Introduction
Acute coronary syndrome (ACS) typically occurs when
coronary artery disease (CAD) results in the obstruction of
coronary arteries. This may lead to myocardial infarction (MI),
heart failure (HF), arrhythmias, cardiac arrest and death (1,2). The
current clinical practice includes rapid patient evaluation and
risk stratification based on clinical and electrocardiographic
characteristics, as well as the assessment of biochemical markers
(3). Cardiovascular diseases, such
as MI, hypertension, hypertrophy or HF, are accompanied by changes
in the composition of the cardiac extracellular matrix (ECM). These
dynamic alterations may determine the mechanical properties of the
damaged heart and directly modulate the inflammatory and reparative
response (4).
Tenascin-C (TNC) is a multifunctional hexameric
glycoprotein that is a major component of the ECM. It is
synthesized by interstitial fibroblasts and its levels are
increased in inflammatory diseases. Upon tissue damage, TNC has a
multitude of functions that mediate inflammatory and fibrotic
processes, in order to enable effective tissue repair. Over the
last decade, accumulating evidence indicated a vital role for TNC
in cardiac and arterial injury, tumor angiogenesis and metastasis,
as well as in the modulation of stem cell behavior (5). Previous in vitro studies
demonstrated that a range of factors implicated in cardiovascular
disease appear to be able to stimulate TNC synthesis by fibroblasts
(6,7).
OX40, also referred to as CD134 or TNFRSF4, is a
member of the tumor necrosis factor (TNF) co-stimulatory receptor
family and is expressed on activated T cells. OX40 is specifically
upregulated by the human T-lymphotropic virus type I (HTLV-1) viral
transactivator, Tax. The ligand of OX40 (OX40L), which belongs to
the TNF superfamily, was first identified as glycoprotein 34 on
HTLV-1-transformed cells and was found to bind OX40. OX40-OX40L
interactions may affect the activity and differentiation of several
types of immune cells (8). It was
recently demonstrated that genetic variants in the OX40L locus are
associated with MI and the severity of CAD in humans. OX40-OX40L
pairs were shown to be involved in ACS (9).
Willett et al(10) demonstrated that the enforced
trimerization of feline CD134L (OX40L) via the introduction of a
subdomain of the TNC oligomerization domain, was able to restore
full ligand-binding activity. The resulting fusion protein was
proven to be as effective as the anti-CD134 monoclonal antibody in
detecting CD134 expression and displayed a strain-specific blocking
of viral entry. Therefore, we surmised that the interaction of TNC
and OX40L is important in atherosclerosis. In the present study, we
investigated the expression of serum TNC and OX40L and assessed the
correlation between their expression and the number of complex
lesions in the patients. In addition, the statistical correlation
between TNC and OX40L or fibrinogen was evaluated.
Patients and methods
Subjects
The patients and healthy controls were involved in
the present study over a period of 18 months. Patients undergoing
clinically indicated diagnostic coronary angiography in the
coronary care unit of The Fourth People’s Hospital of Wuxi, were
consecutively registered. The patients were classified into the ACS
and the stable angina (SA) groups. The ACS group consisted of
patients with unstable angina (UA) and acute myocardial infarction
(AMI). The patients with UA experienced ischemic chest pain at rest
within the preceding 48 h, without enzymatic evidence of myocardial
necrosis; the AMI patients were defined as those with an occurrence
of MI within the preceding 4 weeks. The diagnosis of MI was based
on typical chest pain and ST segment elevation in at least two
contiguous electrocardiographic leads. The patients with SA (n=50)
underwent coronary angiography due to signs and symptoms of
clinical SA. For comparison, 50 gender- and age-matched donors
served as the control group. Patients with concurrent infection,
tumor, liver or kidney diseases were excluded (Table I). All the patients provided written
informed consent prior to their inclusion in the study. This study
was approved by the ethics committee of the Affiliated Hospital of
Jiangnan University.
 | Table IStatistical comparison of demographic
and clinical characteristics of patients with CAD vs. those of
healthy controls. |
Table I
Statistical comparison of demographic
and clinical characteristics of patients with CAD vs. those of
healthy controls.
