Introduction
Cervical cancer was the third most commonly
diagnosed cancer and the fourth leading cause of cancer death in
females worldwide in 2008 (1). Risk
factors for cervical cancer include early onset of sexual activity,
multiple sexual partners, cigarette smoking, high parity, low
socioeconomic status, immuno suppression, high-risk sexual partners
(2) and particularly, human
papillomavirus (HPV) infection (3).
HPV infections are widespread in the general population. However,
only a small proportion of infected women progress to cervical
cancer (4), suggesting that the
development of cervical cancer may be influenced by genetic
factors.
FAS (also known as Apo-1 or CD95), a cell-surface
receptor, plays a key role in apoptotic signaling in many cell
types (5). FAS ligand (FASL) (also
known as CD95L), a member of the tumor necrosis factor super
family, can trigger an apoptotic cascade by cross-linking with its
receptor, FAS (6,7). Single nucleotide polymorphisms in the
promoter regions of FAS and FASL have been associated with the
differential expression of these two genes. It was reported that a
G→A transition at position −1377 and an A→G transition at position
−670 in the promoter region of the FAS gene destroys stimulatory
protein (Sp) 1 and signal transducer and activator of transcription
(STAT) 1 protein-binding element, reducing promoter activity and
decreasing FAS expression (8,9). In
regards to the FASL gene, it has been shown that a T→C transition
at position −844 in a binding motif for CAAT/enhancer-binding
protein β induces a significantly higher basal expression of FASL
(10).
Although evidence of an association between FAS/FASL
polymorphisms and cervical cancer risk has been reported, the
findings remain controversial (11–22).
Therefore, we performed a meta-analysis of all published studies on
the association between the FAS/FASL polymorphisms and cervical
cancer.
Materials and methods
Publication search
A search of the literature was performed using
PubMed, Excerpta Medica Database and the Chinese Biomedical
Literature Database to identify articles that evaluated the
associations between polymorphisms in FAS/FASL and cervical cancer
risk (last search was updated on November 1st, 2012). The search
terms used were: ‘FAS or CD95 or FASL or CD95L’, ‘cervical
carcinoma or cervical cancer or cervix cancer’ and ‘polymorphism or
polymorphisms’. In addition, we checked all the references of
relevant reviews and eligible articles that our search retrieved.
No language restrictions were applied. If more than one article was
published by the same author using the same case series, we
selected the research with the largest sample size.
Inclusion and exclusion criteria
All the studies included in the meta-analysis were
required to meet the following criteria: i) case-control studies;
ii) evaluation of the association between FAS/FASL polymorphisms
and cervical cancer; iii) sufficient data for the estimation of an
odds ratio (OR) with 95% confidence interval (CI). The exclusion
criteria were: i) duplicate data; ii) no controls; iii) no
sufficient data were reported and; iv) abstract, comment, review
and editorial.
Data extraction
Two investigators independently extracted the data
and reached consensus on all items. For each eligible study, the
following were extracted: the first authors’ name, the year of
publication, ethnicity (including Caucasian, Asian, patients of
African descent and Mixed) of the study population, sources of
controls (population- or hospital-based), sample size of cases and
controls, genotyping method, distributions of every genotype and
evidence of Hardy-Weinberg equilibrium (HWE).
Statistical analysis
ORs with 95% CIs were calculated to assess the
strength of the association between FAS/FASL polymorphisms and
cervical cancer risk. To estimate associations with cervical cancer
risk, various genotypic models were explored, including the
following models: i) additive (minor allele vs. major allele); ii)
codominant (heterozygous vs. common homozygous carriers and rare
homozygous vs. common homozygous carriers); iii) dominant (rare
allele carriers vs. common homozygous carriers); iv) recessive
(rare homozygous carriers vs. common allele carriers). Subgroup
analyses were performed by ethnicity and source of controls.
Heterogeneity among studies was assessed by the
χ2-based Q-statistic (23). Heterogeneity was considered
significant for P<0.10. When significant heterogeneity was
detected, the random-effects model (DerSimonian and Laird method)
(23) was used, otherwise the fixed
effects model (Mantel-Haenszel method) (24) was selected. The goodness-of-fit
χ2 test was used to assess the deviation from HWE in
controls, with statistical significance defined as P<0.05
(25).
