Introduction
Ischemic cerebrovascular disease is currently one of
the leading causes of mortality and long-term disability worldwide,
often resulting in irreversible brain damage and subsequent loss of
neuronal function (1,2). The pathophysiological mechanisms
underlying initial and secondary injury following ischemia have not
been fully elucidated. Therefore, it is crucial to investigate the
pathogenesis of cerebral ischemia and develop novel, effective
methods to prevent and/or treat this disease.
It is well known that cell apoptosis is crucial in
neuronal death (3). Currently
available evidence demonstrates that apoptosis is involved in the
injury associated with ischemic cerebral diseases, particularly
cerebral ischemia/reperfusion (I/R) injury (4,5). Due
to its complexity, the precise mechanism of apoptosis induced by
cerebral ischemia remains unclear, although bcl2 and bax were shown
to play important roles (5). Bcl2
and bax belong to bcl2-related protein subfamilies, which are
encoded by several genes affecting cell apoptosis, among which the
bcl2 gene was shown to act as an anti-apoptotic factor and
the bax gene as a promoter of apoptosis (6,7). Novel
drugs, which may decrease the bax and/or increase the
bcl2 gene expression, may exert a protective effect against
neuronal apoptosis induced by I/R injury. Indeed, estradiol,
erythropoietin and SP600125 were reported to inhibit
ischemia-induced neuronal apoptosis through the regulation of
bcl2 and/or bax gene expression (8–10).
Angiotensin (Ang) II is produced from Ang I through
removal of two C-terminal residues by the angiotensin-converting
enzyme. Ang II is best known for its role in the regulation of
blood pressure, fluid balance and neuroendocrine function (11–13).
It was recently demonstrated that Ang II primarily activated
circumventricular neurons, leading to the activation of neurons in
other forebrain regions (14),
indicating that Ang II may exert multiple effects on neuronal
activity. It was also recently suggested that Ang II exerts a
protective effect against cortical neuron injury induced by hypoxia
(15). In this study, an in
vitro ischemia-induced cell model was established to
investigate whether Ang II exerts a protective effect against
neuronal injury and its mechanism of action.
Materials and methods
Materials
Ang II, PD123319 and valsartan were purchased from
Sigma (St. Louis, MO, USA). Cytarabine hydrochloride was purchased
from Changzhou Lab market Co. (Changzhou, China).
Na2S2O4 was purchased from Tianjin
Chemical Reagents Co., Inc. (Xiqing, Tianjin, China). TRIzol was
purchased from the Shanghai Bioengineering Institute (Shanghai,
China). The Annexin V-propidium iodide (PI) apoptosis kit was
purchased from Shenzhen Jingmei Biotechnology Co., Ltd. (Shenzhen,
Guangdong, China).
Animals
Male and female Wistar rats (weighing 220–250 g)
were obtained from the Laboratory Animal Center of Shandong
University. The animals were housed in a light- and
temperature-controlled room (21–22°C, humidity 50–65%) and
maintained on a standard diet and water. All the experiments were
performed in accordance with the Shandong University Guide for the
Care and Use of Laboratory Animals.
Primary neuronal cell culture
The primary neuronal cell cultures were performed as
previously described, with minor modifications (16). Briefly, the neonatal brains were
removed and dissected in D-Hank’s solution. After stripping the
meninges and blood vessels, the brain tissue was minced into
1-mm3 pieces and trypsinized at 37°C for 25 min. The
cells were seeded into 96- or 12-well plates pre-coated with
poly-D-lysine and maintained at 37°C in a humidified atmosphere
containing 5% CO2. Cystine arabinofuranoside was added
to the medium to inhibit the growth of cells other than
neurons.
In vitro ischemia/reperfusion (I/R)
injury model and morphology observation
The experiments were conducted on 7-day-old primary
cultured cortical neurons. Briefly, the primary cultured neurons
were first incubated with Na2S2O4
at a concentration of 1 mM in sugar-free Earle’s solution for 1 h.
