Introduction
Pleomorphic adenoma (PA) is the most common benign
neoplasm of the salivary gland. It occurs most often between the
ages of 30 and 60 years and is detected more commonly in females
than in males. Although the tumor is usually benign, recurrence may
occur when it is inadequately excised. Moreover, 2–17% of the
tumors can progress to malignancy, resulting in carcinoma ex-PA, an
aggressive malignancy that may metastasize and result in death. The
biological behavior of the neoplasm is thought to be associated
primarily with genetic alterations in the tumor cells themselves.
Differential gene expression was validated for the upregulated
gene, Gli2, using real-time PCR and the results were consistent
with those of the cDNA microarray analysis thus verifying the
credibility of the microarray data (1). Gli2 is a nuclear transcription factor
of the Hedgehog (Hh) signaling pathway, a key regulator in
embryogenesis where it affects processes such as cell
proliferation, differentiation and tissue patterning. In adults it
was involved in the maintenance of stem cells and in tissue repair
and regeneration. However, this pathway was also an important in
various types of human cancer where it promoted growth and enabled
the proliferation of tumor stem cells. Results of a recent clinical
study (2) have shown that Hh
signaling is the basis of an important new class of therapeutic
agents with far-reaching implications in oncology. In the present
study, the role of cyclopamine as a Hh signaling pathway inhibitor
in PA cells and in the detection of PA cell apoptosis was
investigated. Additionally, cyclopamine was utilized to detect
expression of apoptotic gene Bcl2. To gain a better understanding
of the human salivary PA of the molecular pathogenesis, we also
investigated the mechanism of the Hh signaling pathway in human
PA-induced apoptosis. Results demonstrated that our data may
provide clues for the identification of new targets for the
diagnosis and therapy of PA.
Materials and methods
Materials
DMEM-F12 joint medium supplemented with 10% fetal
bovine serum (Invitrogen Life Technologies, Carlsbad, CA, USA), 20
ng/ml epidermal growth factor (ProSpec, Rehovot, Israel), 5
μg/ml insulin (Sigma, St. Louis, MO, USA), 0.4 μg/ml
hydrocortisone (ProSpec), penicillin (500 U/ml) and streptomycin
(500 U/ml), and 0.25% trypsin (Sigma) were utilized. Cyclopamine
and dimethyl sulfoxide (DMSO) were purchased from Sigma. Reverse
transcriptase-polymerase chain reaction (RT-PCR) and SYBR-Green
kits were purchased from Takara Bio, Inc. (Tokyo, Japan).
Cyclopamine (1 mg at a purity of ≥98%) was dissolved in 200
μl of DMSO placed in a 60°C water bath box for ∼1 min.
PA cells were cultured in human salivary pleomorphic
adenoma (HSPA). The complete medium was incubated at 37°C 5%
CO2 every 3 to 4 days and passaged at a dilution of 1:2.
Logarithmic phase cells were used in the experiment.
Cells with a density of 5×105/ml were
grown in a petri dish with a diameter of 10 cm. After 24 h in
serum-free medium, cells were synchronized. The medium in the petri
dish was replaced with complete medium (8 ml). Cells were treated
with 10 μmol/l cyclopamine (3) or 0.08% DMSO for 48 h. The blank
control group was not treated with the drug. Group cell numbers and
morphological changes were observed under an inverted
microscope.
RNA extraction
Cells (2×105) were cultivated in 6-well
plates. Cells were synchronized initially in 3 ml complete medium
and 24 h in serum-free medium. The medium was replaced with
complete medium and cyclopamine was added at a final concentration
of 10 μmol/l (3). The DMSO
group was treated and DMSO was adjusted to a final concentration of
0.08%. Total RNA was extracted after 48 h using TRIzol reagent.
Electrophoresis was used to identify the typical RNA bands. Since
the three bands identified were uniform and concentrated, there was
no apparent degradation for RNA. RNA samples from the HSPA cells
were extracted using TRIzol reagent (Invitrogen, Carlsbad, CA,
USA). Total RNA was then reverse-transcribed into cDNA according to
the instructions of the reverse transcription kit (Takara).
