Introduction
Prostate cancer is the second most frequently
diagnosed type of cancer and the sixth leading cause of cancer
mortality among males worldwide (1). Statistics in the USA showed that
∼241,740 men were diagnosed with prostate cancer and 28,170 were
estimated to succumb to the disease in 2012 (2). Prostate cancer is considered a
multifactorial disease. Older age, ethnicity and positive family
history are well-established risk factors (1,3).
Genetic factors were considered important in prostate cancer by
several studies (4–7). Since the 1970s, it has been a matter
of debate whether prostate cancer, or at least a subgroup of
prostate cancer cases, are associated with infection. With the
development of serological assays for the detection of infectious
agents, studies were conducted to investigate the relationship
between prostate cancer and viral infection (8–11).
Herpes virus is one of the viruses most commonly
related to carcinogenesis. It was reported that herpes virus plays
an important role in the pathogenesis of cancer via the inhibition
of cell apoptosis and stimulation of DNA synthesis, which may
ultimately lead to cancer (12).
Previous studies indicated that infection by herpes simplex virus
type 2 (HSV-2) or human herpesvirus 8 (HHV-8) may be associated
with a higher prostate cancer risk (12–14).
However, other epidemiological studies failed to demonstrate such
an association (15–17). Whether HSV-2 or HHV-8 infection is
related to prostate cancer remains controversial. In order to
further assess the correlation between prostate cancer risk and
HSV-2 or HHV-8 infection, a meta-analysis was conducted.
Materials and methods
Literature search
We performed a systematic literature search using
PubMed, Cochrane Library, Web of Science and Scopus in English, as
well as CNKI and CBM in Chinese, to identify the publications
updated up to May, 2012. The medical subject headings (MeSH)
‘Herpesviridae’, ‘Alphaherpesvirinae’, ‘Simplexvirus’, ‘Herpesvirus
2, Human’, ‘Gammaherpesvirinae’, ‘Rhadinovirus’ and ‘Herpesvirus 8,
Human’ were combined with ‘Prostatic Neoplasms’. Common expressions
such as ‘herpes simplex virus 2’, ‘human herpes-virus 8’,
‘prostatic carcinoma’ and ‘prostate cancer’ were also used. In
addition, aliases such as ‘Kaposi’s sarcoma-associated
herpesvirus’, ‘KSHV’, ‘HHV-8’ and ‘HSV-2’ were all included.
Moreover, variant forms of expression were used (presence or
absence of a hyphen or space between adjacent words, Roman numerals
or English words instead of Arabic numerals and presence or absence
of quotation marks). Additional publications were identified from
the reference lists of potentially suitable articles and ‘related
articles’ identified during the search.
Inclusion criteria
Each included publication was reviewed to evaluate
whether the following criteria were met: i) comparative study
assessing the association between infection by HSV-2 or HHV-8 and
prostate cancer risk; ii) detection method restricted to
serological assays; and iii) providing sufficient information to
determine herpes virus infection incidence (number of positive vs.
negative subjects) in the case and control groups. The inclusion
criteria were not restricted by study size, population race or
publication language, date or type. For articles with similar
population resources or overlapping datasets, only the largest or
most recent was included. Studies in which herpes virus was not
detected in either group were excluded, since such studies hold no
weight in meta-analysis and do not affect conclusions.
Data extraction
Two reviewers independently extracted data from all
the potentially qualified articles using a standardized data
extraction form, in order to avoid mistakes or omissions. A third
reviewer was consulted in the case of discrepancies. Data extracted
from the publications included name of the first author, year of
publication, number of case and control subjects, exposure
frequencies in case and control groups, geographical region and
herpes virus subtype.
Statistical analysis
We selected the odds ratio (OR) and 95% confidence
interval (CI) to assess the strength of the association between
infection by HSV-2 or HHV-8 and prostate cancer risk. Subgroup
analyses were performed according to geographical region (North and
South America or Europe). The statistical significance level was
set at 0.05. The extent of heterogeneity among included studies was
quantified using the Q test (18)
and the I2 score (19).
In the Q test, P<0.05 was considered to indicate a statistically
significant difference. High I2 values reflected
increasing heterogeneity. I2 values <25% were
considered as low and values <50% were considered as moderate
(19,20). A fixed-effects model with the
Mantel-Haenszel method was used to provide a summary estimation of
the relationship between herpes virus infection and prostate cancer
risk when heterogeneity was not significant (21). Otherwise, the random-effects model
was utilized (22). Publication
bias was assessed by Egger’s (23)
and Begg’s test (24), with a
statistical significance level of 0.05. The statistical analyses
were performed via STATA version 11 (StataCorp, College Station,
TX, USA).