The cell solution was then replaced by fresh medium, in which the
in vitro I/R model was established. Five groups were then
designed as follows: the control group, in which cortical neurons
were incubated with normal medium; the model group, in which the
solution was replaced with normal cell culture medium; the Ang II
group, in which the medium contained Ang II at a concentration of 1
μM; the Ang II plus PD123319 group, in which the medium contained
Ang II at a concentration of 1 μM and PD123319 at a concentration
of 10 μM; and the Ang II plus valsartan group, in which the medium
contained Ang II at a concentration of 1 μM and valsartan at a
concentration of 10 μM. Following incubation for 12 h, the cell
morphology was observed under an inverted phase contrast microscope
and representative images were selected.
Cell viability
The cell viability was measured with the MTT assay,
as previously described (17).
Briefly, the cortical neurons were incubated as dictated by the
experiment design for 12 h and the medium was then replaced by MTT
solution (200 μg/ml in normal medium) for another 2 h. The medium
was aspirated and the formazan dye crystals were dissolved in 200
μl dimethyl sulfoxide. The cell viability was determined by optical
density values at 570 nm measured by an automatic plate reader.
Annexin V staining
The Annexin V staining assay was performed according
to the manufacturer’s protocol. Briefly, the cells were washed
twice with D-Hank’s solution after treatment and digested with
trypsin. The cells were collected by centrifugation at 300 × g for
5 min and suspended and the density was adjusted to
5×105 cells/ml, in which 195 μl cell suspension and 5 μl
Annexin V-fluorescein isothiocyanate were mixed and incubated for
10 min at room temperature. After washing, the cells were
resuspended in 190 μl pre-diluted binding buffer and stained with
PI (1 μg/ml). The cells were finally analysed by flow cytometry
(FCM) and the apoptosis ratios were calculated.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total mRNA was prepared and RT-PCR was performed as
previously described (18).
Briefly, the cells were washed twice with D-Hank’s solution after
treatment and total RNA was extracted from the cultured cortical
neurons using a TRIzol extraction kit. After RT, the PCR assay was
performed to amplify the bcl2, bax and
β-actin. The PCR products were then subjected to 2% agarose
gel electrophoresis and visualized by staining with ethidium
bromide. The optical density of each band was measured using ImageJ
software and the semi-quantitative measure was expressed as a ratio
compared to that of β-actin. The primer sequences for
bcl2, bax and β-actin are presented in
Table I.
 | Table IPrimer sequences for bcl2,
bax and β-actin. |
Table I
Primer sequences for bcl2,
bax and β-actin.
| Gene | Sequences | Product length |
|---|
| Bcl2 | F:
5′-CTGGTGGACAACATCGCTCTG-3′
R: 5′-GGTCTGCTGACCTCACTTGTG-3′ | 227 bp |
| Bax | F:
5′-GCAGAGGATGATTGCTGATG-3′
R: 5′-CTCAGCCCATCTTCTTCCAG-3′ | 354 bp |
| β-actin | F:
5′-TTGGCACCACACTTTCTACA-3′
R: 5′-TCACGCACGATTTCCCTCTCAG-3′ | 380 bp |
Statistical analysis
Data are expressed as values ± SD and analyzed by
one-way ANOVA. The difference between two groups was assessed by
the Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference between any two groups.