GAPDH, Bcl2 and Gli2 primers were synthesized by
Shanghai Boshang (Shanghai, China). Real-time PCR was performed
using the ABI Prism®7300 real-time PCR system according
to the manufacturer’s instructions. Primer sequences were: GAPDH,
upstream: 5′-AAGGTGAAGGTCGGAGT CAAC-3′ and downstream:
5′-GGGGTCATTGATGGCAAC AATA-3′, with a product length of 102 bp;
Bcl2, upstream: 5′-AAGATTGATGGGATCGTTGC-3′ and downstream:
5′-GCGGAACACTTGATTCTGGT-3′, with a product length of 229 bp; Gli2,
upstream: 5′-CATGGAGCACTACCTCC GTTC-3′ and downstream:
5′-CGACGGTCATCTGGTGG TAAT-3′, with a product length of 172 bp. The
total reaction volume was 20 μl (1 μl cDNA, 10
μl SYBR Premix Ex Taq, 1 μl upstream primer, 1
μl downstream primer and 7 μl ddH2O). The
reaction conditions were: activation at 95°C for 30 sec, 40 cycles
of denaturation at 95°C for 5 sec, primer annealing and extension
at 60°C for 31 sec and further extension at 95°C. Melting curve
analysis of the samples was routinely performed to ascertain that
only the expected products had been generated. mRNA expression
levels of target genes were normalized to the expression of GAPDH
and calculated using the 2-ΔΔCt method.
Logarithmic measurements
Logarithmic phase HSPA cells were inoculated at a
density of 2×105 cells/well in 6-well plates and
cultivated overnight. The cells were synchronized after 24 h in
serum-free medium. The culture medium was removed and the
experimental group was treated with 10 μmol/l cyclopamine.
The DMSO-treated group was treated with 0.08% DMSO. The blank
