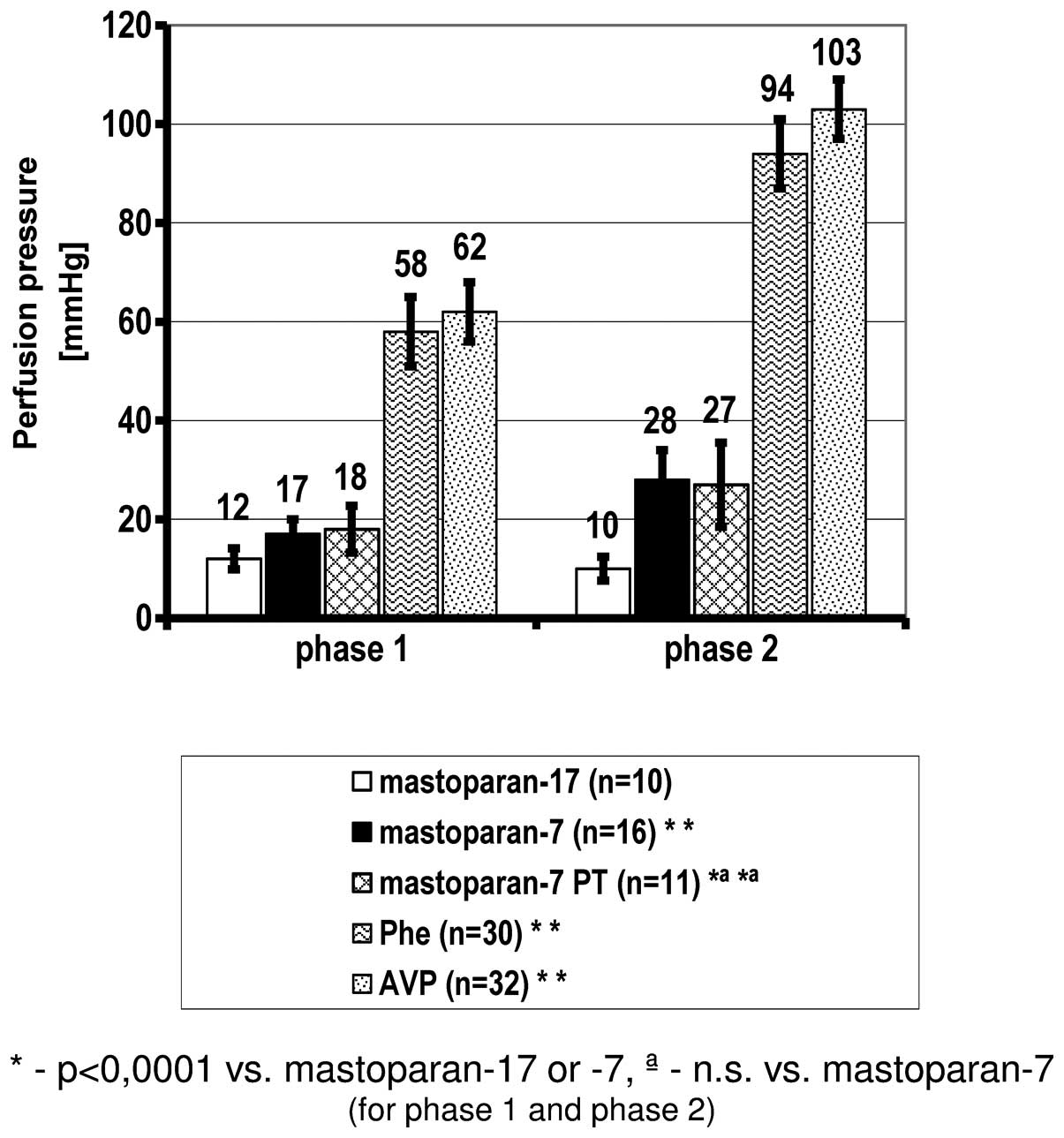
Effect of G-protein activation by mas-7
on perfusion pressure
Analyzing the perfusion pressure as a result of the
contraction induced by Ca2+ influx from the
intracellular Ca2+ stores with mas-7 (phase 1), a
significant increase was observed in comparison to the negative
control mas-17. The same association was observed following
mas-7-induced extracellular Ca2+ influx to the cytoplasm
(phase 2). The presence of PT did not significantly affect the
mas-7-induced contraction. In comparison to phenylephrine and
vasopressin, all the values of perfusion pressure following
stimulation of the G-proteins by mas-7 were significantly lower
(Fig. 2, Table II).