Results
Ang II attenuates the I/R-induced
neuronal damage
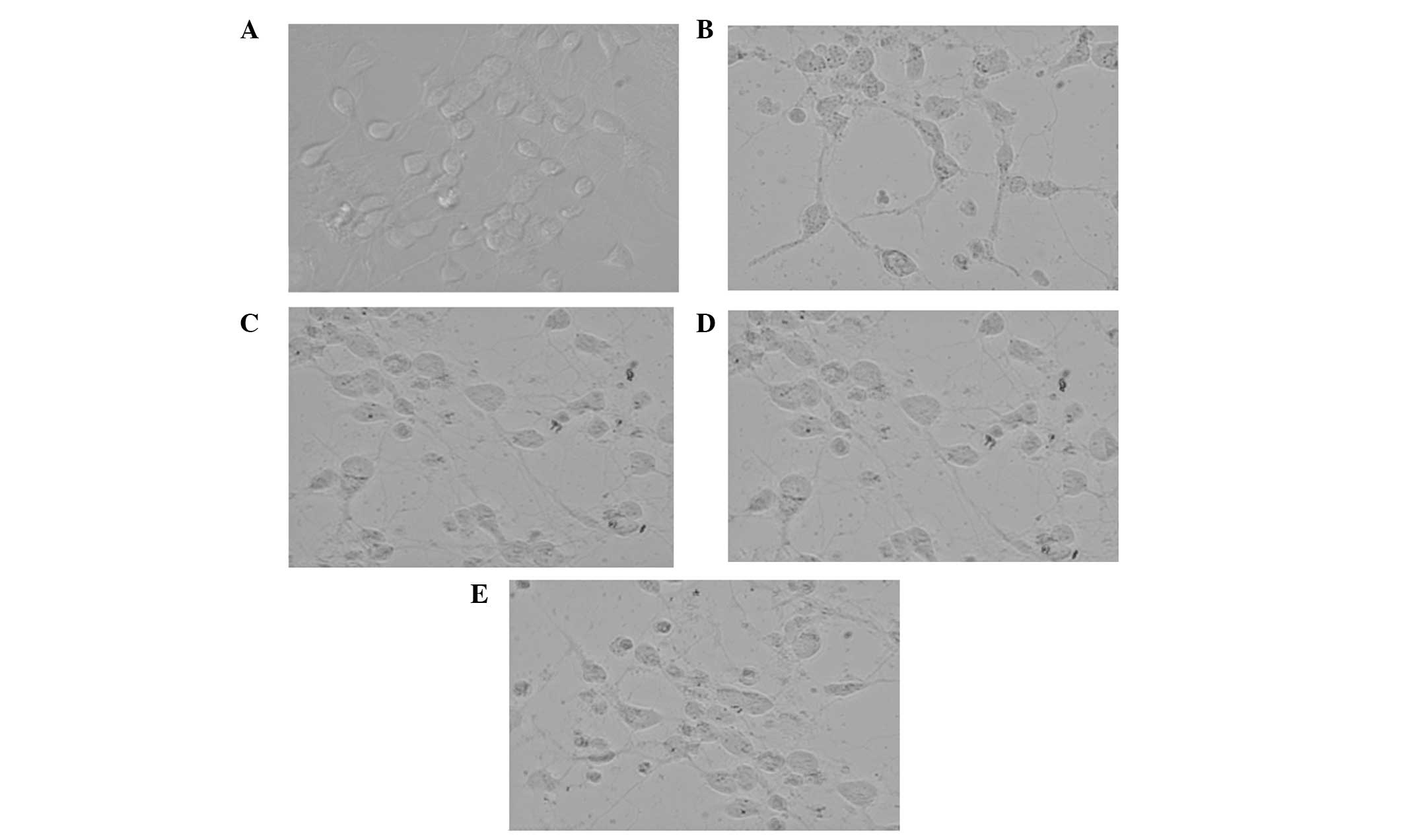
Following observation under an inverted phase
contrast microscope, the primary cultured neurons were
characterized morphologically and biochemically (Fig. 1). The cells in the model group
appeared swollen, with the neurites being contracted or even
severed. However, the cells in the Ang II group appeared to to
exhibit a reconstituted morphology. Similar morphological
characteristics were observed in the presence of Ang II plus
valsartan or PD123319, with Ang II plus valsartan exerting a more
potent protective effect.