control group was not treated with the drug. After 24 h, the single
cell suspension was digested with 0.25% trypsin. Annexin-V-FITC
(annexin V) and pyridine iodide double staining were carried out
according to the manufacturer’s instructions. Cell apoptosis was
detected by flow cytometry and the experimental results were
performed in triplicate.
Statistical analysis
Data were presented as the mean ± standard deviation
and analyzed using SAS 9.13 software. P-values were calculated,
with P<0.01 being statistically significant. Groups were
compared using one-way analysis of variance at an inspection level
of α=0.01.
Results
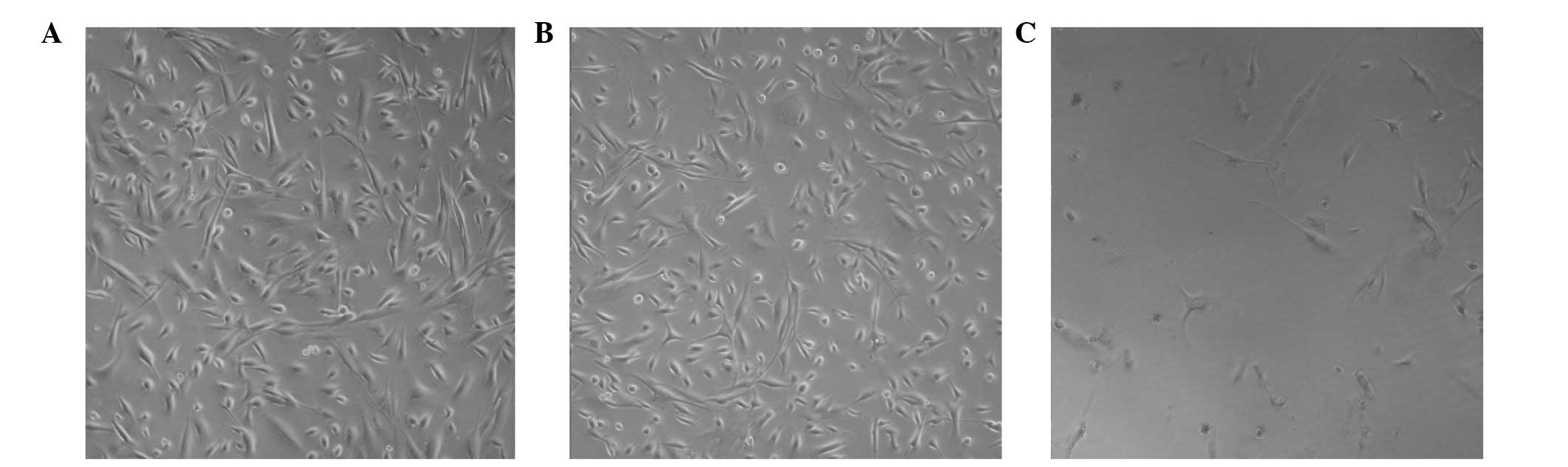
Morphological changes of HSPA cells
Unlike the blank control and DMSO-treated groups,
the addition of 10 μmol/l of cyclopamine to the HSPA cells
in three-dimensional sense of loss, resulted in a decrease in cell
size. The cells formed a circle, while the cell gap increased, as
observed under an inverted microscope. The morphology of HSPA cells
altered significantly following the addition of cyclopamine
(Fig. 1).
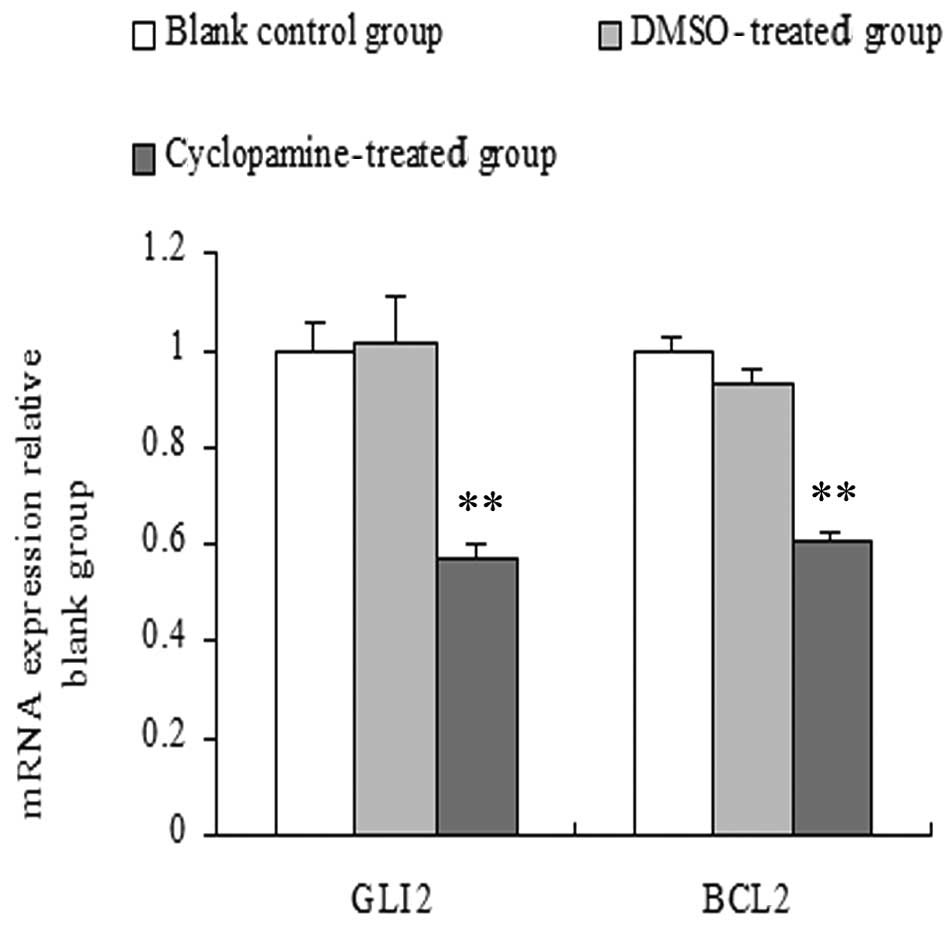
Effects of cyclopamine on mRNA expression
of Gli2 and Bcl2
The amount of mRNA of the target genes relative to
GAPDH was calculated using the formula 2-ΔΔCt, while the
change in fold expression was calculated relative to the blank
control group. The results showed that compared with the blank
control and DMSO-treated groups, Gli2 and Bcl2 mRNA expression
levels of the cyclopamine-treated group were significantly lower.