| Groups | |
|---|
|
| |
|---|
| Control (n=50) | SA (n=50) | UA (n=70) | AMI (n=50) | P-value |
|---|
| Characteristics |
| Age (years) | 53±11.2 | 61±9.3 | 58±8.4 | 65±10.2 | <0.01 |
| Gender |
| Male | 26 | 29 | 42 | 31 | |
| Female | 24 | 21 | 28 | 19 | NS |
| Total cholesterol
(mmol/l) | 4.86±0.71 | 4.91±0.86 | 5.11±0.63 | 5.22±1.12 | <0.01 |
| HDL (cholesterol
mmol/l) | 2.05±0.72 | 1.34±0.52 | 1.14±0.65 | 0.92±0.46 | <0.01 |
| Triglycerides
(mmol/l) | 1.62±0.36 | 1.52±0.42 | 1.76±0.87 | 1.82±0.92 | NS |
| Apolipoprotein B
(g/l) | 0.80±0.31 | 0.92±0.11 | 0.69±0.35 | 0.79±0.26 | NS |
| Lipoprotein (a)
(g/l) | 0.58±0.10 | 0.33±0.18 | 0.41±0.22 | 0.51±0.27 | <0.01 |
| Direct bilirubin
(μmol/l) | 2.85±1.01 | 2.60±0.57 | 2.85±0.86 | 2.59±0.75 | NS |
| Indirect bilirubin
(μmol/l) | 13.24±2.73 | 11.75±3.09 | 12.59±2.47 | 13.79±1.23 | NS |
| Medication (%) |
| Calcium
antagonist | 0 | 9 | 11 | 13 | <0.001 |
| Nitroglycerin | 3 | 12 | 20 | 21 | <0.001 |
| ACEI | 8 | 10 | 17 | 15 | <0.001 |
| β-blockers | 15 | 72 | 78 | 85 | <0.001 |
| HMG-CoA reductase
inhibitors | 2 | 61 | 64 | 77 | <0.001 |
| Aspirin | 1 | 85 | 91 | 98 | <0.001 |
Blood sampling protocol
Peripheral venous blood samples were drawn into
blood collection tubes using a suction catheter (BD Vacutainer,
Franklin Lakes, NJ, USA). The blood to be used for the assessment
of serum TNC levels was collected in tubes containing coagulant and
sent to the clinical laboratory. The blood to be used for the
assessment of plasma OX40L levels was collected in tubes containing
Na2-EDTA at a final concentration of 0.1% and
immediately centrifuged at 1,000 × g for 10 min at 4°C. The
supernatant was stored at −80°C until analysis. The samples were
thawed only once.
The serum TNC levels were measured by a quantitative
automated particle-enhanced immunonephelometric assay (Boehringer
Mannheim, Mannheim, Germany). The plasma levels of OX40L were
determined using a rat anti-human-OX40L ELISA kit (Assay Designs,
Inc., Ann Arbor, MI, USA) according to the manufacturer’s
instructions. Additional laboratory measurements were performed
using standard methods. Fibrinogen was measured using the clotting
method (11).
Coronary angiography
Coronary angiography was performed using a femoral
approach with 6-Fr diagnostic catheters (Cordis Corp., FL, USA).
Images were recorded in multiple projections for the right and left
coronary arteries. All the coronary stenoses with a ≥30% diameter
reduction were assessed by two experienced cardiologists who were
blinded to the levels of TNC and OX40L, as well as to the identity
and clinical characteristics of the patients. Complex lesions were
defined by the following characteristics: i) irregular morphology,
or scalloped borders, or both; ii) overhanging or abrupt edges
perpendicular to the vessel wall; iii) ulceration; and/or iv) the
presence of filling defects consistent with intracoronary thrombus.
The Gensini’s scoring system (12)
was used to quantitatively assess the degree of stenosis for each
coronary vascular lesion.
Statistical analysis
All the numerical data are expressed as means ±
standard deviation. Statistical evaluation was performed with
GraphPad software (Prism 5.0) and SPSS software, version 11.7
(SPSS, Inc., Chicago, IL, USA). The correlation was evaluated using
a regression analysis. The Spearman’s two-way test was used to
assess the correlation between two quantitative variables with
non-normal distribution. The Pearson’s two-way test was used to
assess the correlation between two quantitative variables with
normal distributions. P<0.05 was considered to indicate a
statistically significant difference.
Results
Subject characteristics
The demographic and clinical characteristics of the
study participants are presented in Table I. The study included 170 patients
with angiographically documented CAD. A total of 120 patients
constituted the ACS group (70 patients with UA, without evidence of
myocardial necrosis, and 50 with recent MI) and 50 patients
constituted the SA group. The healthy control group comprised 50
subjects. The patients with CAD were significantly older compared
to the controls (P<0.01). The levels of total cholesterol,
high-density lipoprotein (HDL), cholesterol and lipoprotein (a)
were significantly different between the CAD and the control groups
(P<0.001). The use of medications (calcium antagonists,
nitroglycerine, angiotensin-converting enzyme inhibitors,
β-blockers, 3-hydroxy-3-methylglutaryl-coenzyme A reductase
inhibitors or aspirin) was significantly higher in the ACS and SA
groups compared to that in the control group (P<0.001 for all
the medications).