Results
Description of the meta-analysis
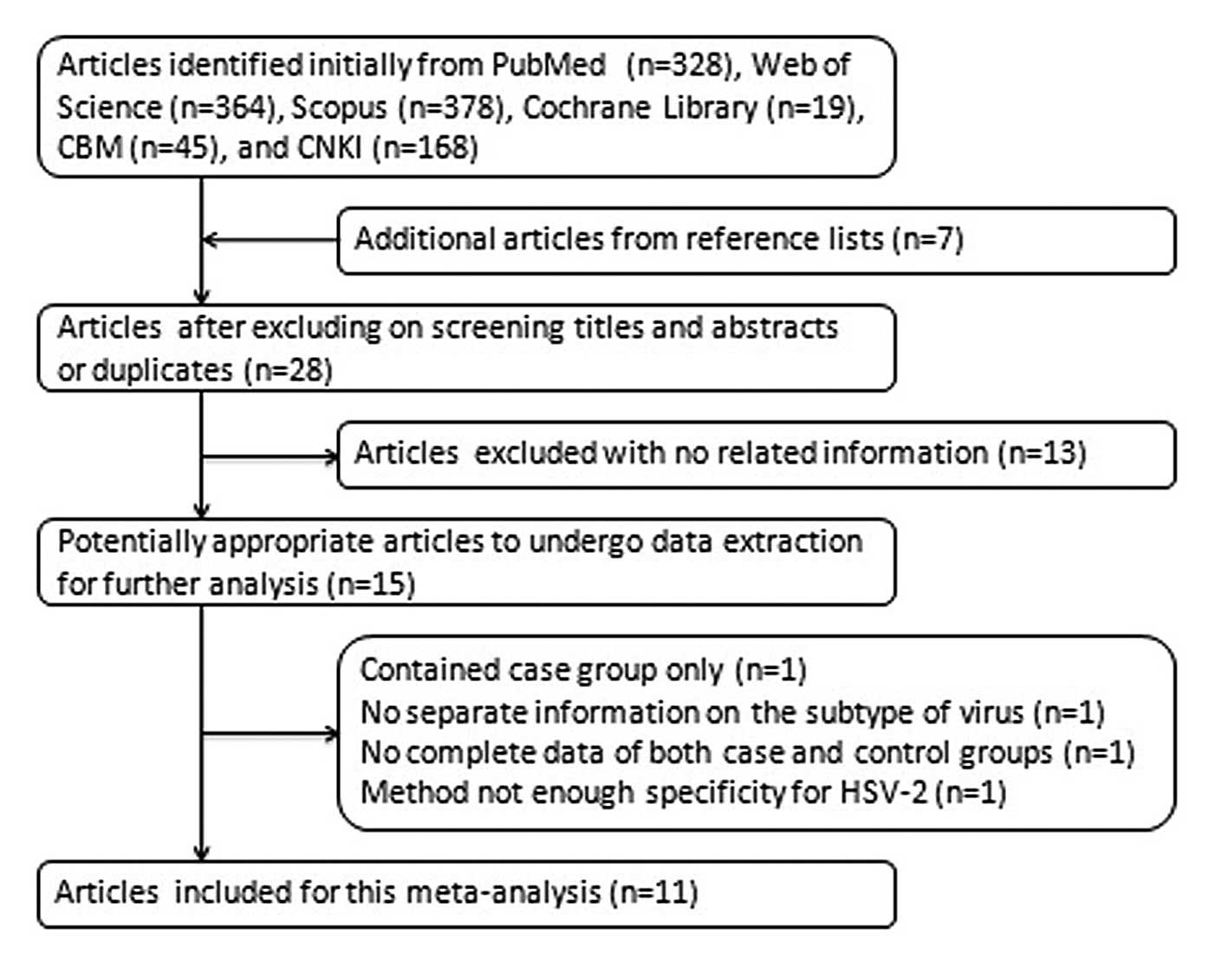
We identified a total of 11 articles on infection by
HSV-2 or HHV-8 and prostate cancer risk, updated up to May 2012
(8–11,14–17,25–27).
The process of identifying and selecting articles is shown in
Fig. 1. Four articles were excluded
at data extraction, since their results were not considered
suitable for this meta-analysis: one of them included a case group
only (28), one provided ambiguous
information regarding the virus subtype (29), one lacked a number of negative
subjects in the case group and of positive subjects in the control
group (30) and one adopted a
detection method of inadequate specificity for HSV-2 (31).
This meta-analysis consisted of 2,996 cases and
3,875 controls. There were two articles evaluating HSV-2 and HHV-8
in the same (15) or different
(11) population groups. One
article detecting HHV-8 consisted of two groups of participants
from either the Republic of Trinidad and Tobago or the USA
(9), whereas another consisted of
four groups from Italy or the USA (16). Studies on different viruses or among
separate population groups in the articles mentioned above, were
treated as independent studies. Therefore, 19 studies from 11
references were included in this meta-analysis, including 8 on
HSV-2 (10,11,14,15,25–27)
and 11 on HHV-8 (8,9,11,15–17).
Detailed characteristics of the eligible studies are listed in
Table I.
 | Table ICharacteristics of the included
studies on herpes simplex virus type 2 (HSV-2) or human herpesvirus
8 (HHV-8) infection and prostate cancer risk. |
Table I
Characteristics of the included
studies on herpes simplex virus type 2 (HSV-2) or human herpesvirus
8 (HHV-8) infection and prostate cancer risk.