 | Table IIMaximal perfusion pressure during
mas-7-induced contraction activated by Ca2+ influx from
intracellular (phase 1) and extracellular (phase 2) Ca2+
stores. |
Table II
Maximal perfusion pressure during
mas-7-induced contraction activated by Ca2+ influx from
intracellular (phase 1) and extracellular (phase 2) Ca2+
stores.
| Intracellular
Ca2+ (phase 1) | Extracellular
Ca2+ (phase 2) |
|---|
|
|
|
|---|
| Compound | nd | Perfusion pressure
(±SE) (mm/Hg) | n | Perfusion pressure
(±SE) (mm/Hg) |
|---|
| Mas-17 | 10 | 11.8 (±2.1) | 10 | 10.1 (±2.4) |
| Mas-7 | 16 | 17.4 (±3.1)a,b | 16 | 28.3 (±5.6)a,b |
| Mas-7+PT | 11 | 18.2 (±4.7)a,c | 11 | 27.4 (±8.5)a,c |
| Phenylephrine | 30 | 57.9 (±7.2) | 30 | 93.6 (±7.8) |
| Vasopressin | 32 | 62.4 (±6.4) | 32 | 103.2 (±6.0) |
Discussion
In the performed experiment, vascular contraction
was induced by mas-7, an activator of G-proteins. The
vasoconstriction triggered by mas-7 exhibited a slower increase
compared to that simulated by phenylephrine or vasopressin. In
response to the stimulation of α1-adrenergic,
vasopressin or angiotensin receptors, vasoconstriction was observed
within a few seconds, whereas the maximum response to mas-7
appeared after 30–40 min. The present study demonstrated that PT
exerted no inhibitory effect on the vasoconstriction stimulated by
mas-7. Kanagy and Webb (10)
previously investigated spiral cutting fragments of the common
carotid artery and demonstrated a measureable response after 10 min
and a maximum response after 30 min following the application of
mas-7 (10−5 M/l) (11).
Furthermore, in rats with hypertension, the arterial reactivity was
significantly higher compared to that of controls. PT and the
phospholipase A2 inhibitor indomethacin did not affect
the response to mas-7. Nifedipine at a concentration of
10−5 M/l was shown to inhibit the contraction of VSMCs
induced by mas-7 at a concentration of 10−7 M/l,
revealing a correlation between voltage-dependent Ca2+
channels and mas-7-induced vasoconstriction. The lack of complete
reversal by nifedipine at higher concentrations of mas-7
(10−5 M/l) suggests that an additional mechanism may be
activated at higher concentrations (10).
In this study, the effect of the G-protein inhibitor
PT on mas-7-induced contraction was investigated. We observed a
notable inhibition of VSMC contraction triggered by G-protein
activation and a proportional perfusion pressure reduction caused
by intra- and extracellular Ca2+ influx.
The vasoconstriction induced by the activation of
metabotropic receptors, such as α-adrenergic receptors, vassopresin
receptors (V1) or angiotensin II receptors type 1, is
conditioned upon the activation of G-proteins. Subsequently,
G-proteins activate PLC, leading to the the hydrolysis of
phosphatidylinositol 4,5-bisphosphate and increased intracellular
concentration of inositol-1,4,5-triphosphate (11–13).
