Introduction
It was estimated that the prevalence of diabetes
among all age groups worldwide was 2.8% in 2000 and is likely to be
4.4% in 2030 (1). The number of
individuals with diabetes is likely to increase to 366 million by
2030. Diabetic nephropathy (DN) is a major cause of morbidity and
mortality, occurring in 20–40% of diabetic patients (2). DN is the single leading cause of
end-stage renal disease (ESRD) (3,4). The
incidence of ESRD is a growing problem in all countries with a
western lifestyle (5). Hypertension
occurs in ~50% of type II diabetes patients and is also a major
factor leading to arterial damage. The resulting arterial damage is
usually progressive and accelerates the development of DN and ESRD
(6).
The renin-angiotensin-aldosterone system (RAAS) is
crucial in the control of blood pressure (BP) and the pathogenesis
of hypertension (7). Blocking the
activity of the RAAS is extensively used in the management of
hypertension. The renal protective effects of angiotensin II type 1
(AT1) receptor blockers (ARBs) have been demonstrated in animal
models of diabetes, including type 1 and 2 diabetic rats (8,9).
Olmesartan medoxomil (OM) is one of the newest additions to the ARB
family and it may be rapidly and completely de-esterified to
olmesartan following oral administration. To the best of our
knowledge, the ability of OM to control DN in animal models of
streptozotocin (STZ)-induced diabetes has not been investigated,
although OM was previously demonstrated to retard the progression
of DN (10). The utilization of OM
in this STZ-induced diabetes animal model appears promising in
elucidating the mechanism underlying DN and advancing translational
research (11,12). The purpose of this study was to
evaluate the efficacy of OM in the treatment of DN by investigating
the renoprotective effects of this drug in an STZ-induced diabetes
rat model.
Materials and methods
Chemicals and instruments
OM was supplied by the Shanghai Sankyo
Pharmaceutical Co., Ltd. (Shanghai, China). The standard STZ was
purchased from Sigma Chemical Co. (St. Louis, MO, USA). Creatinine
(Cr), blood urea nitrogen (BUN), superoxide dismutase (SOD),
malondialdehyde (MDA) and protein test kits were purchased from the
Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All
other chemicals and reagents used were of analytical grade.
Animals
Thirty male Sprague Dawley rats, weighing 180–240 g,
were purchased from the Experimental Animal Center of Luye
Pharmaceutical Company [certificate no. SCXK (Lu) 20030008]. The
rats were kept in a room at a relative humidity of 55% (permissible
range: 30–70%) and a temperature of 23°C (permissible range:
20–26°C) under a 12-h light/dark cycle. The rats were allowed free
access to food and water.
All the experiments in this study were conducted in
accordance with the Guidelines for the Care and Use of Laboratory
Animals of Yantai University and were approved by the Animal Study
Committee.
Experimental design
Following several days of acclimatization, the rats
(n=30) were injected intraperitoneally with STZ dissolved in
citrate buffer (pH 4.5) at a dose of 65 mg/kg body weight. After 3
days, induction of diabetes was confirmed by measuring blood
glucose concentration (≥16.7 mM) (13). The rats with blood glucose levels
>16.7 mM were randomly divided into 2 groups. One group was used
as the DN control (n=10) and the other group (n=10) received OM at
a dose of 10 mg/kg body weight/day via oral gavage. A normal group
of rats (n=10) that underwent sham operation was also included.
During the course of the experiment, the normal and control groups
received physiological saline of equal volumes. The serum glucose
was measured at 0, 4, 8, and 12 weeks after overnight fasting. At
12 weeks, 12-h urine samples were collected using metabolic cages
and the rats were weighed and sacrificed. Blood samples were
obtained from the abdominal aorta. The serum was immediately
separated from the blood by centrifugation at 1,600 × g for 25 min
at 4°C and stored at −80°C. The left kidneys were removed and
weighed following renal perfusion through the renal artery with
ice-cold physiological saline (10,14).
After rinsing with phosphate-buffered saline, kidney sections were
sliced and immersed in 10% formalin for histological evaluation,
whereas the remaining sections were frozen at −80°C.