Serum TNC and plasma OX40L levels
As shown in Table
II, the TNC levels in the serum samples obtained from patients
with ACS were significantly higher (39.39±19.80 ng/ml) compared to
those obtained from patients in the control and SA groups
(28.65±12.32 ng/ml, P<0.01 and 31.22±18.92 ng/ml, P<0.05,
respectively). The OX40L levels were also significantly higher in
the plasma samples obtained from patients with ACS (38.59±15.76
ng/ml) compared to those obtained from patients in the the control
and SA groups (19.42±11.19 ng/ml and 21.52±10.30 ng/ml,
respectively; P<0.001 for both differences).
 | Table IIStatistical comparison between
patients with ACS or SA and healthy controls regarding serum TNC,
OX40L and fibrinogen concentrations. |
Table II
Statistical comparison between
patients with ACS or SA and healthy controls regarding serum TNC,
OX40L and fibrinogen concentrations.
| Groups | |
|---|
|
| |
|---|
| Variables | Control (n=50) | SA (n=50) | ACS (UA and AMI)
(n=120) | P-value |
|---|
| TNC (ng/ml) | 28.65±12.32 | 31.22±18.92 | 39.39±19.80 | <0.05 |
| OX40L (ng/ml) | 19.42±11.19 | 21.52±10.30 | 38.59±15.76 | <0.001 |
| Fibrinogen (g/l) | 16.32±7.87 | 19.00±11.17 | 28.24±16.14 | <0.001 |
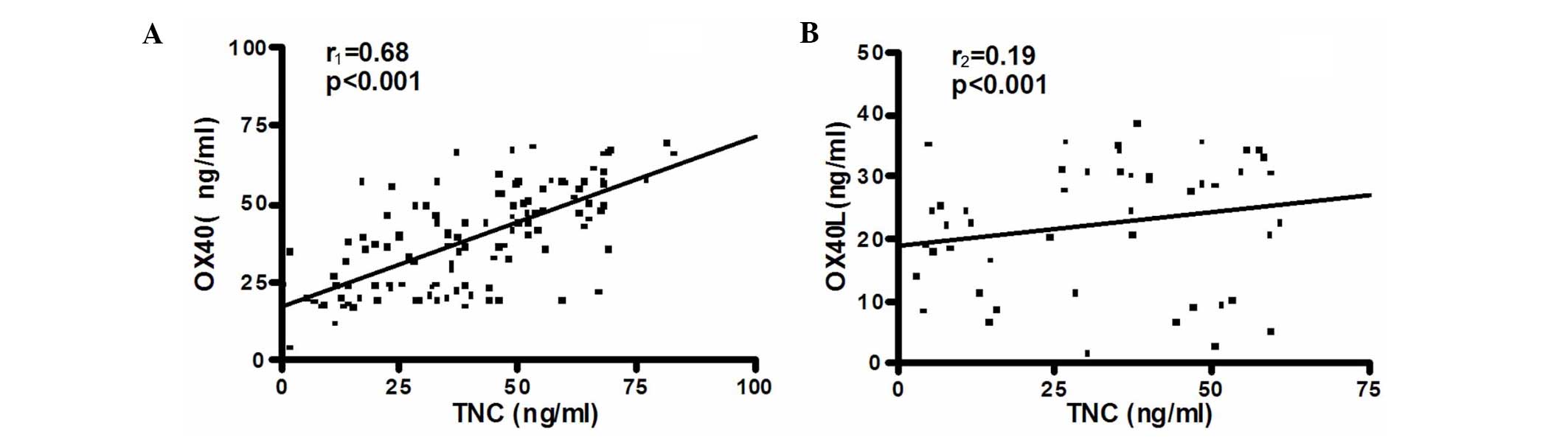
Correlation between TNC and OX40L
expression
The seum levels of TNC in patients with ACS
exhibited a positive correlation with the plasma levels of OX40L
(r1=0.68; P<0.001; Fig.
1A). However, in the SA group, such a correlation was not
observed (r2=0.19; P<0.001; Fig. 1B).