| Year | Authors | Geographical
region | No. of cases | No. of
controls | No. of studies | Herpes virus
subtypes | Refs. |
|---|
| 1976 | Herbert et
al | USA | 28 | 29 | 1 | HSV-2 | (25) |
| 1981 | Baker et
al | USA | 50 | 159 | 1 | HSV-2 | (26) |
| 1981 | Luleci et
al | Turkey | 16 | 35 | 1 | HSV-2 | (27) |
| 2003 | Hoffman et
al | Trinidad &
Tobagoa, USA | 238 (Trinidad and
Tobagoa 138, USA
100) | 317 (Trinidad and
Tobagoa 140, USA
177) | 2 | HHV-8 | (9) |
| 2005 | Korodi et
al | Finland | 163 | 288 | 2 | HSV-2, HHV-8 | (15) |
| 2007 | Sutcliffe et
al | USA | 691 | 691 | 1 | HHV-8 | (17) |
| 2007 | Jenkins et
al | Italy, USA | 250 (Italy 10, USA
African American 41, USA white 104, USA African American
95)b | 287 (Italy 34, USA
African American 98, USA white 80, USA African American 75)b | 4 | HHV-8 | (16) |
| 2008 | Huang et
al | USA | 868 (white 765,
black 103) | 1,282 (white 915,
black 367) | 4 | HSV-2, HHV-8 | (11) |
| 2009 | Dennis et
al | USA | 267 | 267 | 1 | HSV-2 | (14) |
| 2011 | McDonald et
al | Trinidad &
Tobagoa | 96 | 415 | 1 | HHV-8 | (8) |
| 2011 | Hrbacek et
al | Czech Republic | 329 | 105 | 1 | HSV-2 | (10) |
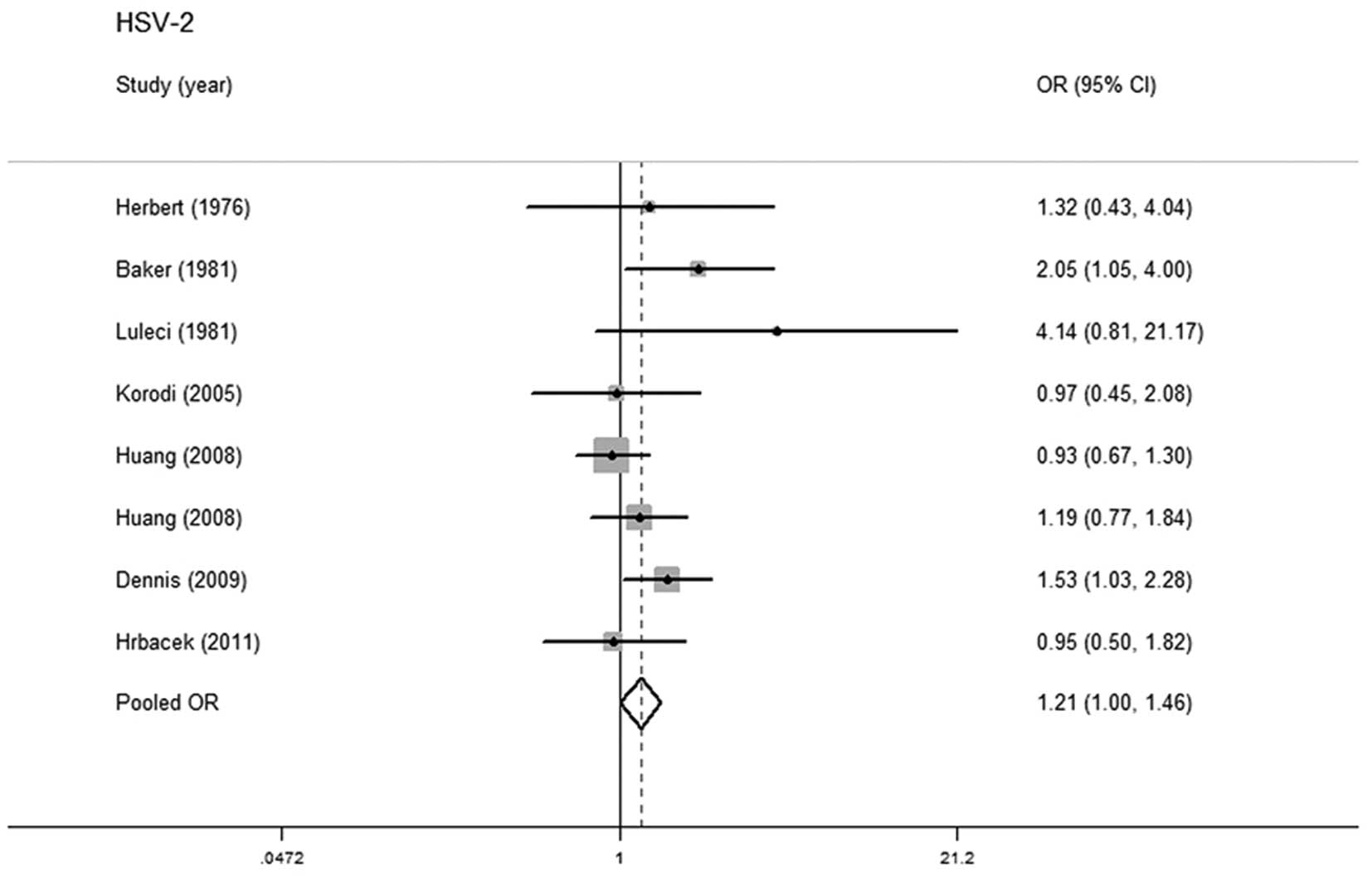
HSV-2 and prostate cancer risk
Eight studies with 1,721 cases and 2,165 controls
were included in this meta-analysis, in order to investigate the
association between HSV-2 infection and prostate cancer risk. Five
studies were located in North and South America, with 1,213 cases
and 1,737 controls (11,14,25,26)
and three were located in Europe, with 508 cases and 428 controls
(10,15,27).