Mas-7 penetrates through biological barriers and binds to the
ligand-binding site of the G-protein-coupled receptor, stimulating
G-proteins in a way similar to an activating receptor. As
demonstrated by biochemical studies, the affinity of mastoparan for
individual G-protein types is significantly different. Mas-7
exhibits a higher affinity for Gi and Go
compared to the Gs protein (14). PT does not affect the VSMC
contraction induced by mas-7 by inhibiting the function of
Gi and Go, indicating that the target in this
process may be Gq. Secondarily to the activation of
Gq and PLC, the metabolism of membrane phospholipids may
be increased (10). Ca2+
channel blockers directly inhibit Ca2+ influx, thereby
decreasing the efficiency of the contraction induced by mas-7,
highlighting the role of Ca2+ channels in this process
(8,10,15,16). A
previous study conducted by Perianin and Snyderman (15) demonstrated that mas-7 may increase
Ca2+ ion concentration in the cytoplasm through
mechanisms which are unrelated to the production of inositol
triphosphate and diacylglycerol.
The affinity for the Gq-protein has not
been specified thus far; however, functional investigations were
performed on the process of G-protein activation with mas-7 in the
VSMCs of the carotid artery in rats (10). The results demonstrated that mas-7
activates Gq-proteins in VSMCs, leading to the increase
of Ca2+ ion concentration in the cytoplasm and resulting
vasoconstriction. Moreover, in rats with genetically determined
hypertension, the contraction of VSMCs was significantly more
prominent compared to the control group (10). Mas-7 may activate phospolipase
A2 at a concentration of 5×10−5 M/l, leading
to the degranulation of mast cells (17). In VSMCs, the process of prostanoid
production does not modify the contraction triggered by mas-7, as
determined by experiments performed in the presence of
indomethacin. No significant effect of indomethacin was
demonstrated in those studies (10,15).
Mas-7 at a concentration of 10−5 M/l may affect
vasoconstriction through additional mechanisms, such as
Ca2+ channel modulation and voltage-independent
Ca2+ channels (15).
Mas-7 also exerts a direct effect on PLC. At low concentrations
(<3×10−6 M/l), PLC activation was inhibited by mas-7,
although at higher concentrations (>5×10−6 M/l)
direct activation was observed (18,19).
In our study, a lower concentration of mas-7
(3×10−10-10−6 M/l) was used, which was not
sufficiently high to affect elements of signaling pathway other
than the G-proteins. The vasoconstriction induced by mas-7 depends
on the intra- and extracellular Ca2+ pool, which may
also affect apoptosis. The results of this process were higher
values of perfusion pressure.
The constriction of VSMCs induced by mas-7 was
significantly lower in comparison to that induced by phenylephrine
and vasopressin, as was previously confirmed by Kanagy and Webb
(10). The effects observed for
mas-7 were similar to the effect observed following activation by
partial receptor agonists, rather than full agonists such as
phenylephrine or vasopressin. Similar perfusion pressure values
were reported with the α2-receptor agonist clonidine
(20).
In conclusion, our results suggest that mas-7
significantly induces VSMC contraction. The binding site for mas-7
is different from that for PT; therefore, PT does not affect VSMC
contraction. The tissue effect of this stimulation is comparable to
the effect of stimulation with partial receptor agonists. Our
current knowledge regarding the apoptosis pathway demonstrates the
significance of Ca2+ ions involved in this process.
Thus, mas-7 may induce apoptosis through an increase in the
cytoplasmic Ca2+ concentration; however, the use of this
mechanism in anticancer therapy must be preceded by a molecule
modification that eliminates the vasoconstrictive effect.
Abbreviations:
|
CRC
|
concentration response curve
|
|
EC50
|
half maximal effect concentration
|
|
Emax
|
maximal tissue response
|
|
mas-7
|
mastoparan-7
|
|
PLC
|
phospholipase C
|
|
PT
|
pertussis toxin
|
References
|
1
|
King TP, Jim SY and Wittkowski KM:
Inflammatory role of two venom components of yellow jackets
(Vespula vulgaris): a mast cell degranulating peptide
mastoparan and phospholipase A1. Int Arch Allergy Immunol.