Publication bias was evaluated using Begg’s funnel
plot (26) and Egger’s linear
regression test (27). Analyses
were performed using the software Stata version 12.0 (Stata
Corporation, College Station, TX, USA). P<0.05 was considered to
indicate a statistically significant difference and all the
P-values were two sided.
Results
Eligible studies
Based on a literature search and selection, a total
of 10 publications (11–19,22)
comparing the FAS/FASL polymorphism and cervical cancer
susceptibility were identified. Among these studies, 1 study was in
Chinese (22) and 1 study contained
data on two different ethnicities (18). As a result, 11 case-control studies
were included in this meta-analysis. Ten studies on FAS-670A/G
polymorphism, five on FAS-1377G/A and six studies on FASL-844T/C
polymorphisms met the inclusion criteria (Table I). The genotype distribution in the
controls of the studies was consistent with HWE, with the exception
of 2 studies for FAS-670 A/G polymorphism (17,18)
and 1 study for FASL-844 T/C polymorphism (11) (Table
I).
 | Table ICharacteristics of studies considered
in the meta-analysis. |
Table I
Characteristics of studies considered
in the meta-analysis.
| First author | Year | Ethnicity | Source of
controls | Polymorphism | Genotype method | Sample size | Cases/controls | HWE | Refs. |
|---|
|
|
|---|
| Cases | Controls | AA | GA | GG |
|---|
| Sun | 2005 | Asian | Population-based | FAS-670 A/G | PCR-RFLP | 314 | 615 | 138/268 | 144/272 | 32/75 | 0.641 | (11) |
| Lai | 2005 | Asian | Hospital-based | FAS-670 A/G | TaqMan | 318 | 318 | 121/91 | 137/161 | 60/66 | 0.736 | (12) |
| Zoodsma | 2005 | Caucasian | Population-based | FAS-670 A/G | TaqMan | 670 | 607 | 143/155 | 363/290 | 164/162 | 0.274 | (13) |
| Chen | 2006 | Asian | Population-based | FAS-670 A/G | MAMA-PCR | 194 | 138 | 68/32 | 94/76 | 32/30 | 0.232 | (22) |
| Ueda | 2006 | Asian | Population-based | FAS-670 A/G | RCR-RFLP | 83 | 95 | 15/23 | 38/54 | 30/18 | 0.172 | (14) |
| Kang | 2007 | Asian | Hospital-based | FAS-670 A/G | RCR-RFLP | 154 | 160 | 48/53 | 73/84 | 33/23 | 0.264 | (15) |
| Zucchi | 2008 | Mixed | Hospital-based | FAS-670 A/G | RCR-RFLP | 91 | 176 | 18/43 | 52/92 | 21/41 | 0.545 | (16) |
| Tamandani | 2008 | Asian | Hospital-based | FAS-670 A/G | RCR-RFLP | 200 | 200 | 26/55 | 167/121 | 7/24 | 0.001 | (17) |
| Chatterjee | 2009 | Black | Hospital-based | FAS-670 A/G | RCR-RFLP | 103 | 99 | 1/2 | 35/43 | 67/54 | 0.047 | (18) |
| Chatterjee | 2009 | Mixed | Hospital-based | FAS-670 A/G | RCR-RFLP | 332 | 321 | 53/39 | 132/146 | 147/136 | 0.985 | (18) |
| | | | | | | | GG | AG | AA | | |
| Sun | 2005 | Asian |
Population-based | FAS-1377G/A | RCR-RFLP | 314 | 615 | 144/282 | 144/277 | 26/56 | 0.304 | (11) |
| Lai | 2005 | Asian | Hospital-based | FAS-1377G/A | TaqMan | 318 | 318 | 127/99 | 138/165 | 53/54 | 0.293 | (12) |