No statistically significant heterogeneity was detected in pooled
analysis (P=0.243, I2=23.4%) and the fixed-effects model
was adopted. A marginally positive relationship was observed
between HSV-2 infection and prostate cancer (OR=1.209; 95% CI,
1.003–1.456) (Fig. 2). No
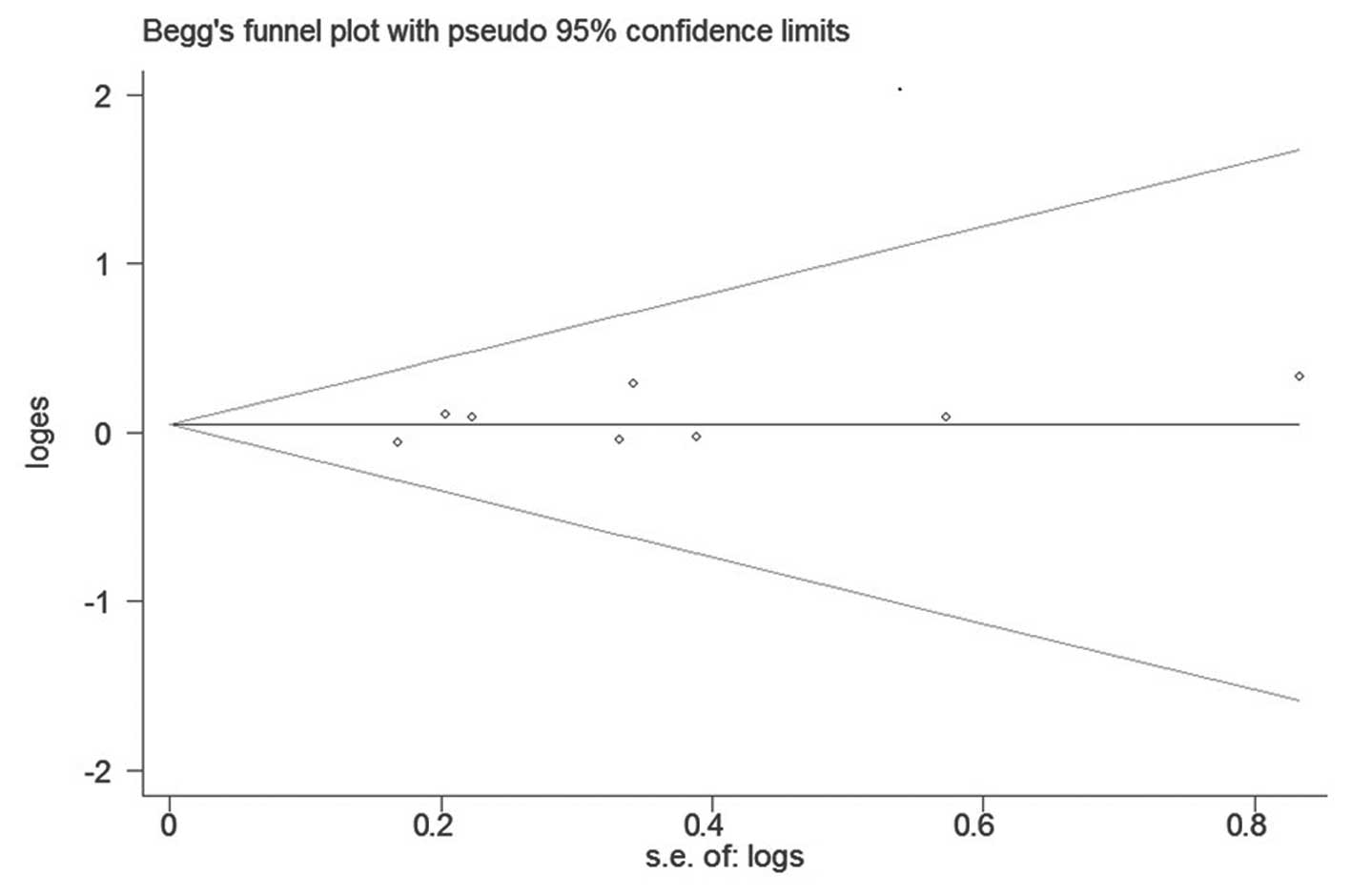
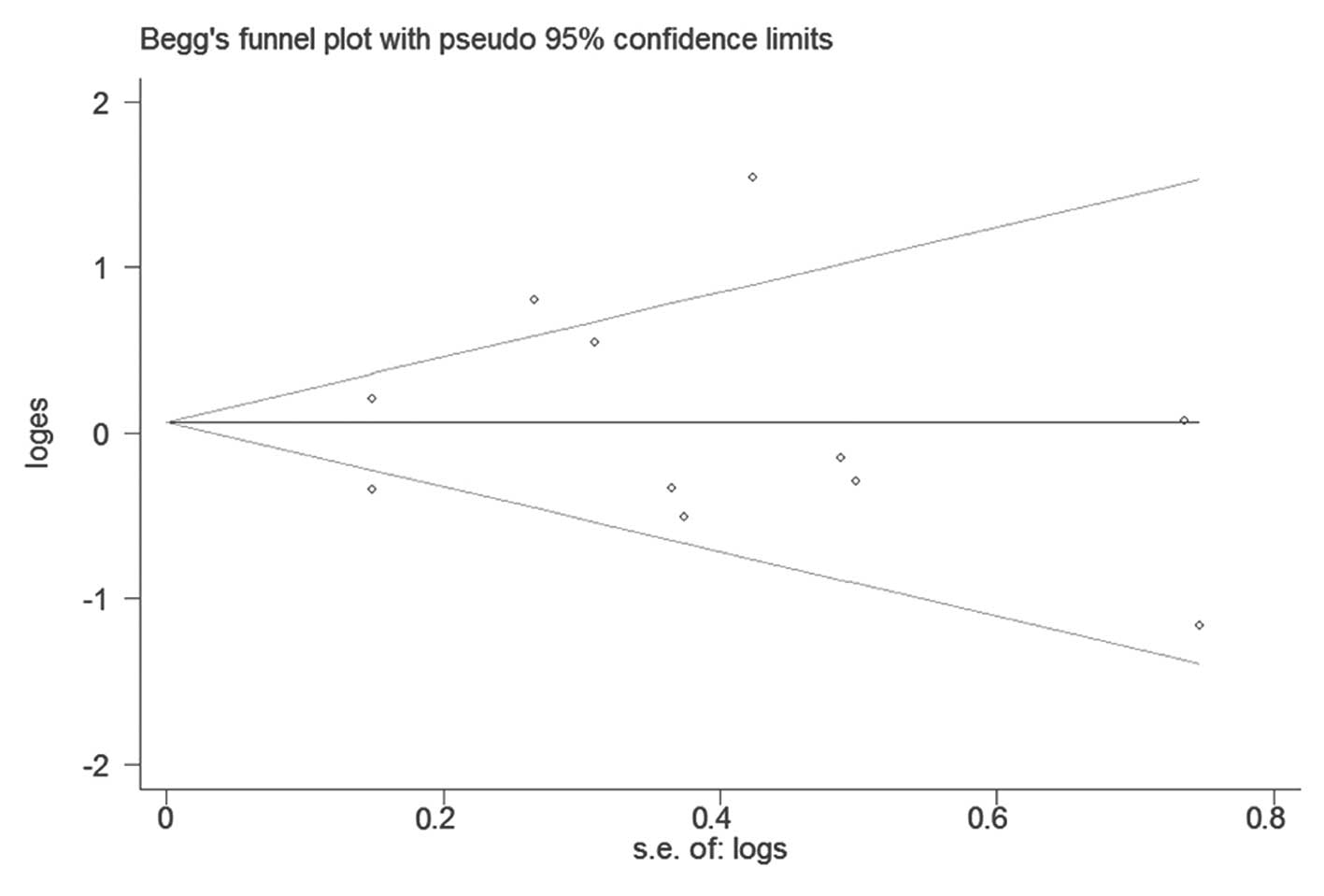
publication bias was found with Begg’s or Egger’s test (P=0.386 and
P=0.291, respectively). Begg’s funnel plot is shown in Fig. 3.