Biochemical measurement
The plasma samples were used for the measurement of
glucose, Cr concentration, BUN, SOD, MDA and albumin. The indices
were examined with an ultraviolet-visible spectrophotometer using
commercial reagents. Urine samples were collected using metabolic
cages and the supernatant was used for examination of the urinary
protein concentration. The Cr clearance (CCr) was calculated using
the equation: CCr (ml/kg bw/min) = [UCr (mg/dl) × urine
volume (ml)/SCr (mg/dl)] × [1000/bw (g)] × [1/720
(min)], where UCr is the urinary creatinine,
SCr is the serum creatinine and bw is the body weight.
The levels of microalbumin (mALB) were quantified by ELISA (YfSwBio
Shanghai, China).
Histological analysis
Renal tissues were fixed in 10% formalin solution
and embedded in paraffin. Sections (2 μm) were obtained by a
Polycut microtome (CM1950; Leica, Mannheim, Germany) and stained
with hematoxylin and eosin. The sections were then examined under a
light microscope.
Statistical analysis
Data are expressed as means ± standard deviation.
Significant differences within the groups were calculated by
one-way ANOVA. Statistical differences between groups were
evaluated by the Student’s unpaired t-test, using SPSS statistical
software, version 11.5 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Body and kidney weight changes
The effects of OM administered by oral gavage on the
body and kidney weight of the rats are presented in Table I. The body weights of the rats in
the treatment group was increased compared to that of the DN rats.
Furthermore, the body weight gain of normal rats was 4.95 times
higher compared to the DN control rats. In the treatment group,
there was a 1.94-fold increase in the body weight. However, the
kidney weight of DN control rats was 2.0 times higher compared to
the normal group, indicating reduced enlargement due to OM
administration.
 | Table IChanges in body and renal weight. |
Table I
Changes in body and renal weight.
| Groups | Body weight (g) | Renal weight (g/100 g
body weight) |
|---|
|
|---|
| Initial | Final | Gain (12 weeks) |
|---|