| Kang | 2007 | Asian | Hospital-based | FAS-1377G/A | RCR-RFLP | 154 | 158 | 54/56 | 69/82 | 31/20 | 0.233 | (15) |
| Chatterjee | 2009 | black | Hospital-based | FAS-1377G/A | RCR-RFLP | 96 | 90 | 74/75 | 21/14 | 1/1 | 0.707 | (18) |
| Chatterjee | 2009 | Mixed | Hospital-based | FAS-1377G/A | RCR-RFLP | 290 | 295 | 186/196 | 87/86 | 1713 | 0.37 | (18) |
| | | | | | | | TT | TC | CC | | |
| Sun | 2005 | Asian |
Population-based | FASL -844T/C | RCR-RFLP | 314 | 615 | 10/40 | 111/291 | 193/284 | 0.002 | (11) |
| Lai | 2005 | Asian | Hospital-based | FASL -844T/C | TaqMan | 303 | 316 | 21/27 | 119/132 | 163/157 | 0.92 | (12) |
| Ivansson | 2007 | Caucasian |
Population-based | FASL -844T/C | PCR-RFLP | 1,284 | 280 | 119/24 | 531/112 | 634/144 | 0.738 | (19) |
| Kang | 2007 | Asian | Hospital-based | FASL -844T/C | RCR-RFLP | 154 | 160 | 7/7 | 66/63 | 81/90 | 0.327 | (15) |
| Chatterjee | 2009 | Black | Hospital-based | FASL -844T/C | RCR-RFLP | 103 | 100 | 70/74 | 31/23 | 2/3 | 0.469 | (18) |
| Chatterjee | 2009 | Mixed | Hospital-based | FASL -844T/C | RCR-RFLP | 327 | 315 | 143/139 | 144/136 | 40/40 | 0.457 | (18) |
Analysis for FAS-670 A/G
polymorphism
A total of 2,127 cases and 2,408 controls were
identified for the analysis on FAS-670 A/G polymorphism and
cervical cancer risk. The overall result suggested no statistically
significant association of this polymorphism with cervical cancer
susceptibility (for G vs. A: OR=1.016; 95% CI, 0.898–1.149; P=0.028
for heterogeneity; for GG vs. AA: OR=0.944; 95% CI, 0.729–1.222;
P=0.058 for heterogeneity; for GA vs. AA: OR=1.034; 95% CI,
0.768–1.393; P=0.000 for heterogeneity; for GG+GA vs. AA: OR=1.043;
95% CI, 0.797–1.366; P=0.000 for heterogeneity; for GG vs. GA/AA:
OR=0.999; 95% CI, 0.784–1.274; P=0.004 for heterogeneity; Table II). In the subgroup analyses by
ethnicity and source of controls, no significant associations were
found for the genetic models (Table
II). Following the exclusion of studies deviating from HWE in
controls (17,18), the non-associations in the
above-mentioned genetic models remained.
 | Table IIMeta-analysis of FAS/FASL
polymorphisms and cervical cancer risk. |
Table II
Meta-analysis of FAS/FASL
polymorphisms and cervical cancer risk.
| Variables | Na | G vs. A | GG vs. AA | GA vs. AA | GG/GA vs. AA | GG vs. GA/AA |
|---|
|
|
|
|
|
|---|
| OR (95% CI) | P-valueb | OR (95% CI) | P-valueb | OR (95% CI) | P-valueb | OR (95% CI) | P-valueb | OR (95% CI) | P-valueb |
|---|
| FAS-670 A/G | | | | | | | | | | | |
| Overall | 10 | 1.016
(0.898–1.149)c | 0.028 | 0.944
(0.729–1.222) | 0.058 | 1.034
(0.768–1.393) | 0.000 | 1.043
(0.797–1.366) | 0.000 | 0.999
(0.784–1.274) | 0.004 |
| Studies with
HWE | 8 | 0.984
(0.860–1.125) | 0.029 | 0.960
(0.727–1.267) | 0.034 | 0.911
(0.711–1.167) | 0.010 | 0.932
(0.738–1.176) | 0.012 | 1.019
(0.828–1.254) | 0.078 |
| Ethnicity | | | | | | | | | | | |
| Asian | 6 | 0.996