No significant differences were observed in the DMSO-treated and
blank control groups (P>0.05) (Fig.
2).
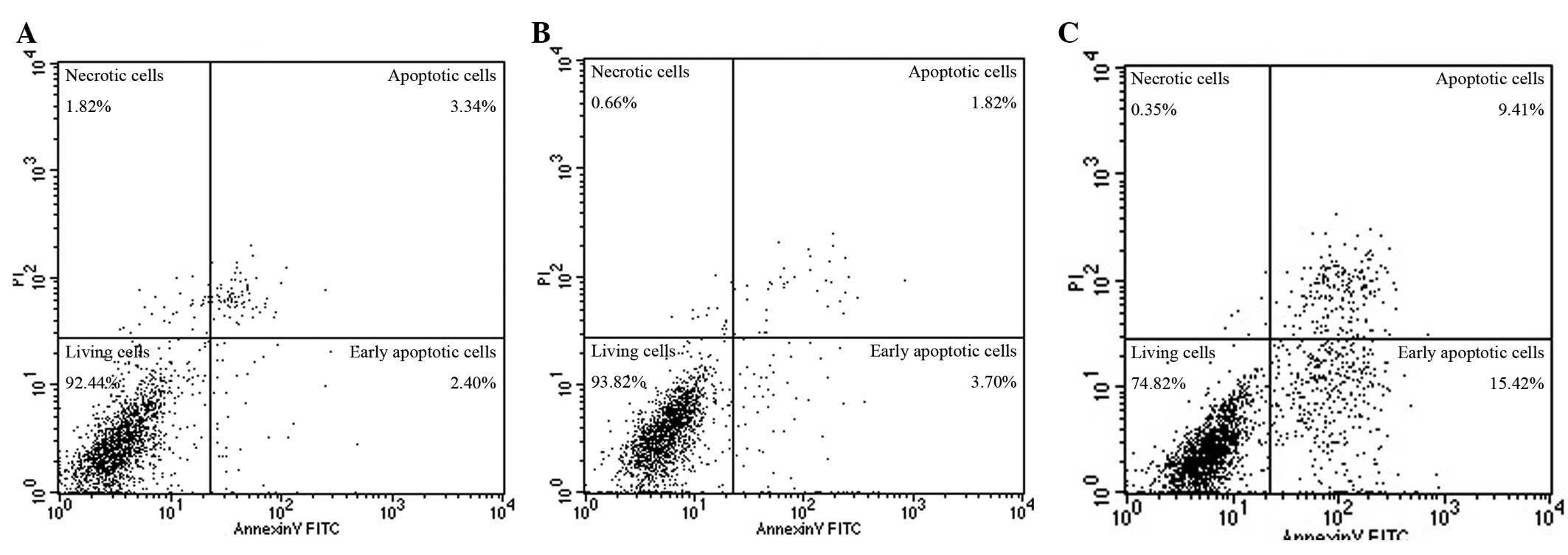
Effect of cyclopamine on HSPA cells
The apoptotic rate of the blank control group
(apoptosis rate = early apoptosis + late apoptosis rate) was
(7.10±1.23)%, the DMSO-treated group was (8.18±1.98)%, and the 10
μmol/l cyclopamine-treated group was (20.45±3.27)%. The
apoptotic rate of the cyclopamine group was significantly higher
compared with that of the blank control and DMSO-treated groups
(F=40.94, P<0.01). No significant differences were detected in
the DMSO-treated and blank control groups (P>0.05) (Fig. 3).