| A (normal) | 265.8±7.5 | 360.1±14.1 | 95.2±7.8 | 0.35±0.04 |
| B (DN control) | 252.3±5.4b | 271.5±6.3b | 19.2±5.6b | 0.71±0.05b |
| C (OM) | 256.9±6.9a,b | 304.8±5.7a,b | 48.9±6.3a,b | 0.56±0.03a,b |
Serum indices and BP
As shown in Table
II, the administration of OM affected serum glucose and albumin
levels to a certain extent. The serum glucose level in the DN
control group was 34.40 mmol/l, which was significantly higher
compared to the normal group. However, the serum glucose level
decreased to 22.00 mmol/l following administration of OM. The serum
albumin level in the DN control rats was 30.32 g/l, which was lower
compared to the normal rats. These levels were significantly
increased by 5.0% following treatment with OM. The changes in blood
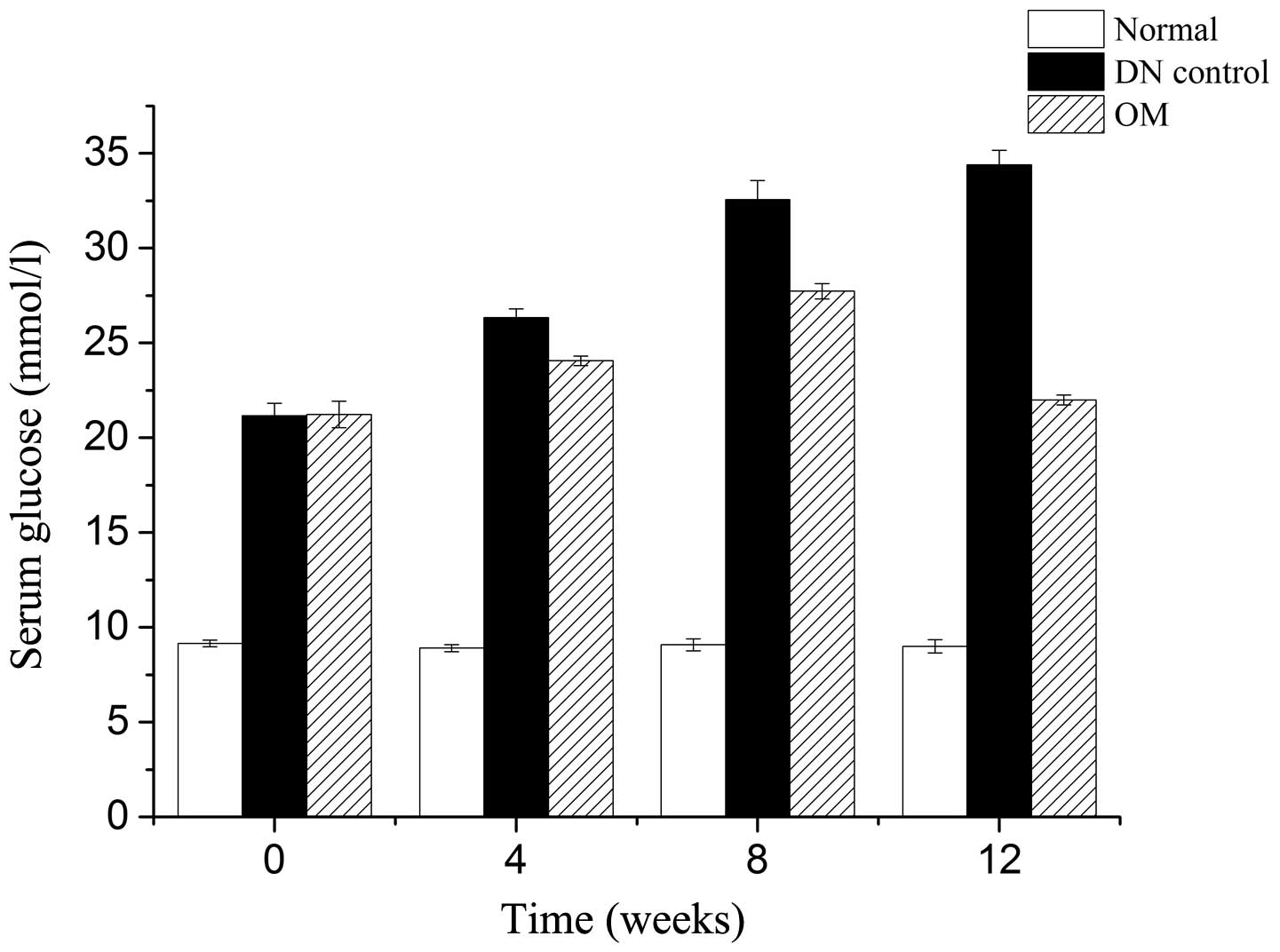
glucose during 12 weeks are shown in Fig. 1. The level of hyperglycemia was
significantly reduced by OM at 12 weeks. However, at 4 and 8 weeks
there was only a minor decrease in hyperglycemia.
 | Table IIEffects of OM on blood pressure and
biological indices in serum and urine. |
Table II
Effects of OM on blood pressure and
biological indices in serum and urine.
| Variables | A (normal) | B (DN control) | C (OM) |
|---|
| BP (mmHg) | 100.25±1.91 | 118.12±2.13b | 102.18±2.42a,b |
| Serum |
| Glucose
(mmol/l) | 9.00±0.35 | 34.40±0.75b | 22.00±0.26a,b |
| Albumin (g/dl) | 35.65±0.45 | 30.32±0.26b | 31.84±0.21a,b |
| Creatinine
(μmol/l) | 7.43±2.01 | 78.08±1.95b | 61.45±1.83a,b |
| BUN (mmol/l) | 9.35±0.37 | 18.97±1.31b | 14.28±0.97a,b |
| SOD (U/mg prot) | 1.20±0.05 | 0.98±0.02b | 1.11±0.03a,b |
| MDA (nmol/ml) | 3.01±0.27 | 5.76±0.31b | 4.31±0.28a,b |
| Urine |
| CCr (ml/kg body
weight/min) | 25.45±1.23 | 16.94±1.38b | 20.95±0.89a,b |
| Protein excretion
(mg/day) | 8.34±0.61 | 43.26±5.93b | 23.83±3.34a,b |
| mALB (ng/ml) | 16.53±0.54 | 35.04±0.38b | 28.93±0.86a,b |
The serum SOD concentration in DN rats was lower
compared to that in normal rats and the SOD concentration was
increased by 13.27% with OM treatment. The serum MDA level was
increased by 1.91-fold in DN rats and decreased with OM treament.