In the stratified analysis by geographical region,
no statistically significant heterogeneity was observed in either
subgroup (Table II). Therefore, the
fixed-effects model was adopted. A marginally positive association
between HSV-2 infection and increased prostate cancer risk was
demonstrated in the North and South American subgroup (OR=1.226;
95% CI, 1.000–1.503). However, no statistically positive result was
observed in the European subgroup (OR=1.222; 95% CI, 0.704–1.787).
No publication bias was found in the subgroup analyses, as shown in
Table II (funnel plot not
shown).
 | Table IIStratified pooled OR and 95% CI for
HSV-2 or HHV-8 infection and prostate cancer risk. |
Table II
Stratified pooled OR and 95% CI for
HSV-2 or HHV-8 infection and prostate cancer risk.
| | | Heterogeneity
| Publication bias
P-value
|
|---|
| Subgroup | No. of studies
(Refs.) | OR (95% CI) | P-value | I2
(%) | Begg’s test | Egger’s test |
|---|
| HSV-2 by
geographical region | | | | | | |
| North and South
America | 5 (11,14,25,26) | 1.226
(1.000–1.503) | 0.192 | 34.4 | 0.624 | 0.284 |
| Europe | 3 (10,15,27) | 1.122
(0.704–1.787) | 0.241 | 29.6 | 0.117 | 0.085 |
| HHV-8 by
geographical region | | | | | | |
| North and South
America | 9 (8,9,11,16,17) | 1.142
(0.759–1.718) | 0.000 | 78.4 | 0.677 | 0.770 |
| Europe | 2 (15,16) | 0.839
(0.374–1.882) | 0.682 | 0.0 | 0.317 | - |
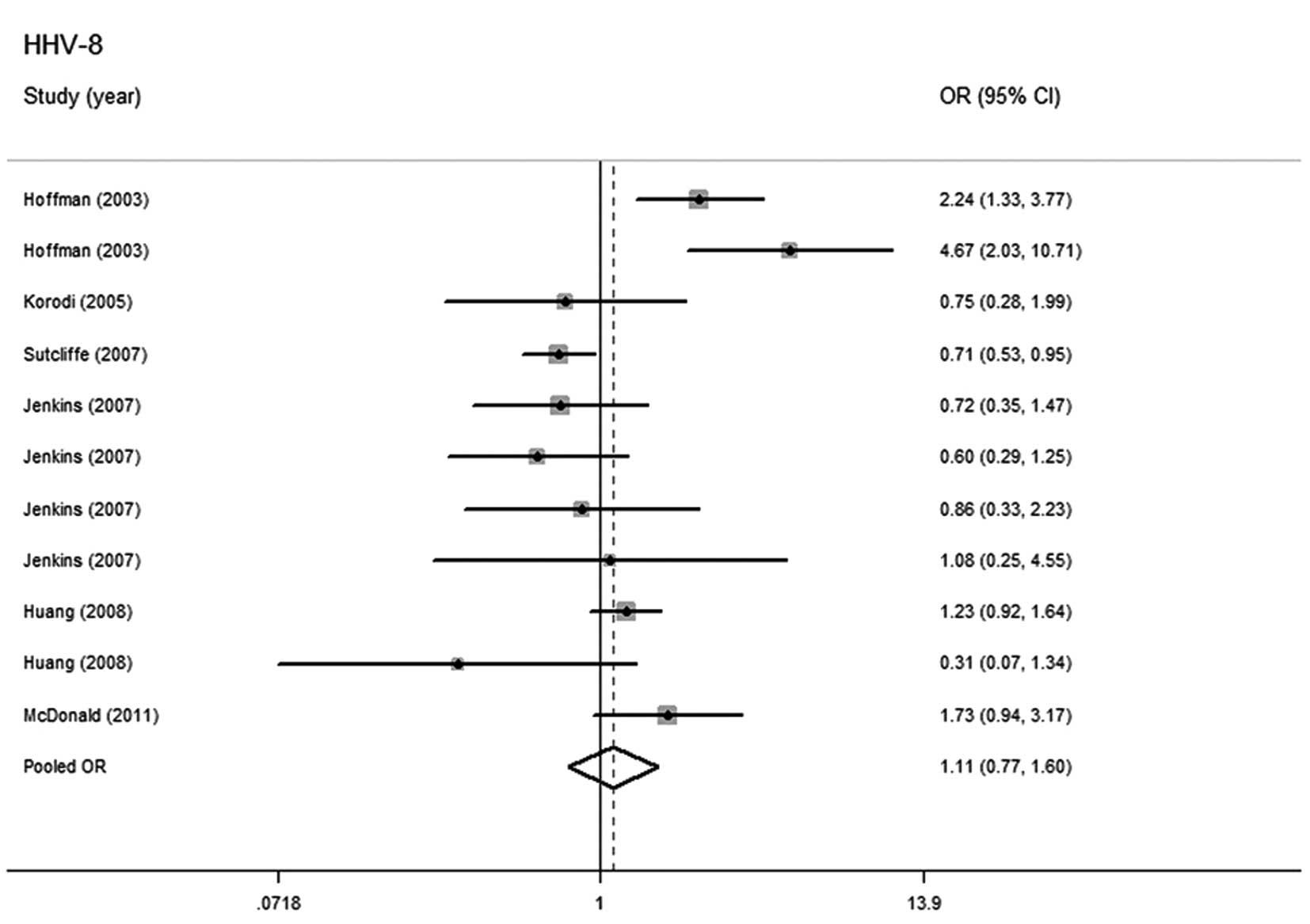
HHV-8 and prostate cancer risk
There were 11 studies with 2,306 cases and 3,280
controls evaluating the correlation between HHV-8 infection and
prostate cancer risk. There were nine studies in North and South
America, with 2,133 cases and 2,958 controls (8,9,11,16,17)
and two in Europe, with 173 cases and 322 controls (15,16).