Discussion
Parotid PA is a borderline tumor that is treated
mainly by surgical resection. The tumor capsule is unevenly thick
and incomplete, thus tumor cells usually infiltrate both within and
outside the capsule, resulting in poor adhesion between the tumor
and its capsule. Surgical resection also has numerous defective
impacts including facial scarring as well as complications such as
Frey’s syndrome, earlobe numbness, recurrence and malignant
sequelae. Consequently, studies have focused on basic investigation
into PA. The Hh signaling pathway is considered a key regulator in
embryogenesis where it regulates processes such as cell
proliferation, differentiation and tissue patterning (4,5). In
adults it is involved in the maintenance of stem cells and in
tissue repair and regeneration. It is also involved in several
types of human cancer, promoting the growth and proliferation of
tumor stem cells. Effector molecules involved in tumor cell
proliferation, such as BMP and cyclin, have been proven to be
target genes of the Hh pathway or downstream molecules (6). Crosstalk exists between the Hh
signaling pathway and other signaling pathways, including the Notch
and Wnt, Ras-of Erk pathways (6–8).
Cyclopamine is the most potent specific inhibitor of
the Hh signaling pathway. It is able to downregulate the activity
of this pathway, thereby inhibiting the excessive activation of
tumor cell proliferation.
Hh signaling pathway comprises three Hh ligands
[Sonic Hh (Shh), Indian Hh (Ihh) and Desert Hh (Dhh)], two
transmembrane protein receptors [Patched (Ptch) and Smoothened
(Smo)], three nuclear transcription factors (Gli1, -2 and -3) and
downstream target genes (Ptch, Gli, Wnt, EGF and cyclins). Gli2,
which has a length of 3,678 bp and is localized at 2q14, is a zinc
finger transcription factor of the Hh signaling transduction
pathway. Recent studies have confirmed (1) that the Gli2 gene mRNA expression in PA
was significantly higher compared with the normal tissue adjacent
to the tumor, suggesting that Gli2 is capable of inducing the
proliferation of PA cells. Prostate cancer cells using
Gli2-specific small hairpin RNA knock down Gli2 in these cells,
resulting in the downregulation of the Hh signaling pathway and
inhibition of the growth of tumor tissue xenografts in vivo
(9). Conversely, the ectopic
expression of Gli2 in non-tumorigenic prostate epithelial cells
resulted in accelerated cell cycle progression, particularly
transition through G2-M and increased proliferation (9).
Apoptotic signaling is activated via the death
receptor and mitochondrial pathways. The death receptor pathway is
triggered through ligand activation of the abnormal cell surface
death receptors, including CD95/Fas, tumor necrosis factor receptor
and death receptor. Ligand binding with the receptor, combined with
the Fas-associated death domain protein generates a death-inducing
signaling complex. Additionally, activating caspase cascade is
likely to cause irreversible damage in cells. The mitochondrial
pathway is mainly triggered by oxidative stress, DNA damage, cancer
chemotherapeutic drugs and ionizing radiation, which can increase
the permeability of the mitochondrial membrane, and promote the
release of cytochrome C. Cytochrome C can be combined with
apoptotic protease activation factor-1 and activate caspase 9
precursor. Additionally, the formation of apoptotic bodies and
activation of caspase downstream effectors is able to induce DNA
fragmentation and cell death (10–12).
Bcl2 family proteins, divided into anti-apoptotic
(Bcl2, Bcl-G) and pro-apoptotic (Bax, Bad, Bid) proteins, regulate
the activity of the mitochondrial pathway. Bid and Bax proteins
promote the mitochondrial release of Cyt-C and induce apoptosis.