Over the course of this study, BP was elevated in DN compared to
normal rats and treatment with OM lowered BP to almost normal
levels (Table II).
Parameters of renal function
The effects of OM on serum and urinary parameters of
renal function are presented in Table
II. BUN, urinary protein excretion and serum Cr levels were
higher in the DN control compared to the normal group. Following
oral administration of OM, the BUN and serum Cr levels were
decreased. In addition, the use of OM decreased urinary protein
excretion from 43.26 to 23.83 mg/day. The urinary mALB
concentration was increased significantly in DN rats and was
decreased significantly with OM (P<0.05).
Histopathological changes
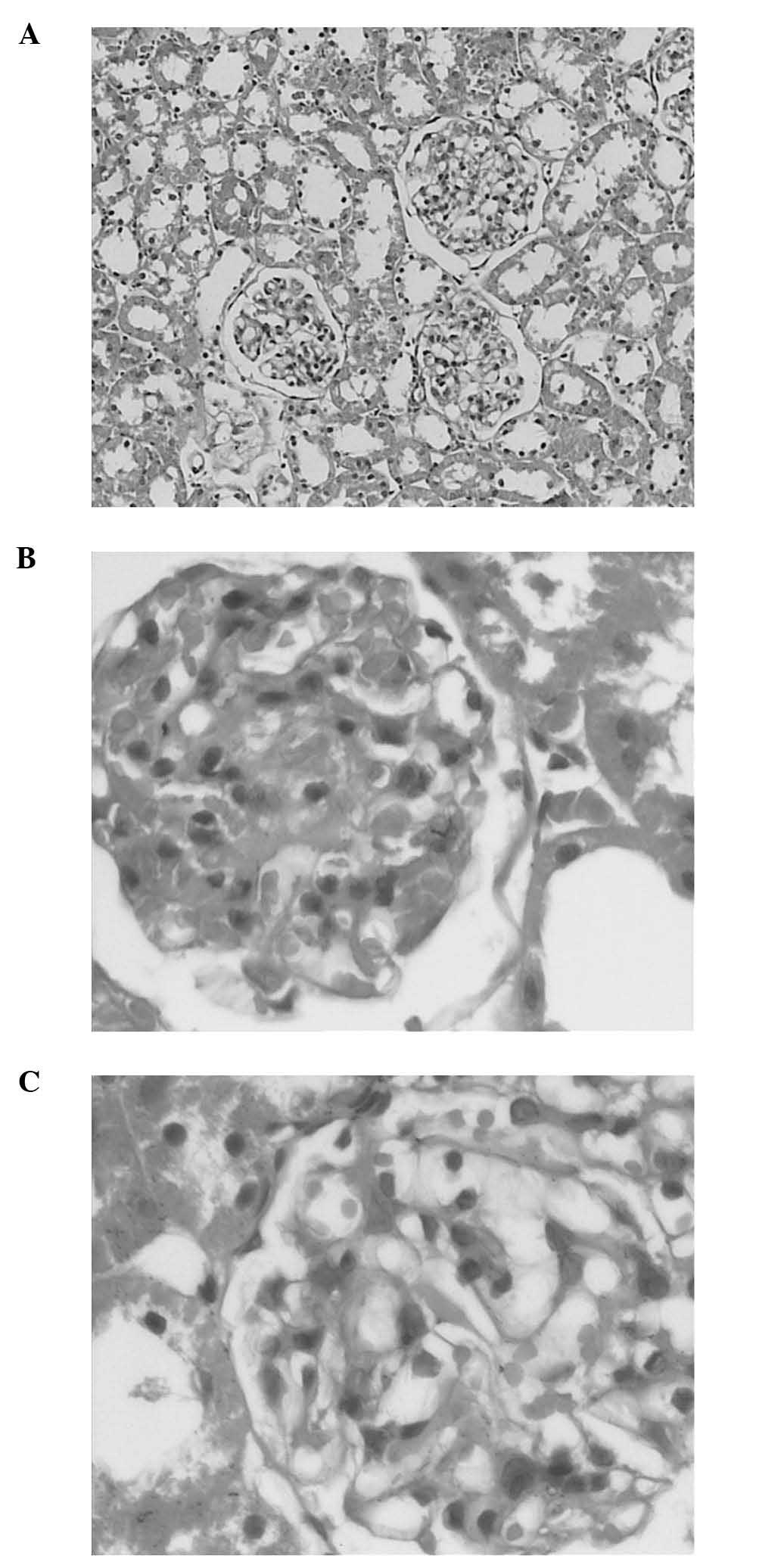
Light microscopy examination revealed lesions and an
increase of the mesangial matrix in the DN control group. In
addition, glomerular hypertrophy in the DN group was more prominent
compared to that observed in normal rats. The OM treatment group
exhibited minimal exudative renal lesions compared to those in the
DN control group (Fig. 2) and
presented with decreased glomerular hypertrophy to a certain
extent.
Discussion
STZ is toxic to pancreatic β cells (15,16)
and has been widely used to induce diabetes in animal models
(17). There are various animal
models of diabetes with different origin, characteristics and
underlying causes (18). However,
STZ-induced experimental diabetes exhibits sustained hyperglycemia
and well-characterized diabetic complications with a low incidence
of mortality (15). In this study,
an STZ-induced diabetes animal model was selected to investigate
the ability of OM to control DN.
DN is characterized by excessive amassing of
extracellular matrix, with thickening of the glomerular and tubular
basement membranes and an increased amount of mesangial matrix
(19). The STZ-injected rats
exhibited the main characteristics of diabetes mellitus and the
changes in the DN markers in our study were similar to those
previously reported (20–22). In the present study, OM was able to
improve these parameters in DN rats.
Hypertension is one of the most important