(0.815–1.217) | 0.009 | 0.909
(0.595–1.386) | 0.020 | 1.011
(0.660–1.547) | 0.000 | 1.032
(0.695–1.531) | 0.000 | 0.931
(0.590–1.469) | 0.001 |
| Mixed | 2 | 0.996
(0.822–1.206) | 0.547 | 0.897
(0.601–1.340) | 0.347 | 0.915
(0.459–1.824) | 0.084 | 0.892
(0.624–1.275) | 0.131 | 1.061
(0.806–1.396) | 0.794 |
| Source of
controls | | | | | | | | | | | |
| PB | 4 | 0.998
(0.795–1.254) | 0.018 | 0.981
(0.610–1.577) | 0.022 | 1.005
(0.718–1.408) | 0.041 | 0.997
(0.712–1.397) | 0.027 | 0.991
(0.671–1.464) | 0.030 |
| HB | 6 | 1.002
(0.892–1.124) | 0.127 | 0.880
(0.680–1.138) | 0.268 | 1.097
(0.646–1.864) | 0.000 | 1.112
(0.699–1.770) | 0.001 | 1.001
(0.703–1.424) | 0.013 |
| FAS-1377 G/A | | | | | | | | | | | |
| | A vs. G | | AA vs. GG | | AG vs. GG | | AA/AG vs. GG | | AA vs. AG/GG | |
| Overall | 5 | 0.993
(0.880–1.122) | 0.254 | 1.000
(0.757–1.322) | 0.402 | 0.926
(0.781–1.097) | 0.153 | 0.950
(0.808–1.117) | 0.167 | 1.105
(0.853–1.432) | 0.492 |
| Ethnicity | | | | | | | | | | | |
| Asian | 3 | 0.952
(0.830–1.092) | 0.195 | 0.949
(0.701–1.284) | 0.201 | 0.854
(0.699–1.044) | 0.149 | 0.879
(0.726–1.063) | 0.165 | 1.076
(0.814–1.420) | 0.215 |
| Source of
controls | | | | | | | | | | | |
| HB | 4 | 1.000
(0.860–1.162) | 0.150 | 1.043
(0.747–1.458) | 0.279 | 0.878
(0.710–1.085) | 0.111 | 0.925
(0.756–1.131) | 0.100 | 1.201
(0.882–1.634) | 0.480 |
| FASL -844 T/C | | | | | | | | | | | |
| | C vs. T | | CC vs. TT | | TC vs. TT | | CC/TC vs. TT | | CC vs. TC/TT | |
| Overall | 6 | 1.116
(0.914–1.364) | 0.006 | 1.168
(0.903–1.510) | 0.165 | 1.111
(0.896–1.378) | 0.858 | 1.131
(0.922–1.385) | 0.445 | 1.114
(0.829–1.498) | 0.008 |
| Studies with
HWE | 5 | 1.007
(0.895–1.134) | 0.656 | 0.994
(0.749–1.319) | 0.863 | 1.074
(0.856–1.347) | 0.892 | 1.055
(0.851–1.308) | 0.850 | 0.982
(0.829–1.162) | 0.722 |
| Ethnicity | | | | | | | | | | | |
| Asian | 3 | 1.221
(0.877–1.699) | 0.012 | 1.215
(0.714–2.068) | 0.171 | 1.266
(0.823–1.949) | 0.801 | 1.477
(0.979–2.229) | 0.387 | 1.269
(0.825–1.952) | 0.005 |
| Source of
controls | | | | | | | | | | | |
| PB | 2 | 1.235
(0.713–2.139) | 0.000 | 1.503
(0.501–4.504) | 0.010 | 1.113
(0.751–1.649) | 0.294 | 1.332
(0.590–3.010) | 0.052 | 1.306
(0.656–2.599) | 0.000 |
| HB | 4 | 1.048
(0.904–1.214) | 0.648 | 1.061
(0.744–1.513) | 0.815 | 1.110
(0.859–1.436) | 0.842 | 1.099
(0.861–1.403) | 0.824 | 1.029
(0.823–1.287) | 0.644 |
Analysis for FAS-1377 G/A
polymorphism
A total of 1,172 cases and 1,476 controls were
identified for the analysis on FAS-1377 G/A polymorphism and
cervical cancer risk. The overall result showed that there was no
statistically significant association between this polymorphism and
cervical cancer susceptibility (Table
II). Subsequent subgroup analyses revealed that there was no
statistically significant association in each subgroup by ethnicity
and source of controls in the genetic models (Table II).