However, Bcl2 inhibited mitochondrial-releasing cytochrome C and
apoptosis. In this study, the Hh pathway inhibitor cyclopamine
decreased activity of the Hh pathway and downregulated Gli2 and
Bcl2 mRNA expression levels. We hypothesized that the
cyclopamine-inducing apoptosis mechanism in HSPA cells may be
associated with the process of the decreased expression of Bcl2
following the downregulation of Gli2. As the decreased expression
of Bcl2 activates the mitochondrial pathway of apoptosis, it is
likely to increase the release of mitochondrial cytochrome C and
activate the downstream caspase cascade, leading to irreversible
cell damage. Previous studies have shown that Bcl2 is a Gli target
gene of the Hh signaling pathway (13–15).
Gli is able to regulate the expression of Bcl2 (16). In a study on basal cell carcinoma,
Bigelow et al (15) observed
that the Bcl2 promoter has seven potential Gli anchor points. Gli
proteins regulate Bcl2 activity through transcriptional regulation
of the Bcl2 promoter. Bar et al (14) found that in medulloblastoma samples,
Gli1 was clearly correlated with Bcl2 mRNA levels. Improving the
transfection of Gli1 mRNA expression yielded a corresponding
increase in Bcl2 mRNA. Thus, when cyclopamine blocks Hh signaling
pathway activity to decrease Gli1 mRNA expression, a corresponding
decrease in Bcl2 mRNA occurs, thereby promoting apoptosis of tumor
cells. The Bcl2 gene has two promoters, E1P1+ and E2P2. The E1P1+
sequence contains three nucleic acid sequences (CGCCACCCA,
GACCACCAA and GCACACCCA) which are highly homologous with the Gli
family of protein-binding elements. Restructuring of the Gli1
protein can be combined with the Bcl2 promoter fragment containing
the GACCACCAA sequence, suggesting that Bcl2 is regulated by Gli1
target genes. Similar events also occur in the Gli2 gene.
Activation of Bcl2 by Gli2 is stronger compared with that of Gli1,
whose gene expression can be strengthened >10-fold (13). Hepatocellular carcinoma studies have
shown that cyclopamine inhibits Hh signaling which highly expresses
the Gli signaling factor. By downregulating the expression of Bcl2,
Hh signaling induces apoptosis of hepatoma cells (17). Studies on pancreatic cancer
(18), prostate cancer (19), colon cancer (20), gastric cancer (21), medulloblastoma (22) and basal cell carcinoma (15) obtained similar results, thus Hh
signaling occurs via the regulation of Bcl2 gene to manipulate
tumor cell apoptosis.
Findings of recent studies suggest that cyclopamine
is a new method for the treatment of tumors by inducing apoptosis
of malignant cells (23). The Hh
signaling pathway constitutes an important target. Findings of this
study have demonstrated that inhibition of transduction of the Hh
signaling pathway results in an enhanced HSPA cell apoptotic rate,
obvious morphological changes of apoptosis and decrease of the
anti-apoptotic factor Bcl2 mRNA expression. These findings are
crucial in the pathogenesis of PAs, as well as the promotion of
tumor cell apoptosis and drug targets. Therefore, cyclopamine is
potentially of great significance to the prevention, treatment and
prognosis of PA.
Acknowledgements
This study was supported by grant nos.
08JC1412900 and 10DZ1951300 from the Science and Technology
Commission of Shanghai Municipality and grant no. S30206 from the
Shanghai Leading Academic Discipline Project.
References
|
1
|
Song M, Xiao C, Wang T, et al: Study of
the differentially expressed genes in pleomorphic adenoma using
cDNA micro-arrays. Pathol Oncol Res. 17:765–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kelleher FC, Cain JE, Healy JM, et al:
Prevailing importance of the hedgehog signaling pathway and the
potenial for treatment advancement in sarcoma. Pharmacol Ther.
136:153–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mimeault M, Moore E, Moniaux N, et al:
Cytotoxic effects induced by a combination of cyclopamine and
gefitinib, the selective hedgehog and epidermal growth factor
receptor signaling inhibitors, in prostate cancer cells. Int J
Cancer. 118:1022–1031. 2006. View Article : Google Scholar
|
|
4
|
Pasca di Magliano M and Hebrok M: Hedgehog
signalling in cancer formation and maintenance. Nat Rev Cancer.