contributing factors to DN. Therefore, BP needs to be tightly
regulated. The angiotensin-converting enzyme inhibitors are a group
of drugs that are beneficial in the treatment of DN (23). The angiotensin II (Ang II) receptor
blockers (ARBs) have been shown to retard the progression of
nephropathy in patients with diabetes. The renoprotective effects
of ARBs in rat models were previously reported (24,25).
OM, one of the most potent ARBs, was demonstrated to increase
plasma renin activity in rats. Therefore, the renoprotective
actions demonstrated in this study may be attributed to the
systemic and intrarenal blockade of the renin-angiotensin system
(26). In this study, it was
demonstrated that OM was able to decrease BP. It was also
demonstrated that the serum glucose concentration in diabetic rats
was by 3.78-fold higher compared to that in normal rats and
treatment with OM significantly inhibited hyperglycemia. OM also
reduced renal weight and increased body weight. The mechanism of
the inhibition of hyperglycemia by OM has not been elucidated,
although it may be through the blockade of RAS or the inhibition of
oxidative stress.
The common characteristic in the development of DN
is the decrease in the glomerular filtration rate, which may
reflect serum Cr and CCr levels and lead to proteinuria. The DN
rats exhibited significant increases in serum Cr, BUN and urinary
protein excretion, whereas the CCr level was significantly
decreased compared to that in normal rats. According to a previous
study by Oktem et al(22),
proteinuria is an important indicator of early-stage DN and
accelerates the occurrence of tubular cell damage. Another previous
study (27) reported that mALB is a
sensitive and specific predictor of DN and it has been widely used
as a clinical index of early-stage DN. ARBs were reported to reduce
proteinuria in a number of animal models of diabetes (9,28). OM
significantly inhibited the development of mALB in DN rats. The
increasing levels of BUN and serum Cr may indicate progressive
renal damage and the present study demonstrated that OM positively
affected these parameters.