Ang II increases the viability of
oxygen-glucose deprivation-treated neurons
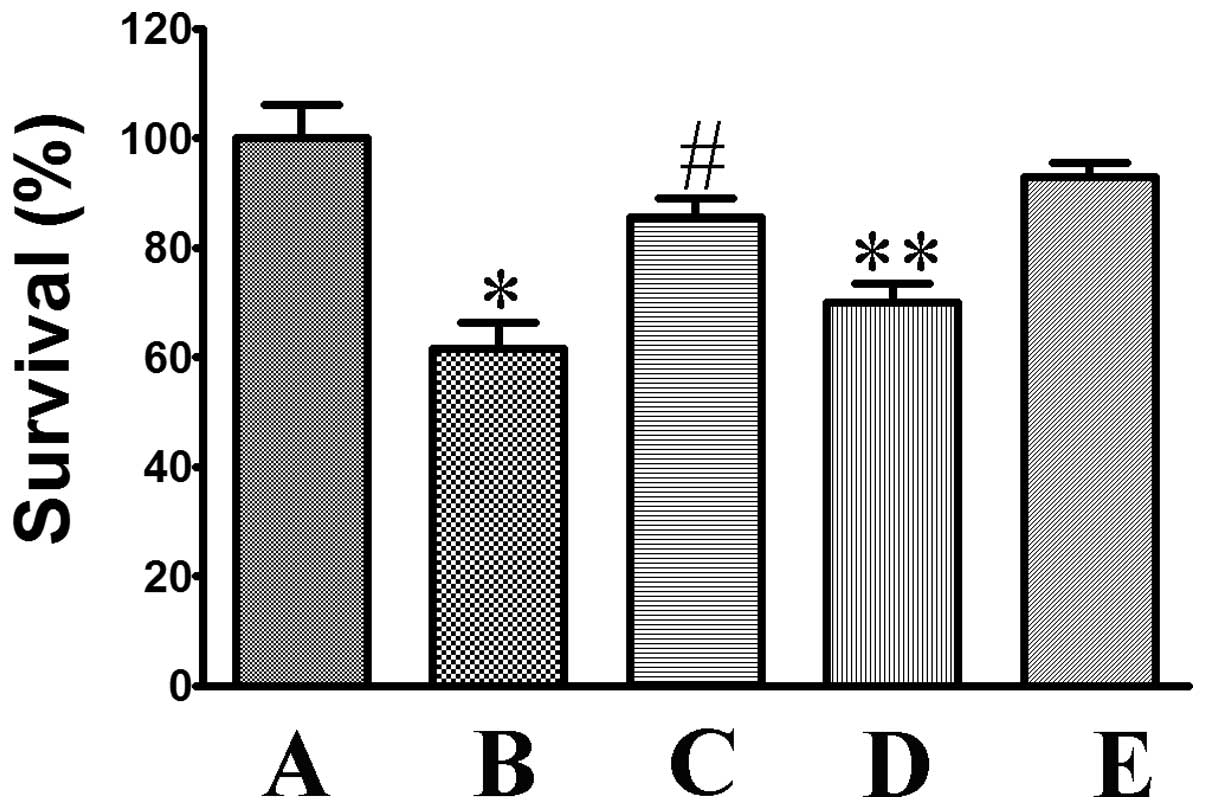
MTT was commonly used to assess cell viability. As
shown in Fig. 2, I/R injury
significantly reduced cell viability to 61.65±4.67% (P<0.05,
compared to that in the control group). However, incubation with
Ang II significantly attenuated cell death to 85.55±3.54%
(P<0.05, compared to that in the model group). The protective
activity of Ang II was significantly attenuated by co-incubation
with PD123319, with the relative survival being reduced to
70.10±3.38% (P<0.05, compared to that in the Ang II group).
Co-treatment with valsartan was shown to further increase the
protective activity of Ang II to 92.95±2.26%.