3:903–911. 2003.PubMed/NCBI
|
|
5
|
Marini KD, Payne BJ, Watkins DN and
Martelotto LG: Mechanisms of Hedgehog signaling in cancer. Growth
Factors. 29:221–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paiva KB, Silva-Valenzuela MD, Massironi
SM, et al: Differential Shh, Bmp and Wnt gene expressions during
craniofacial development in mice. Acta Histochem. 112:508–517.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi I, Hashemi Sadraei N, Duan ZH and Shi
T: Aberrant signaling pathways in squamous cell lung carcinoma.
Cancer Inform. 10:273–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Min TH, Kriebel M, Hou S and Pera EM: The
dual regulator Sufu integrates Hedgehog and Wnt signals in the
early Xenopus embryo. Dev Biol. 358:262–276. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiyagarajan S, Bhatia N, Reagan-Shaw S,
et al: Role of GLI2 transcription factor in growth and
tumorigenicity of prostate cells. Cancer Res. 67:10642–10646. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iannolo G, Conticello C, Memeo L and De
Maria R: Apoptosis in normal and cancer stem cells. Crit Rev Oncol
Hematol. 66:42–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiao L and Wong BC: Targeting apoptosis as
an approach for gastrointestinal cancer therapy. Drug Resist Updat.
12:55–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen XL, Cao LQ, She MR, et al: Gli-1
siRNA induced apoptosis in Huh7 cells. World J Gastroenterol.
14:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Regl G, Kasper M, Schnidar H, et al:
Activation of the BCL2 promoter in response to Hedgehog/GLI signal
transduction is predominantly mediated by GLI2. Cancer Res.
64:7724–7731. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bar EE, Chaudhry A, Farah MH and Eberhart
CG: Hedgehog signaling promotes medulloblastoma survival via Bc/II.
Am J Pathol. 170:347–355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bigelow RL, Chari NS, Unden AB, et al:
Transcriptional regulation of Bcl-2 mediated by the sonic hedgehog
signaling pathway through gli-1. J Biol Chem. 279:1197–1205. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katoh Y and Katoh M: Hedgehog target
genes: mechanisms of carcinogenesis induced by aberrant hedgehog
signaling activation. Curr Mol Med. 9:873–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen XL, Cheng QY, She MR, et al:
Expression of sonic hedgehog signaling components in hepatocellular
carcinoma and cyclopamine-induced apoptosis through Bcl-2
downregulation in vitro. Arch Med Res. 41:315–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mo W, Xu X, Xu L, et al: Resveratrol
inhibits proliferation and induces apoptosis through the hedgehog
signaling pathway in pancreatic cancer cell. Pancreatology.
11:601–609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Farooqi AA, Mukhtar S, Riaz AM, et al: Wnt
and SHH in prostate cancer: trouble mongers occupy the TRAIL
towards apoptosis. Cell Prolif. 44:508–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mazumdar T, DeVecchio J, Shi T, et al:
Hedgehog signaling drives cellular survival in human colon
carcinoma cells. Cancer Res. 71:1092–1102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han ME, Lee YS, Baek SY, et al: Hedgehog
signaling regulates the survival of gastric cancer cells by
regulating the expression of Bcl-2. Int J Mol Sci. 10:3033–3043.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCall TD, Pedone CA and Fults DW:
Apoptosis suppression by somatic cell transfer of Bcl-2 promotes
Sonic hedgehog-dependent medulloblastoma formation in mice. Cancer
Res. 67:5179–5185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurita S, Mott JL, Cazanava SC, et al:
Hedgehog inhibition promotes a switch from Type II to Type I cell
death receptor signaling in cancer cells. PLoS One. 6:e183302011.
View Article : Google Scholar : PubMed/NCBI
|