It was previously demonstrated that increased
oxidative stress and reactive oxygen species are involved in the
pathogenesis of diabetes (29).
Oxidative stress may increase the production of free oxygen
radicals, promote the formation of lipid peroxidation products and
reduce the level of antioxidant enzymes, such as SOD. SOD is a
scavenger of free radicals and the protective effect of SOD on
renal function is directly associated with its ability to alleviate
oxidative stress. MDA is an end-product of lipid peroxidation and
may reflect the degree of oxidation in renal tissues. OM was
reported to improve endothelin-induced hypertension and oxidative
stress in rats (10). According to
Fujimoto et al(30), OM may
inhibit superoxide production and oxidative stress, independent of
its BP-lowering effect. The data in our study demonstrated that the
serum SOD concentrations in diabetic rats were lower compared to
those in normal rats and the levels of SOD were increased, whereas
MDA levels were decreased following treatment with OM.
In conclusion, our results suggest that OM exerts a
beneficial effect on DN via different pathways and it may be a
potential renoprotective pharmaceutical for the treatment of
DN.
Acknowledgements
This study was supported by the Programs for Science
and Technology Development and Plan of Yantai (no. 2012076) and
Science Foundation for The Excellent Youth Scholars of Shandong
Province (no. 2008BS02027).
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053.
2004.PubMed/NCBI
|
|
2
|
Tan AL, Forbes JM and Cooper ME: AGE,
RAGE, and ROS in diabetic nephropathy. Semin Nephrol. 27:130–143.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dalla Vestra M, Saller A, Bortoloso E,
Mauer M and Fioretto P: Structural involvement in type 1 and type 2
diabetic nephropathy. Diabetes Metab. 26(Suppl 4): 8–14.
2000.PubMed/NCBI
|
|
4
|
American Diabetes Association. Standards
of medical care in diabetes. Diabetes Care. 28(Suppl 1): 4–36.
2005. View Article : Google Scholar
|
|
5
|
Ritz E, Rychlik I, Locatelli F and Halimi
S: End-stage renal failure in type 2 diabetes: a medical
catastrophe of worldwide dimensions. Am J Kidney Dis. 34:795–808.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pugsley MK: The angiotensin-II (AT-II)
receptor blocker olmesartan reduces renal damage in animal models
of hypertension and diabetes. Proc West Pharmacol Soc. 48:35–38.
2005.PubMed/NCBI
|
|
7
|
de Gasparo M, Catt KJ, Inagami T, Wright
JW and Unger T: International union of pharmacology. XXIII. The
angiotensin II receptors. Pharmacol Rev. 52:415–472.
2000.PubMed/NCBI
|
|
8
|
Kohzuki M, Yasujima M, Kanazawa M, et al:
Antihypertensive and renal-protective effects of losartan in
streptozotocin diabetic rats. J Hypertens. 13:97–103. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uehara Y, Hirawa N, Kawabata Y, et al:
Angiotensin II subtype-1 receptor antagonists improve hemodynamic
and renal changes without affecting glucose metabolisms in genetic
rat model of non-insulin-dependent diabetes mellitus. Am J
Hypertens. 12:21–27. 1999. View Article : Google Scholar
|
|
10
|
Yao L, Kobori H, Rahman M, et al:
Olmesartan improves endothelin-induced hypertension and oxidative
stress in rats. Hypertens Res. 27:493–500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adams AJ: Bench to bedside and bedside to
bench: where are we? Optom Vis Sci. 83:125–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelly A, Li C, Gao Z, Stanley CA and
Matschinsky FM: Glutaminolysis and insulin secretion: from bedside
to bench and back. Diabetes. 51(Suppl 3): 421–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai GL, He JK, Xie Y, Han R, Qin ZH and
Zhu LJ: Therapeutic potential of Naja naja atra venom in a
rat model of diabetic nephropathy. Biomed Environ Sci. 25:630–638.