Ang II attenuates cell apoptosis induced
by oxygen-glucose deprivation and reperfusion
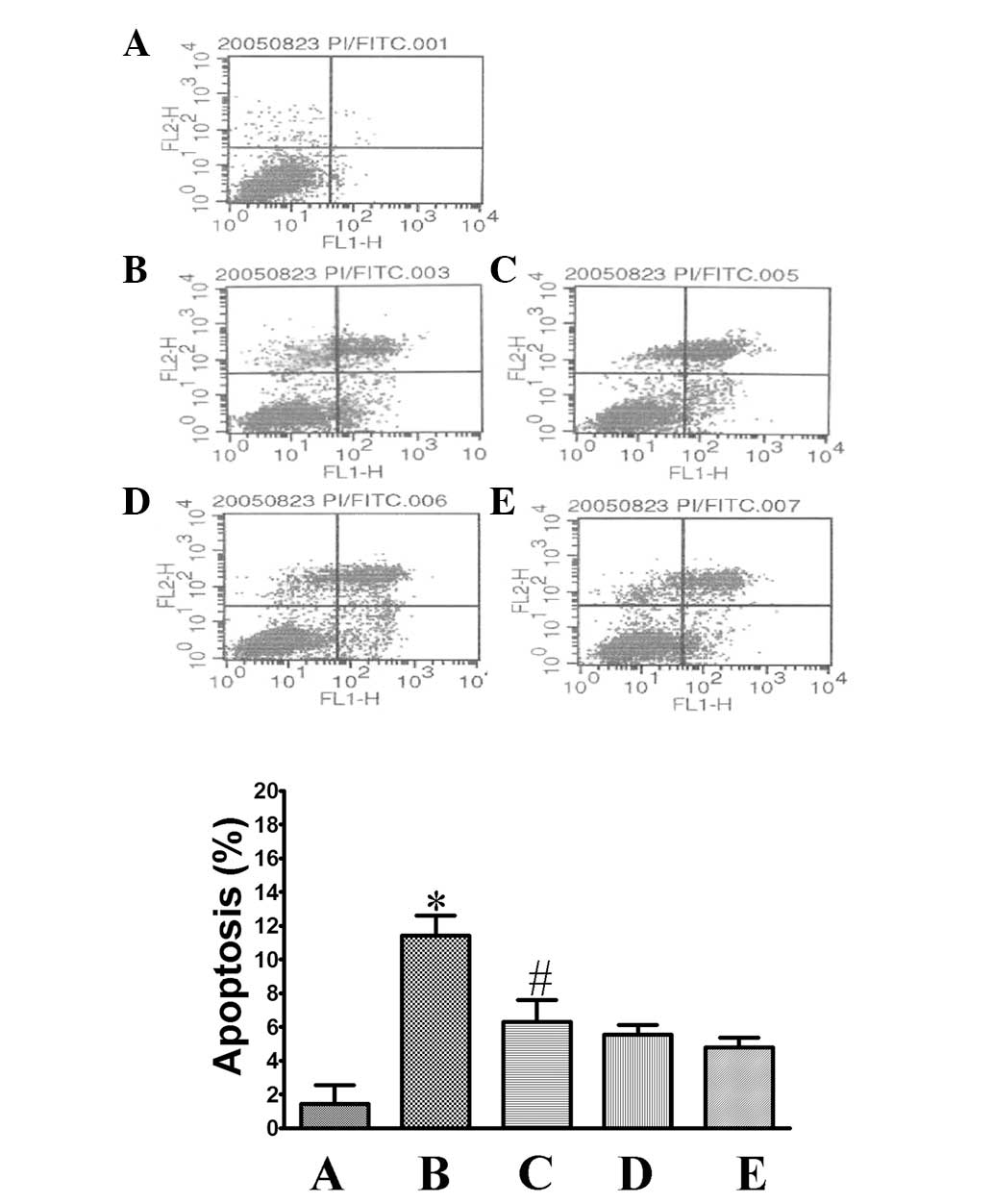
The Annexin V staining assay was used to detect cell
apoptosis by FCM. As shown in Fig.
3, the apoptosis ratio in the control group was 1.44±1.11%.
After the cells were exposed to oxygen-glucose deprivation and
reperfusion, that ratio increased to 11.4±1.20% (P<0.05,
compared to that in the control group), which indicated that the
cells were undergoing apoptosis. Treatment of the cells with Ang II
significantly attenuated cell apoptosis, with an apoptosis ratio of
5.27±0.55% (P<0.05, compared to that in the model group).
Co-treatment with PD123319 or valsartan was shown to inhibit cell
apoptosis with a similar tendency (apoptosis ratio, 5.55±0.58 and
4.77±0.62%, respectively).