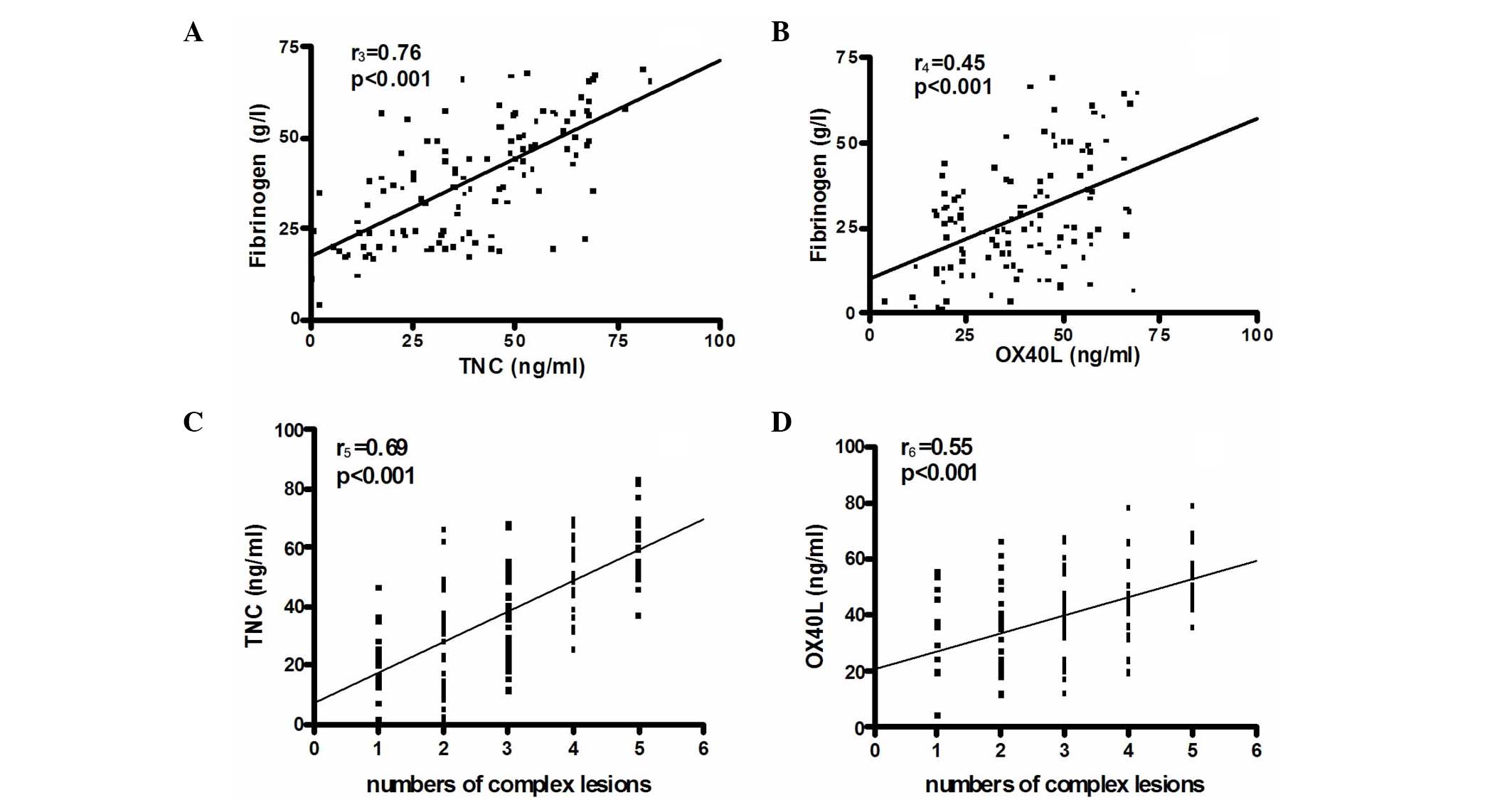
Correlation of TNC and OX40L with
fibrinogen and coronary artery stenosis
In patients with ACS, the levels of TNC and OX40L
were significantly correlated with the levels of fibrinogen
(r3=0.76 and r4=0.45, respectively,
P<0.001) (Fig. 2A and B). There
was a significant correlation between the number of complex lesions
and the TNC (r5=0.69; P<0.001; Fig. 2C) and OX40L levels
(r6=0.55; P<0.001; Fig.
2D).