The random-effects model was used for summarizing the estimation
when heterogeneity was demonstrated by the pooled analysis
(P=0.000, I2=73.4%). No significant relationship was
identified between HHV-8 infection and higher prostate cancer risk
(OR=1.106; 95% CI, 0.765–1.598) (Fig.
4).
Stratified analysis was also performed according to
geographical region. Heterogeneity was observed in the North and
South America subgroup (P=0.000, I2=78.4%) and the
random-effects model was adopted. No association was observed in
either subgroup (Table II).
There was no publication bias in the overall
(Fig. 5) or stratified analysis
(funnel plot not shown). The results of Begg’s and Egger’s test are
presented in Table II.
Sensitivity test
We performed sensitivity analyses by sequentially
excluding studies one by one, in order to examine the influence of
a single study on the overall estimate or on any stratum. The
results demonstrated that no single study considerably affected the
summary of risk estimates in this meta-analysis (data not
shown).
Discussion
Viral infection is a potential risk factor for
carcinogenesis. Several studies indicated that these infectious
agents may elicit an immune response, creating a cytokine tissue
environment that leads to chronic inflammation, DNA damage, cell
proliferation, angiogenesis and ultimately prostate cancer
(32–35). An in vitro study suggested
that components of viral and other infectious agents may shift the
balance towards altered homeostasis in cells that have already
deviated from normal gene expression and may thus play a role in
malignant transformation (36).
Several epidemiological studies evaluated the association between
herpes virus infection and prostate cancer risk, although results
were inconsistent (9,14,16,17).
Therefore, an elaborate and comprehensive demonstration of the
association between herpes virus infection and prostate cancer risk
is of significance. To the best of our knowledge, this
meta-analysis is the first to focus on the relationship between
HSV-2 or HHV-8 infection and prostate cancer risk.
Prostate cancer incidence and HSV-2 prevalence are
affected by geographical region and age. Prostate cancer incidence
rates vary by >25-fold worldwide, with the highest rates
recorded primarily in the developed countries of Oceania, Europe
and North America (1). In a global
review, the HSV-2 prevalence is highest in parts of Africa and
North and South America, whereas it tends to be lower in Asia
(37). In our meta-analysis, the
geographical factor was considered and used for study
stratification prior to further analysis. However, no studies from
Africa or Asia were included, which restricted a thorough
comparison among different areas. By contrast, older age is a
well-established risk factor for prostate cancer (1). The median age of prostate cancer cases
in the USA is 67 years, according to the statistics updated up to
2012 (38). Furthermore, HSV-2
prevalence is also strongly associated with age, increasing from
negligible levels in children <12 years to as high as 80% among
older age populations (37). We
aimed to conduct a stratified analysis according to age. However,
of all the studies included, only a few provided the mean or median
age (10,14,26,30).
Moreover, a study demonstrated that the association with HSV-2 was
strengthened when earlier sera analyses were restricted to
specimens collected at least five years prior to diagnosis and
suggested a long latency period from HSV-2 infection to prostate
cancer development (14). We aimed
to investigate this association stratified according to the period
between serum sample collection and diagnosis. However, due to the
insufficient information provided, this investigation could not be
conducted.
Some of the studies reported that the HHV-8 DNA
sequence was detected in both normal and cancerous prostatic
tissues (39–41), whereas others did not (42–45).
Moore and Chang (46) reported that
HHV-8 expresses viral interleukin-6, a homolog of human
interleukin-6, which was suggested to elicit prostate cancer cell
proliferation by Platz and De Marzo (35). Several epidemiological studies
demonstrated no association between HHV-8 and prostate cancer
(8,11,15,16),
which is consistent with the results of this meta-analysis
(OR=1.106; 95% CI, 0.765–1.598). Hoffman et al(9) conducted two studies among participants
from either the Republic of Trinidad and Tobago or the USA, which
demonstrated elevated HHV-8 seropositivity among prostate cancer
patients. The consistent results from two independent laboratories
with reliable assays and algorithms were the first documented to
indicate the positive association. However, not all prostate cancer
patients in the two studies were HHV-8 seropositive, suggesting
that the virus is not always associated with prostate cancer.