Ang II increases the ratio of bcl2/bax in
mRNA expression
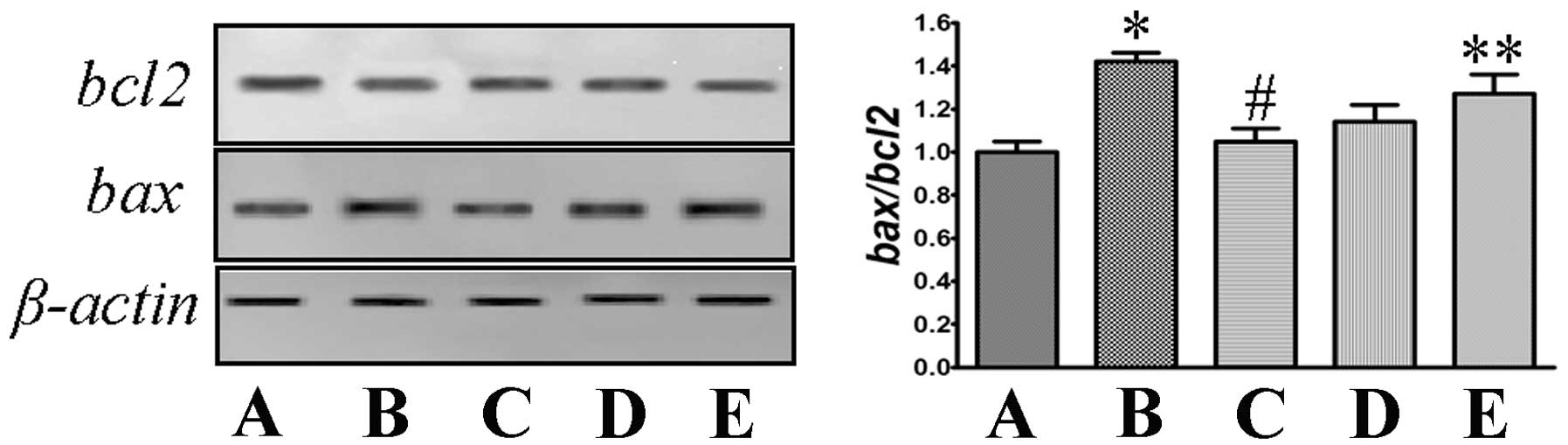
Total mRNA was prepared and RT-PCR was performed. As
shown in Fig. 4, the model group
exhibited increased mRNA expression of bax and decreased
mRNA expression of bcl2, with a significant increase of the
bcl2/bax ratio (P<0.05, compared to that in the control
group). Co-incubation with Ang II attenuated the increased
bax and decreased bcl2, which reduced the changes in
the bcl2/bax ratio (P<0.05, compared to that in the model
group). Co-treatment with PD123319 or valsartan exhibited a similar
tendency.
Discussion
The cerebral vascular event (stroke) caused by
ischemia or hemorrhage is the most common type of cerebrovascular
disease and the main cause of disability and mortality worldwide
(1). Since the pathophysiological
mechanism of initial and secondary injury after ischemia has not
been fully elucidated, it is crucial to investigate its
pathogenesis and develop novel treatment methods.
The central nervous system is vulnerable to the
effects of disease and toxic or metabolic insults, such as oxygen
and glucose deprivation, which often occurs during stroke and
cardiac arrest (19,20). Although thrombolysis has been
approved as an effective therapy for acute ischemic stroke with
specific time windows, early reperfusion may aggravate edema
formation (21). In this study,
Na2SO4 was used to establish the hypoxic
environment through clearing oxygen from the culture medium,
followed by medium re-incubation, which mimics the process of
cerebral I/R injury. Our data clearly demonstrated that the I/R
injured the primary cultured neurons, which were observed to be
swollen, with the neurites being contracted, or even severed.
Incubation with Ang II was able to reconstitute the morphology of
the injured cells, which indicated that Ang II exerted a protective
effect against I/R-induced injury in neurons. Consistently, I/R
injury significantly decreased cell survival, as was detected by
the MTT assay. Treatment with Ang II was able to attenuate cell
death induced by I/R injury, which also indicated that Ang II may
be used against ischemic cerebral vascular disease.