131:25–32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoshina MM, Santos LD, Palma MS and
Marin-Morales MA: Cytotoxic, genotoxic/antigenotoxic and
mutagenic/antimutagenic effects of the venom of the wasp Polybia
paulista. Toxicon. 72:64–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yordanova ZP, Woltering EJ,
Kapchina-Toteva VM and Iakimova ET: Mastoparan-induced programmed
cell death in the unicellular alga Chlamydomonas
reinhardtii. Ann Bot. 111:191–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin CH, Hou RF, Shyu CL, Shia WY, Lin CF
and Tu WC: In vitro activity of mastoparan-AF alone and in
combination with clinically used antibiotics against
multiple-antibiotic-resistant Escherichia coli isolates from
animals. Peptides. 36:114–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sowa NA, Street SE, Vihko P and Zylka MJ:
Prostatic acid phosphatase reduces thermal sensitivity and chronic
pain sensitization by depleting phosphatidylinositol
4,5-bisphosphate. J Neurosci. 30:10282–10293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hajnoczky G, Davies E and Madesh M:
Calcium signaling and apoptosis. Biochem Biophys Res Commun.
304:445–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newmeyer DD and Ferguson-Miller S:
Mitochondria: releasing power for life and unleashing the
machineries of death. Cell. 112:481–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grześk G, Wiciński M, Malinowski B, Grześk
E, Manysiak S, Odrowąż-Sypniewska G, Darvish N and Bierwagen M:
Calcium blockers inhibit cyclosporine A-induced hyperreactivity of
vascular smooth muscle cells. Mol Med Rep. 5:1469–1474.
2012.PubMed/NCBI
|
|
9
|
Grześk G, Kozinski M, Navarese EP,
Krzyzanowski M, Grześk E, Kubica A, Siller-Matula JM, Castriota F
and Kubica J: Ticagrelor, but not clopidogrel and prasugrel,
prevents ADP-induced vascular smooth muscle cell contraction: a
placebo-controlled study in rats. Thromb Res. 130:65–69.
2012.PubMed/NCBI
|
|
10
|
Kanagy NL and Webb RC: Enhanced vascular
reactivity to mastoparan, a G protein activator, in genetically
hypertensive rats. Hypertension. 23:946–950. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Birnbaumer L: The discovery of signal
transduction by G proteins: a personal account and an overview of
the initial findings and contributions that led to our present
understanding. Biochim Biophys Acta. 1768:756–771. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cotecchia S: The α1-adrenergic
receptors: diversity of signaling networks and regulation. J Recept
Signal Transduct Res. 30:410–419. 2010.
|
|
13
|
Bylund DB, et al: Adrenoceptors. The
IUPHAR Compendium of Receptor Characterization and Classification.
2nd edition. IUPHAR Media; London: pp. 88–103. 2000
|
|
14
|
Higashijima T, Burnier J and Ross EM:
Regulation of Giand Goby mastoparan, related
amphiphilic peptides, and hydrophobic amines. Mechanism and
structural determinants of activity. J Biol Chem. 265:14176–14186.
1990.
|
|
15
|
Perianin A and Snyderman R: Mastoparan, a
wasp venom peptide, indentifies two discrete mechanisms for
elevating cytosolic calcium and inositol triphosphates in human
polymorphonuclear leukocytes. J Immunol. 143:1669–1673. 1989.
|
|
16
|
Dostal DE, Murahashi T and Peach MJ:
Regulation of cytosolic calcium by angiotensins in vascular smooth
muscle. Hypertension. 15:815–822. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Argiolas A and Pisano JJ: Facilitation of
phospholipase A2 activity by mastoparans, a new class of mast cell
degranulating peptides from wasp venom. J Biol Chem.
258:13697–13702. 1983.PubMed/NCBI
|
|
18
|
Wallace MA and Carter HR: Effects of the
wasp venom peptide, mastoparan, on a phosphoinositide-specific
phospholipase C purified from rabbit brain membranes. Biochim
Biophys Acta. 1006:311–316. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hiramatsu Y, Horn VJ, Baum BJ and Ambudkar
IS: Characterization of polyphosphoinositide-specific phospholipase
C in rat parotid gland membranes. Arch Biochem Biophys.
297:368–376. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grześk G and Szadujkis-Szadurski L:
Modyfikacja reaktywnosci receptorow α-adrenergicznych przez
8Br-cGMP i angiotensyne II. Ann Acad Med Bydg. 17:5–10. 2003.(In
Polish).
|