Another study included in this meta-analysis indicated an inverse
association between HHV-8 infection and prostate cancer risk
(17). The confounding factor of
Mediterranean heritage in the participants enrolled and the
differences in assay sensitivity and specificity were considered by
the authors as a possible eplanation of the inverse findings, along
with the skewing of the immune response mediated by HHV-8
chemokines, which was hypothesized to exert a potentially
protective effect on prostate cancer (46). McDonald et al(8) suggested that HHV-8 might segregate
patients with manifest and emergent prostate cancer into two
groups, HHV-8 sero-positive prostate cancer detected immediately
and HHV-8 sero-negative prostate cancer detected later, which may
explain the opposite positive and negative HHV-8 associations
observed.
Previous studies detected herpes virus DNA (41,47) or
anti-virus antibody in tissue samples (13). However, herpes virus detection in
tissues was not frequently performed, since blood samples were
easier to collect from the participants compared to tissue samples
and the serological assays were more convenient to conduct compared
to DNA detection. Thus, serological assays were widely used in
studies, in order to guarantee an adequate study size and ensure
data reliability to a certain extent. There is no evidence strong
enough at present to support that the results of viral infection
from blood samples were equal to or comparable with those from
tissue samples, therefore, the detection methods for HSV-2 and
HHV-8 in this meta-analysis were restricted to serological
assays.
The pooled ORs were estimated based on all the
studies obtained from systematic search, providing a relatively
high statistical power. However, certain limitations should be
considered. Firstly, this being a meta-analysis, no original data
were obtained. Since prostate cancer is a multifactorial disease
and herpes virus infection is related to individual behavior,
several covariates, including age, sexual behavior, smoking status
and ethnicity, should be taken into consideration to make this
meta-analysis more reliable. However, such information could not be
summarized without the original data of the recruited studies,
which made it difficult to further assess potential confounding
factors. Secondly, although different databases were used to cover
publications from different areas, there existed a distinction
between study quantity and size among different geographical
groups, which may bias the conclusions. No studies from Africa or
Asia were included; therefore, assessment regarding these areas was
not possible. Furthermore, in HSV-2-related analyses, there were
five studies with 1,213 cases and 1,737 controls in the North and
South American group (11,14,25,26)
and three studies with 508 cases and 428 controls in the European
group (10,15,27).
With fewer studies and participants, the results from the European
group may not be representative or stable. Thirdly, the selected
databases covered parts of unpublished articles; however, we did
not specifically search for these gray data, although no
publication bias was observed by tests.
In conclusion, the meta-analysis demonstrated a
potential association between HSV-2 infection and a higher prostate
cancer risk, confined to the North and South American group, as
determined by stratified analysis. An association between HHV-8
infection and prostate cancer was not detected. The results of this
study failed to explain the roles that HSV-2 or HHV-8 infection may
play in prostate cancer. Further investigations and large-sample
studies are required to elucidate the potential mechanism
underlying viral carcinogenesis and the relationship between herpes
virus infection and prostate cancer risk.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
3
|
Gunderson K, Wang CY and Wang R: Global
prostate cancer incidence and the migration, settlement, and
admixture history of the Northern Europeans. Cancer Epidemiol.
35:320–327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Wang S, Lin YW, et al:
Angiotensin-converting enzyme insertion/deletion polymorphism and
the risk of prostate cancer in the Han population of China. Med
Oncol. 29:1964–1971. 2012. View Article : Google Scholar
|
|
5
|
Giovannucci E, Stampfer MJ, Krithivas K,
et al: The CAG repeat within the androgen receptor gene and its
relationship to prostate cancer. Proc Natl Acad Sci USA.
94:3320–3323. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merrill RM and Lyon JL: Explaining the
difference in prostate cancer mortality rates between white and
black men in the United States. Urology. 55:730–735. 2000.
View Article : Google Scholar
|
|
7
|
Souiden Y, Mahdouani M, Chaieb K, Elkamel
R and Mahdouani K: Polymorphisms of glutathione-S-transferase M1
and T1 and prostate cancer risk in a Tunisian population. Cancer
Epidemiol. 34:598–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McDonald AC, Jenkins FJ, Bunker CH, Wilson
JW, Patrick AL and Weissfeld JL: A case-cohort study of human
herpesvirus 8 seropositivity and incident prostate cancer in
Tobago. Infect Agent Cancer. 6:252011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoffman LJ, Bunker CH, Pellett PE, et al:
Elevated seroprevalence of human herpesvirus 8 among men with
prostate cancer. J Infect Dis. 189:15–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hrbacek J, Urban M, Hamsikova E, et al:
Serum antibodies against genitourinary infectious agents in
prostate cancer and benign prostate hyperplasia patients: a
case-control study. BMC Cancer. 11:532011. View Article : Google Scholar
|
|
11
|
Huang WY, Hayes R, Pfeiffer R, et al:
Sexually transmissible infections and prostate cancer risk. Cancer
Epidemiol Biomarkers Prev. 17:2374–2381. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ewald PW: An evolutionary perspective on
parasitism as a cause of cancer. Adv Parasitol. 68:21–43. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haid M and Sharon N: Immunofluorescent
evidence of prior herpes simplex virus type-2 infection in prostate
carcinoma. Urology. 24:623–625. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dennis LK, Coughlin JA, McKinnon BC, et
al: Sexually transmitted infections and prostate cancer among men
in the U.S. military. Cancer Epidemiol Biomarkers Prev.
18:2665–2671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Korodi Z, Wang X, Tedeschi R, Knekt P and
Dillner J: No serological evidence of association between prostate
cancer and infection with herpes simplex virus type 2 or human
herpesvirus type 8: a nested case-control study. J Infect Dis.