It is well known that neuronal death is associated
with apoptosis and currently available evidence demonstrates that
apoptosis is involved in the injury associated with ischemic
cerebral diseases, particularly cerebral ischemia/reperfusion (I/R)
injury (3,4). Apoptosis may be the response to a
cellular ‘insult’, such as chemical or physical damage. The insult
initiates a cascade of events leading to DNA breaks and the
destruction of the cell, which are easily detected and analyzed by
several methods, such as the TUNEL staining assay, electron
microscope scanning and Annexin V staining (22,23).
In this study, we performed the Annexin V staining assay and
analyzed the apoptosis ratio in neurons with flow cytometry. Our
data demonstrated that I/R injury induced neuronal apoptosis, as
indicated by the ratio of PI staining-positive cells. Treatment
with Ang II attenuated cell apoptosis induced by the ischemic
stress, which indicated that the protective effect of Ang II may be
partially mediated though preventing the neurons from undergoing
apoptosis.
The mechanism of apoptosis induced by ischemic
injury in neurons has not been fully elucidated, although the bcl2
family was shown to play an important role (6). Based on structural and functional
characteristics, the protein families were divided into
anti-apoptotic members, such as bcl2, and pro-apoptotic members,
such as bax (7). After the neurons
were exposed to I/R injury, the mRNA levels of bax were
observed to increase and the mRNA levels of bcl2 were
decreased. The disruption of balance between anti- and
pro-apoptotic effects induced cell apoptosis. However, treatment
with Ang II was shown to increase the reduced bcl2
expression and decrease the elevated bax expression, which
in turn attenuated the neuronal apoptosis induced by I/R
injury.
Ang II is best known for its role in the regulation
of cardiovascular behavior and fluid homeostasis, through binding
with its receptors, of which there were two major isoforms, Ang II
type 1 (AT1) receptor and AT2 receptor (24,25).
These isoforms are considered to account for the diverse actions of
Ang II in peripheral organs, as well as in the nervous system.
Based on our results, the protective effect of Ang II against
neuronal death induced by I/R injury may be inhibited by
co-incubation with PD123319, but not with valsartan, indicating
that this protective effect of Ang II was exerted in an
AT2-dependent manner. Of note, co-treatment with valsartan did not
decrease, but rather increased cell survival. This finding was
consistent with previous findings stating that chronic blockade of
AT1 receptors with losartan prevented bax upregulation and
normalized apoptosis in these cells, independently of its
hemodynamic effect (26). The exact
molecular mechanism through which Ang II displays its protective
activity and its effects in vivo remains unclear and
requires further investigation.
In summary, to the best of our knowledge, this was
the first study to demonstrate that Ang II exerts a protective
effect against ischemia-induced neuronal injury in an AT2-dependent
manner, which is associated with the inhibition of neuronal
apoptosis via the regulation of the ratio of bax/bcl2 mRNA
expression by Ang II. Therefore, Ang II may be used as a
therapeutic target in the future.
References
|
1
|
Read SJ, Hirano T, Davis SM and Donnan GA:
Limiting neurological damage after stroke: a review of
pharmacological treatment options. Drugs Aging. 14:11–39. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baron JC: Perfusion thresholds in human
cerebral ischemia: historical perspective and therapeutic
implications. Cerebrovasc Dis. 11(Suppl 1): 2–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okouchi M, Ekshyyan O, Maracine M and Aw
TY: Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal.
9:1059–1096. 2007. View Article : Google Scholar
|
|
4
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niizuma K, Yoshioka H, Chen H, et al:
Mitochondrial and apoptotic neuronal death signaling pathways in
cerebral ischemia. Biochim Biophys Acta. 1802:92–99. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shamas-Din A, Kale J, Leber B and Andrews
DW: Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb
Perspect Biol. 5:a0087142013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ayaloglu-Butun F, Terzioglu-Kara E,
Tokcaer-Keskin Z and Akcali KC: The effect of estrogen on bone
marrow-derived rat mesenchymal stem cell maintenance: inhibiting
apoptosis through the expression of Bcl-xL and Bcl-2. Stem Cell
Rev. 8:393–401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Warren JS, Zhao Y, Yung R and Desai A:
Recombinant human erythropoietin suppresses endothelial cell
apoptosis and reduces the ratio of Bax to Bcl-2 proteins in the
aortas of apolipoprotein E-deficient mice. J Cardiovasc Pharmacol.