Analysis for FASL-844 T/C
polymorphism
A total of 2,485 cases and 1,786 controls were
identified for the analysis on FASL-844 T/C polymorphism and
cervical cancer risk. The overall result showed that there was no
statistically significant association between this polymorphism and
cervical cancer susceptibility (Table
II). Following exclusion of the study deviating from HWE in
controls (11), the results did not
alter substantially in any of the genetic models. Subsequent
subgroup analyses revealed that there was no statistically
significant association in each subgroup by ethnicity and source of
controls in the genetic models (Table
II).
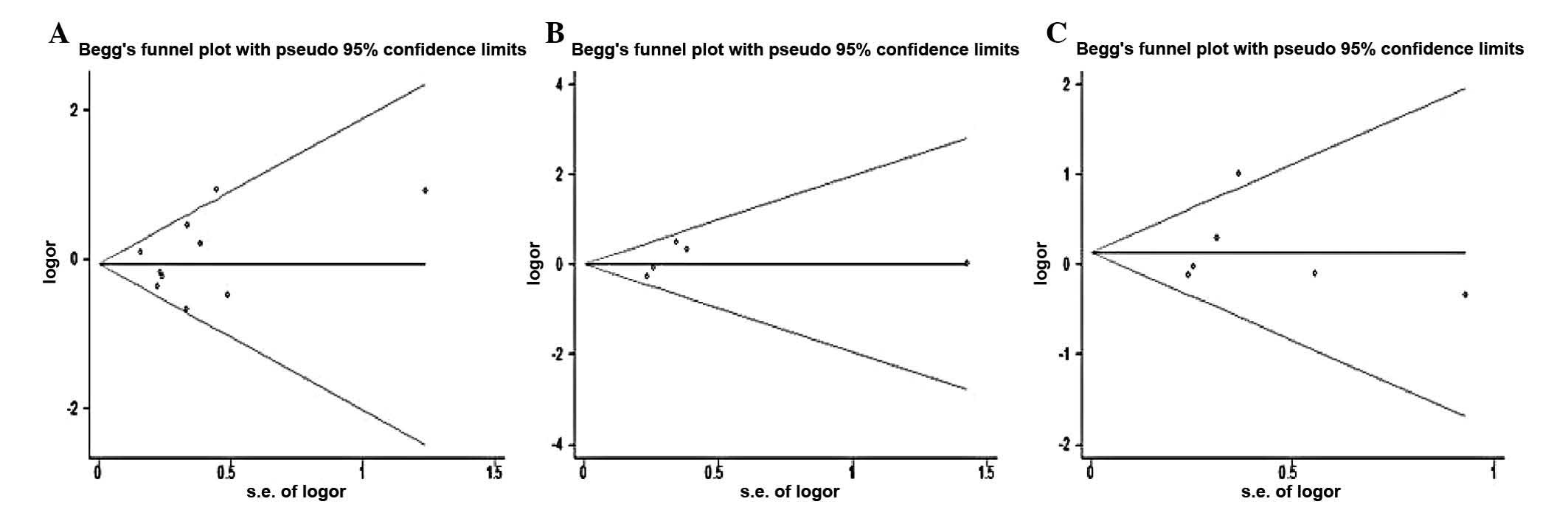
Publication bias
Both Begg’s funnel plot and Egger test were
performed to assess the publication bias of the studies. The shape
of the funnel plot did not reveal any evidence of obvious asymmetry
(Fig. 1). The Egger test was used
to provide statistical evidence of funnel plot symmetry. The
results again did not suggest any obvious evidence of publication
bias for the genetic models (all P>0.05).
Discussion
Genetic variants of the FAS/FASL gene in the
etiology of several types of cancer have drawn increasing
attention. A number of studies revealed that polymorphic variants
of the FAS/FASL polymorphisms were associated with etiology of
cervical cancer. However, the results are inconclusive. To gain a
better understanding of the association between these polymorphisms
and cervical cancer risk, a pooled analysis with a large sample of
patients, as well as a subgroup analysis were performed. The aim of
our study was to investigate the relationship between FAS/FASL
polymorphisms and cervical cancer susceptibility.