191:2008–2011. 2005. View
Article : Google Scholar
|
|
16
|
Jenkins FJ, Hayes RB, Jackson A, et al:
Human herpesvirus 8 seroprevalence among prostate cancer case
patients and control subjects. J Infect Dis. 196:208–211. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sutcliffe S, Giovannucci E, Gaydos CA, et
al: Plasma antibodies against Chlamydia trachomatis, human
papillomavirus, and human herpesvirus type 8 in relation to
prostate cancer: a prospective study. Cancer Epidemiol Biomarkers
Prev. 16:1573–1580. 2007.PubMed/NCBI
|
|
18
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
22
|
Fleiss JL: The statistical basis of
meta-analysis. Stat Methods Med Res. 2:121–145. 1993. View Article : Google Scholar
|
|
23
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Herbert JT, Birkhoff JD, Feorino PM and
Caldwell GG: Herpes simplex virus type 2 and cancer of the
prostate. J Urol. 116:611–612. 1976.PubMed/NCBI
|
|
26
|
Baker LH, Mebust WK, Chin TD, Chapman AL,
Hinthorn D and Towle D: The relationship of herpesvirus to
carcinoma of the prostate. J Urol. 125:370–374. 1981.PubMed/NCBI
|
|
27
|
Luleci G, Sakizli M, Gunalp A, Erkan I and
Remzi D: Herpes simplex type 2 neutralization antibodies in
patients with cancers of urinary bladder, prostate, and cervix. J
Surg Oncol. 16:327–331. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siracusano F, Tarro G and Biviano D:
TAF-test: a tumor diagnosis device in oncologic urology. Cancer.
50:2215–2217. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Csata S, Dan P, Kulcsar G, Horvath J, Nasz
I and Verebelyi A: Viral examinations in malignant tumours of the
urogenital system. Acta Chir Hung. 25:201–205. 1984.PubMed/NCBI
|
|
30
|
Mandel JS and Schuman LM: Sexual factors
and prostatic cancer: results from a case-control study. J
Gerontol. 42:259–264. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sabin AB and Tarro G: Herpes simplex and
herpes genitalis viruses in etiology of some human cancers. Proc
Natl Acad Sci USA. 70:3225–3229. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Marzo AM, Platz EA, Sutcliffe S, et al:
Inflammation in prostate carcinogenesis. Nat Rev Cancer. 7:256–269.
2007.
|
|
34
|
Palapattu GS, Sutcliffe S, Bastian PJ, et
al: Prostate carcinogenesis and inflammation: emerging insights.
Carcinogenesis. 26:1170–1181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Platz EA and De Marzo AM: Epidemiology of
inflammation and prostate cancer. J Urol. 171:S36–S40. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kundu SD, Lee C, Billips BK, et al: The
toll-like receptor pathway: a novel mechanism of infection-induced
carcinogenesis of prostate epithelial cells. Prostate. 68:223–229.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smith JS and Robinson NJ: Age-specific
prevalence of infection with herpes simplex virus types 2 and 1: a
global review. J Infect Dis. 186(Suppl 1): S3–S28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
National Cancer Institute; Howlader N,
Noone AM, Krapcho M, et al: SEER Cancer Statistics Review,
1975–2009 (Vintage 2009 Populations). http://seer.cancer.gov/csr/1975_2009_pops09/uri.
Accessed July 22, 2012.
|
|
39
|
Diamond C, Brodie SJ, Krieger JN, et al:
Human herpesvirus 8 in the prostate glands of men with Kaposi’s
sarcoma. J Virol. 72:6223–6227. 1998.PubMed/NCBI
|
|
40
|
Staskus KA, Zhong W, Gebhard K, et al:
Kaposi’s sarcoma-associated herpesvirus gene expression in
endothelial (spindle) tumor cells. J Virol. 71:715–719. 1997.
|
|
41
|
Monini P, de Lellis L, Fabris M, Rigolin F
and Cassai E: Kaposi’s sarcoma-associated herpesvirus DNA sequences
in prostate tissue and human semen. N Engl J Med. 334:1168–1172.
1996.
|
|
42
|
Rubin MA, Parry JP and Singh B: Kaposi’s
sarcoma associated herpesvirus deoxyribonucleic acid sequences:
lack of detection in prostatic tissue of human immunodeficiency
virus-negative immunocompetent adults. J Urol. 159:146–148.
1998.
|
|
43
|
Diamond C, Huang ML, Kedes DH, et al:
Absence of detectable human herpesvirus 8 in the semen of human
immunodeficiency virus-infected men without Kaposi’s sarcoma. J
Infect Dis. 176:775–777. 1997.PubMed/NCBI
|
|
44
|
Corbellino M, Bestetti G, Galli M and
Parravicini C: Absence of HHV-8 in prostate and semen. N Engl J
Med. 335:1237author reply 1238–1239. 1996. View Article : Google Scholar
|
|
45
|
Tasaka T, Said JW, Morosetti R, et al: Is
Kaposi’s sarcoma - associated herpesvirus ubiquitous in urogenital
and prostate tissues? Blood. 89:1686–1689. 1997.
|
|
46
|
Moore PS and Chang Y: Kaposi’s
sarcoma-associated herpesvirus. Fields’ Virology. 2nd edition.
Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B and
Straus SE: Lippincott Williams & Wilkins; Philadelphia: pp.
2803–2833. 2001
|
|
47
|
Boldogh I, Baskar JF, Mar EC and Huang ES:
Human cytomegalovirus and herpes simplex type 2 virus in normal and
adenocarcinomatous prostate glands. J Natl Cancer Inst. 70:819–826.
1983.PubMed/NCBI
|