57:424–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramin M, Azizi P, Motamedi F, Haghparast A
and Khodagholi F: Inhibition of JNK phosphorylation reverses memory
deficit induced by β-amyloid (1–42) associated with decrease of
apoptotic factors. Behav Brain Res. 217:424–431. 2011.PubMed/NCBI
|
|
11
|
Wysocki J, Ye M, Rodriguez E, et al:
Targeting the degradation of angiotensin II with recombinant
angiotensin-converting enzyme 2: prevention of angiotensin
II-dependent hypertension. Hypertension. 55:90–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reis WL, Saad WA, Camargo LA, Elias LL and
Antunes-Rodrigues J: Central nitrergic system regulation of
neuroendocrine secretion, fluid intake and blood pressure induced
by angiotensin-II. Behav Brain Funct. 6:642010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grobe JL, Grobe CL, Beltz TG, et al: The
brain renin-angiotensin system controls divergent efferent
mechanisms to regulate fluid and energy balance. Cell Metab.
12:431–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao X, Peterson JR, Wang G, et al:
Angiotensin II-dependent hypertension requires cyclooxygenase
1-derived prostaglandin E2 and EP1 receptor signaling in the
subfornical organ of the brain. Hypertension. 59:869–876. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCarthy CA, Vinh A, Broughton BR, Sobey
CG, Callaway JK and Widdop RE: Angiotensin II type 2 receptor
stimulation initiated after stroke causes neuroprotection in
conscious rats. Hypertension. 60:1531–1537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pont-Lezica L, Colasse S and Bessis A:
Depletion of microglia from primary cellular cultures. Methods Mol
Biol. 1041:55–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Yu P, Gou H, et al:
Cardioprotective effects of 20(S)-ginsenoside Rh2 against
doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid Based
Complement Alternat Med. 2012:5062142012.PubMed/NCBI
|
|
18
|
Wang H, Yu P, Bai J, et al: Ocotillol
enhanced the antitumor activity of doxorubicin via p53-dependent
apoptosis. Evid Based Complement Alternat Med.
2013:4685372013.PubMed/NCBI
|
|
19
|
Xilouri M and Stefanis L: Autophagy in the
central nervous system: implications for neurodegenerative
disorders. CNS Neurol Disord Drug Targets. 9:701–719. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou T, Jiang J, Zhang M, Fu Y, Yang Z and
Jiang L: Protective effect of mild hypothermia on oxygen-glucose
deprivation injury in rat hippocampal neurons after hypoxia. Mol
Med Rep. 7:1859–1864. 2013.PubMed/NCBI
|
|
21
|
Dominguez C, Delgado P, Vilches A, et al:
Oxidative stress after thrombolysis-induced reperfusion in human
stroke. Stroke. 41:653–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Chen Y, Jenkins LW, Kochanek PM
and Clark RS: Bench-to-bedside review: Apoptosis/programmed cell
death triggered by traumatic brain injury. Crit Care. 9:66–75.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krysko DV, Vanden Berghe T, D’Herde K and
Vandenabeele P: Apoptosis and necrosis: detection, discrimination
and phagocytosis. Methods. 44:205–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaschina E and Unger T: Angiotensin
AT1/AT2 receptors: regulation, signalling and function. Blood
Press. 12:70–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldenberg I, Grossman E, Jacobson KA,
Shneyvays V and Shainberg A: Angiotensin II-induced apoptosis in
rat cardiomyocyte culture: a possible role of AT1 and AT2
receptors. J Hypertens. 19:1681–1689. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fortuno MA, Ravassa S, Etayo JC and Diez
J: Overexpression of Bax protein and enhanced apoptosis in the left
ventricle of spontaneously hypertensive rats: effects of AT1
blockade with losartan. Hypertension. 32:280–286. 1998. View Article : Google Scholar : PubMed/NCBI
|