In this meta-analysis, we failed to find any
association between FAS-670 A/G, FAS-1377 G/A and FASL-844 T/C
polymorphisms and cervical cancer risk in the overall analysis. In
the subgroup analysis, no association was identified between the
three polymorphisms and cervical cancer. These findings indicate
that the three polymorphisms may not be risk factors for the
development of cervical cancer.
Apoptosis is an important regulatory mechanisms
occurring in multicellular organisms that is involved in normal
development and tissue homeostasis (28). FAS plays a crucial role in apoptotic
signaling and interacts with its natural ligand, FASLG, to initiate
the death signal cascade, which results in cell death. Findings of
Ueda et al (14) on
germ-line polymorphism of Fas promoter -670 demonstrated that the
frequency of GG genotype or G allele increased the risk of cervical
cancer. Lai et al (12)
detected an association between the Fas-670 polymorphism and
cervical cancer susceptibility. Zoosdma et al (13) have demonstrated that Fas-670
polymorphism might be involved in the development of adenocarcinoma
of the cervix but not in the development of squamous cell
carcinoma. Engelmark et al (21), Sun et al (11) and Chatterjee et al (18) did not show a significant association
of the FAS/FASL polymorphisms with cervical cancer. Qiu et
al (29) indicated that
significantly increased risks in FAS-1377 AA carriers were found in
the breast cancer subgroup but not in the lung cancer subgroup. Liu
et al (30) indicated that
the FASL-844C allele was associated with a significantly increased
cancer risk overall, but in the stratified analysis by cancer
types, significant associations were still not observed in the
genetic models. In contrast to these studies, our data failed to
reveal significant associations between FAS/FASL polymorphisms and
cervical cancer. The cause of these varied results remains unclear,
however, discrepancies may exist due to different mechanisms of
carcinogenesis in different types of cancer. Cervical cancer is a
multi-factorial disease and individual exposures to various
environmental factors in combination with genetic susceptibility
may have contributed to discrepancies.
However, this study has several limitations. First,
significant between-study heterogeneity was detected in some of the
comparisons, distorting the findings of the meta-analysis. Second,
subgroup analyses concerning other risk factors such as smoking
status and HPV infection have not been included in the present
study due to insufficient data from the primary literature. Third,
further studies estimating the effect of gene-environment
interactions may eventually provide an improved, comprehensive
understanding of the association between the FAS/FASL polymorphisms
and cervical cancer risk. Fourth, relatively small samples of
subpopulation may render the interpretation of negative results
difficult, requiring more studies with a larger sample size to
elucidate those effects. Fifth, the meta-analysis remains a
retrospective study that is subject to the methodological
deficiencies of the included studies.
In conclusion, our meta-analysis suggests that
FAS-670 A/G, FAS-1377 G/A and FASL-844 T/C polymorphisms were not
associated with cervical cancer risk. However, large sample studies
including different ethnic groups with a careful matching between
cases and controls are necessary to confirm our findings.
Acknowledgements
This study was granted by Anhui
Provincial Science and Technology Agency Foundation of China (nos.
11070403061 and 09020303042).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Wallin KL, Wiklund F, Luostarinen T, et
al: A population-based prospective study of Chlamydia
trachomatis infection and cervical carcinoma. Int J Cancer.
101:371–374. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiffman M, Castle PE, Jeronimo J,
Rodriguez AC and Wacholder S: Human papillomavirus and cervical
cancer. Lancet. 370:890–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
zur Hausen H: Papillomaviruses and cancer:
from basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002.PubMed/NCBI
|
|
5
|
Andera L: Signaling activated by the death
receptors of the TNFR family. Biomed Pap Med Fac Univ Palacky
Olomouc Czech Repub. 153:173–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim R, Emi M, Tanabe K, Uchida Y and Toge
T: The role of Fas ligand and transforming growth factor beta in
tumor progression: molecular mechanisms of immune privilege via
Fas-mediated apoptosis and potential targets for cancer therapy.