Discussion
To the best of our knowledge, this study was the
first to assess the correlation between serum TNC and plasma OX40L
levels and angiographically demonstrated complex stenosis in
patients with ACS. The results of this study demonstrated that the
TNC and OX40L levels were increased as clinical severity increased
from SA to ACS. We also observed that there was a positive
correlation between TNC and OX40L expression and the number of
complex lesions in patients with ACS.
Atherosclerosis is a multifactorial disease, in
which inflammatory processes play an important role. Inflammation
underlies lesion evolution at all stages, from plaque establishment
to rupture and thrombosis (13–15).
ACSs (UA, AMI and ischemic sudden death) may result from the
disruption of atherosclerotic plaques, leading to coronary
thrombosis. The potential cellular mechanisms involved in plaque
disruption are also complex (16).
We hypothesized that the levels of TNC and OX40L in patients with
ACS reflect the activity of endothelial cells in coronary plaques,
which may result in higher levels of adhesion molecules,
proinflammatory cytokines and monocyte chemotactic proteins in the
circulation during plaque rupture. Circulating plasma OX40L may
pass through the damaged atherosclerotic endothelium and come into
direct contact with the cells inside the lesion, increasing the
inflammation of the coronary plaque.
TNC is involved in the physiological as well as the
pathological remodelling of blood vessels. TNC is expressed in the
heart in the early embryo, where it contributes to the development
of the myocardium, valves and coronary vessels (6). In cardiovascular pathology, TNC is
involved in post-MI remodelling, neointimal hyperplasia and
restenosis following percutaneous transluminal coronary angioplasty
and bypass grafting, as well as pulmonary vascular disease and
hypertension (17). Under normal
conditions, only low levels of TNC are found in adult tissue.
However, higher levels of TNC expression were previously reported
in areas of wound healing, cancer development and cardiovascular
disease, where the level of expression appears to be a reliable
biomarker of disease progression and poor patient prognosis
(18). Emerging evidence (6) has highlighted a number of novel roles
for TNC in cardiovascular disease.
Although a previous study indicated that the
OX40/OX40L pathway is crucial in atherosclerosis (19), the precise function of the OX40L
system in ACS has not been fully elucidated. The present study
demonstrated that the OX40L expression levels were increased in
patients with ACS. The regression analysis indicated that the serum
levels of TNC, which degrades the connective tissue matrix protein,
ultimately resulting in plaque rupture and the development of ACS,
were significantly associated with the levels of OX40L. These
findings suggested that TNC and OX40L expression in patients with
ACS may affect the development of atherosclerosis and the
instability of atherosclerotic plaques. However, further studies
are required to verify these findings.
Compared to patients with SA, those with UA and AMI
exhibited a higher number of complex coronary lesions (20). In our study population, complex
coronary stenosis morphology was found in ~68% of the patients with
UA, 60% of the patients with MI and ~24% of the patients with
chronic SA. A positive correlation between TNC and OX40L expression
and the number of complex lesions was observed in patients with
ACS. However, in SA patients, such a correlation was not observed.
Therefore, our findings suggest that the increased expression of
serum TNC and plasma OX40L in patients with ACS may be a marker of
immune activation and may also be involved in the underlying
pathogenic processes. The interruption of the OX40-OX40L
interaction was shown to ameliorate several autoimmune-like
diseases (21,22), indicating that the downregulation of
the OX40-OX40L interaction and TNC and OX40L expression may
represent a novel therapeutic approach for patients with ACS.
However, the results of this study should be interpreted with
caution, due to the relatively limited patient sample. In addition,
coronary angiography has certain limitations compared to angioscopy
and intravascular ultrasound and further investigation is required
to clarify its role in ACS.
In conclusion, our results suggest that the
concentrations of serum TNC and plasma OX40L may be of value as
clinical markers of the severity of CAD and the downregulation of
TNC and OX40L expression may represent a novel therapeutic approach
for patients with ACS. However, the precise mechanisms underlying
the involvement of TNC and OX40L in ACS have not been fully
elucidated. Further investigation is required to determine whether
TNC and OX40L may be used as biomarkers in the clinical setting to
guide intervention strategies in patients with CAD, or serve as
markers under emergency conditions to diagnose ACS more
efficiently.
Acknowledgements
This study was supported by the Medical Science
Foundation of Wuxi, Jiangsu province (grant no. YGM1111).
References
|
1
|
Lin Y, Pan W, Ning S, Song X, Jin Z and Lv
S: Prevalence and management of hypertension in patients with acute
coronary syndrome vary with gender: Observations from the Chinese
registry of acute coronary events (CRACE). Mol Med Rep. 8:173–177.