2012.
|
|
14
|
Yamabe N, Yokozawa T, Oya T and Kim M:
Therapeutic potential of (−)-epigallocatechin 3-O-gallate on renal
damage in diabetic nephropathy model rats. J Pharmacol Exp Ther.
319:228–236. 2006.
|
|
15
|
Ozturk Y, Altan VM and Yildizoglu-Ari N:
Effects of experimental diabetes and insulin on smooth muscle
functions. Pharmacol Rev. 48:69–112. 1996.PubMed/NCBI
|
|
16
|
Junod A, Lambert AE, Orci L, Pictet R,
Gonet AE and Renold AE: Studies of the diabetogenic action of
streptozotocin. Proc Soc Exp Biol Med. 126:201–205. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jones RB, Dickinson K, Anthony DM, Marita
AR, Kaul CL and Buckett WR: Evaluation of BTS 67 582, a novel
antidiabetic agent, in normal and diabetic rats. Br J Pharmacol.
120:1135–1143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Srinivasan K and Ramarao P: Animal models
in type 2 diabetes research: an overview. Indian J Med Res.
125:451–472. 2007.PubMed/NCBI
|
|
19
|
Kanwar YS, Wada J, Sun L, et al: Diabetic
nephropathy: mechanisms of renal disease progression. Exp Biol Med
(Maywood). 233:4–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakagawa T, Sato W, Glushakova O, et al:
Diabetic endothelial nitric oxide synthase knockout mice develop
advanced diabetic nephropathy. J Am Soc Nephrol. 18:539–550. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ota T, Takamura T, Ando H, Nohara E,
Yamashita H and Kobayashi K: Preventive effect of cerivastatin on
diabetic nephropathy through suppression of glomerular macrophage
recruitment in a rat model. Diabetologia. 46:843–851. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oktem F, Ozguner F, Yilmaz HR, Uz E and
Dundar B: Melatonin reduces urinary excretion of
N-acetyl-beta-D-glucosaminidase, albumin and renal oxidative
markers in diabetic rats. Clin Exp Pharmacol Physiol. 33:95–101.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis EJ, Hunsicker LG, Bain RP and Rohde
RD: The effect of angiotensin-converting enzyme inhibition on
diabetic nephropathy. The Collaborative Study Group. N Engl J Med.
329:1456–1462. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noda M, Matsuo T, Fukuda R, et al: Effect
of candesartan cilexetil (TCV-116) in rats with chronic renal
failure. Kidney Int. 56:898–909. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ueno T, Kaname S, Takaichi K, et al:
LOX-1, an oxidized low-density lipoprotein receptor, was
upregulated in the kidneys of chronic renal failure rats. Hypertens
Res. 26:117–122. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou A, Yu L, Li J, Zhang J and Wang H:
Renal protective effects of blocking the intrarenal
renin-angiotensin system: angiotensin II type I receptor antagonist
compared with angiotensin-converting enzyme inhibitor. Hypertens
Res. 23:391–397. 2000. View Article : Google Scholar
|
|
27
|
Tabaei BP, Al-Kassab AS, Ilag LL, Zawacki
CM and Herman WH: Does microalbuminuria predict diabetic
nephropathy? Diabetes Care. 24:1560–1566. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Inada Y, Murakami M, Tazawa S and Akahane
M: KRH-594, a new angiotensin AT1 receptor antagonist, ameliorates
nephropathy and hyperlipidaemia in diabetic spontaneously
hypertensive rats. Clin Exp Pharmacol Physiol. 27:270–276. 2000.
View Article : Google Scholar
|
|
29
|
Baynes JW and Thorpe SR: Role of oxidative
stress in diabetic complications: a new perspective on an old
paradigm. Diabetes. 48:1–9. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujimoto S, Satoh M, Horike H, et al:
Olmesartan ameliorates progressive glomerular injury in subtotal
nephrectomized rats through suppression of superoxide production.
Hypertens Res. 31:305–313. 2008. View Article : Google Scholar
|