Cancer. 100:2281–2291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suda T, Takahashi T, Golstein P and Nagata
S: Molecular cloning and expression of the Fas ligand, a novel
member of the tumor necrosis factor family. Cell. 75:1169–1178.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang QR, Morris D and Manolios N:
Identification and characterization of polymorphisms in the
promoter region of the human Apo-1/Fas (CD95) gene. Mol Immunol.
34:577–582. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sibley K, Rollinson S, Allan JM, et al:
Functional FAS promoter polymorphisms are associated with increased
risk of acute myeloid leukemia. Cancer Res. 63:4327–4330.
2003.PubMed/NCBI
|
|
10
|
Wu J, Metz C, Xu X, et al: A novel
polymorphic CAAT/enhancer- binding protein beta element in the FasL
gene promoter alters Fas ligand expression: a candidate background
gene in African American systemic lupus erythematosus patients. J
Immunol. 170:132–138. 2003. View Article : Google Scholar
|
|
11
|
Sun T, Zhou Y, Li H, et al: FASL -844C
polymorphism is associated with increased activation-induced T cell
death and risk of cervical cancer. J Exp Med. 202:967–974. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai HC, Lin WY, Lin YW, et al: Genetic
polymorphisms of FAS and FASL (CD95/CD95L) genes in cervical
carcinogenesis: an analysis of haplotype and gene-gene interaction.
Gynecol Oncol. 99:113–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zoodsma M, Nolte IM, Schipper M, et al:
Interleukin-10 and Fas polymorphisms and susceptibility for
(pre)neoplastic cervical disease. Int J Gynecol Cancer. 15(Suppl
3): 282–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ueda M, Terai Y, Kanda K, et al: Fas gene
promoter -670 polymorphism in gynecological cancer. Int J Gynecol
Cancer. 16(Suppl 1): 179–182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang S, Dong SM, Seo SS, Kim JW and Park
SY: FAS-1377 G/A polymorphism and the risk of lymph node metastasis
in cervical cancer. Cancer Genet Cytogenet. 180:1–5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zucchi F, da Silva ID, Ribalta JC, et al:
Fas/CD95 promoter polymorphism gene and its relationship with
cervical carcinoma. Eur J Gynaecol Oncol. 30:142–144.
2009.PubMed/NCBI
|
|
17
|
Kordi Tamandani DM, Sobti RC and Shekari
M: Association of Fas-670 gene polymorphism with risk of cervical
cancer in North Indian population. Clin Exp Obstet Gynecol.
35:183–186. 2008.
|
|
18
|
Chatterjee K, Engelmark M, Gyllensten U,
et al: Fas and FasL gene polymorphisms are not associated with
cervical cancer but differ among Black and Mixed-ancestry South
Africans. BMC Research Notes. 2:2382009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ivansson EL, Gustavsson IM, Magnusson JJ,
et al: Variants of chemokine receptor 2 and interleukin 4 receptor,
but not inter-leukin 10 or Fas ligand, increase risk of cervical
cancer. Int J Cancer. 121:2451–2457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ueda M, Hung YC, Terai Y, et al: Fas gene
promoter -670 polymorphism (A/G) is associated with cervical
carcinogenesis. Gynecol Oncol. 98:129–133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Engelmark MT, Renkema KY and Gyllensten
UB: No evidence of the involvement of the Fas-670 promoter
polymorphism in cervical cancer in situ. Int J Cancer.
112:1084–1085. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YQ, Lu LG, Tian QF, et al: The
research of Fas-670 polymorphism and cervical cancer
susceptibility. Natl Med J China. 86:2792–2794. 2006.
|
|
23
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
25
|
Haber M: Exact significance levels of
goodness-of-fit tests for the Hardy-Weinberg equilibrium. Hum
Hered. 31:161–166. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reed JC: Mechanisms of apoptosis. Am J
Pathol. 157:1415–1430. 2000. View Article : Google Scholar
|
|
29
|
Qiu LX, Shi J, Yuan H, et al: FAS -1,377
G/A polymorphism is associated with cancer susceptibility: evidence
from 10,564 cases and 12,075 controls. Hum Gen. 125:431–435. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Wen Q-J, Yin Y, et al: FASLG
polymorphism is associated with cancer risk. Eur J Cancer.
45:2574–2578. 2009. View Article : Google Scholar : PubMed/NCBI
|