2013.PubMed/NCBI
|
|
2
|
Goodacre S, Thokala P, Carroll C, et al:
Systematic review, meta-analysis and economic modelling of
diagnostic strategies for suspected acute coronary syndrome. Health
Technol Assess. 17:1–188. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hou HW, Li XG, Yan M, Hu ZQ and Song YE:
Increased leukocyte Rho-kinase activity in a population with acute
coronary syndrome. Mol Med Rep. 8:250–254. 2013.PubMed/NCBI
|
|
4
|
Dobaczewski M, Gonzalez-Quesada C and
Frangogiannis NG: The extracellular matrix as a modulator of the
inflammatory and reparative response following myocardial
infarction. J Mol Cell Cardiol. 48:504–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohno Y, Izumi M, Yoshioka K, Ohori M,
Yonou H and Tachibana M: Prognostic significance of tenascin-C
expression in clear cell renal cell carcinoma. Oncol Rep.
20:511–516. 2008.PubMed/NCBI
|
|
6
|
Golledge J, Clancy P, Maguire J, Lincz L
and Koblar S: The role of tenascin C in cardiovascular disease.
Cardiovasc Res. 92:19–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trescher K, Thometich B, Demyanets S, et
al: Type A dissection and chronic dilatation: tenascin-C as a key
factor in destabilization of the aortic wall. Interact Cardiovasc
Thorac Surg. 17:365–370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueki T, Murata S, Kitamura N, Mekata E and
Tani T: Pre-treatment with cyclophosphamide or OX40 (CD134)
costimulation targeting regulatory T cell function enhances the
anti-tumor immune effect of adoptively transferred CD8+T
cells from wild-type mice. Mol Med Rep. 2:615–620. 2009.PubMed/NCBI
|
|
9
|
Chen Y, Zhang L, Huang H, et al:
Association of OX40 and OX40L gene polymorphisms with acute
coronary syndrome in a Han Chinese population. DNA Cell Biol.
30:597–602. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Willett BJ, McMonagle EL, Logan N,
Schneider P and Hosie MJ: Enforced covalent trimerisation of
soluble feline CD134 (OX40)-ligand generates a functional
antagonist of feline immunodeficiency virus. Mol Immunol.
46:1020–1030. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Committee for Clinical Laboratory
Standards (NCCLS). Procedure for the Determination of Fibrinogen in
Plasma; Approved Guideline. NCCLS document H30-A. 2nd edition.
Wayne, PA: pp. 991999
|
|
12
|
Gensini GG: A more meaningful scoring
system for determining the severity of coronary heart disease. Am J
Cardiol. 51:6061983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Profumo E, Buttari B, Saso L, Capoano R,
Salvati B and Riganò R: T lymphocyte autoreactivity in inflammatory
mechanisms regulating atherosclerosis. ScientificWorldJournal.
2012:1575342012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nicholls SJ, Andrews J, Puri R, Uno K and
Kataoka Y: Imaging progression of coronary atherosclerosis. Circ J.
77:3–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reriani MK, Flammer AJ, Jama A, Lerman LO
and Lerman A: Novel functional risk factors for the prediction of
cardiovascular events in vulnerable patients following acute
coronary syndrome. Circ J. 76:778–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christenson E and Christenson RH: The role
of cardiac biomarkers in the diagnosis and management of patients
presenting with suspected acute coronary syndrome. Ann Lab Med.
33:309–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niebroj-Dobosz I: Tenascin-C in human
cardiac pathology. Clin Chim Acta. 413:1516–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohtsuka M, Yamamoto H, Oshiro R, et al:
Concurrent expression of C4.4A and Tenascin-C in tumor cells
relates to poor prognosis of esophageal squamous cell carcinoma.
Int J Oncol. 43:439–446. 2013.PubMed/NCBI
|
|
19
|
Nakano M, Fukumoto Y, Satoh K, et al: OX40
ligand plays an important role in the development of
atherosclerosis through vasa vasorum neovascularization. Cardiovasc
Res. 88:539–546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koch KC, Schaefer WM, Ersahin K, et al:
Haemodynamic significance of stent lesions compared to native
coronary lesions: a myocardial perfusion imaging study. Heart.
90:691–692. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaur D and Brightling C: OX40/OX40 ligand
interactions in T-cell regulation and asthma. Chest. 141:494–499.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Croft M, So T, Duan W and Soroosh P: The
significance of OX40 and OX40L to T-cell biology and immune
disease. Immunol Rev. 229:173–191. 2009. View Article : Google Scholar : PubMed/NCBI
|