Introduction
Epigenetic changes do not alter the nucleotide gene
sequence, however, alteration of the phenotype leads to heritable
changes in gene expression. The main types of epigenetic changes
are DNA methylation, chromatin restructuring, histone modification
and RNA editing. In mammals, DNA methylation is the most common
natural chemical modification and is important in adjusting gene
expression and maintaining the normal differentiation of cells.
Recent studies have demonstrated that abnormal DNA methylation is
important in the development of tumor progression (1). Abnormal DNA methylation mainly occurs
in the gene promoter region (CpG island); the CpG island in gene
promoter region of normal tissue is unmethylated. Thus, when
abnormal methylation occurs in the 5′-cytosine of the CpG island
sequence, it can lead to suppression of gene transcription. This,
in turn, leads to the inhibition of the anticancer gene, resulting
in tumor formation (2,3). The WW domain-containing oxidoreductase
(WWOX) gene is a newly identified tumor suppressor gene that
crosses the chromosomal fragile site, FRA16D (4). Abnormal expression of the WWOX gene in
bladder, breast, prostate and stomach cancer, as well as other
tumors, is associated with abnormal DNA methylation (5–7). The
aim of this study was to examine the association between the
aberrant methylation status of WWOX gene promoter CpG islands and
epithelial ovarian cancer.
Materials and methods
Patient samples
Between October 2009 and June 2011, we collected 48
surgically-resected epithelial ovarian cancer tissues (serous
cystadenocarcinoma, 29; mucinous cystadenocarcinoma, 19), 18
borderline epithelial ovarian tumors, 26 epithelial benign tumors
and 33 normal ovarian tissues (due to myoma of the uterus, these
patients underwent hysterectomy and bilateral salpingo-oophorectomy
and subsequently the tissues were pathologically confirmed as
normal ovarian tissues) obtained from patients in the Affiliated
Hospital of Xuzhou Medical College, Xuzhou Maternity and Child
Centers and Xuzhou Tumor Hospital. Fresh surgical specimens were
cultured using a common method, then stored at −80°C to investigate
the methylation status of WWOX gene CpG islands.
The study was approved by the Ethics Committee of
Xuzhou Medical College, Xuzhou, Jiangsu, China. Informed consent
was obtained from the patients prior to the study.
Experimental reagents and primers
Reagents used in the study included the Ezup column
animal genome DNA extraction kit (Shanghai Shengong Bioengineering
Co., Ltd., Shanghai, China), EpiTect Bisulfite kit (used for DNA
modification; Qiagen, Hilden, Germany) and the EpiTect
methylation-specific PCR (MSP) kit [used for MSP; Qiagen ]. Primers
were purchased from Invitrogen (Shanghai, China). The methylation
primer sequence of the WWOX gene was: forward,
5′-TATGGGCGTCGTTTTTTTAGTT-3′; and reverse,
5′-CAATCTCCGCAATATCGCGACA-3′ (product length, 347 bp). The
unmethylated primer sequence used was: forward,
5′-TATGGGTGTTGTTTTTTTAGTT-3′; and reverse, 5′-CAATCTCCACAATATCAC
AACA-3′ (product length, 347 bp).
DNA extraction
Approximately 30 mg tissue specimen was obtained
from the surgically resected samples, i.e., epithelial ovarian
cancer, borderline epithelial ovarian tumor, benign ovarian
epithelial tumor and normal ovary tissue samples. Samples were
triturated in liquid nitrogen and DNA was extracted using an Ezup
column animal genome DNA extraction kit (Shanghai Shengong
Bioengineering Co., Ltd.), according to the manufacturer’s
instructions. The extracted DNA was subjected to agarose gel
electrophoresis to obtain DNA bands and was visualized under light
microscopy. DNA purity was estimated using an ultraviolet
spectrophotometer. The adsorption (A) value was measured as
A260/A280:1.8–2.0.
Modification and purification of DNA
sulfite
Reagent was prepared according to the manufacturer’s
instructions (EpiTect Bisulfite kit, Qiagen). DNA (15 μl)
was added to RNase-free water, until a total volume of 20 μl
was obtained, and then to a bisulfite mix (85 μl
dissolved Bisulfite Mix and 35 μl DNA Protect buffer;
EpiTect Bisulfite kit) and amplified using polymerase chain
reaction (PCR) according to the manufacturer’s instructions. The
modified DNA was moved into an EpiTect centrifugal column, and
underwent maximum speed centrifugation for 1 min. The liquid waste
was then discarded and the sulfonation liquid was added, followed
by desalination and purification. Finally, 30 μl DNA was
obtained by washing with Buffer EB.
MSP
MSP was carried out in a total volume of 50
μl (EpiTect Master Mix, 25 μl), with modified DNA (4
μl) in RNase-free water. The reaction conditions for MSP
were: initial denaturation at 95°C for 10 min, followed by 94°C for
30 sec; 58°C for 45 sec; 72°C for 45 sec for 40 cycles; and 72°C
for 10 min for both methylated and unmethylated reactions. The PCR
products (6 μl) were electrophoresed, verified in 1.5%
agarose gel and observed under UV light. Target gene amplification
of methylation primers was considered fully methylated, while that
of unmethylated primers was considered partially methylated.
Statistical analysis
Data were analyzed using SPSS 13.0 statistical
software. A comparison of the various sample rates and the
differences of the methylation rates between the two groups was
carried out using χ2 test. Statistical significance was
defined as P<0.05.
Results
Methylation status of WWOX gene promoter
CpG islands status in various ovarian tissues
The methylation rate of WWOX gene promoter CpG
islands in epithelial ovarian cancer, borderline ovarian epithelial
tumors and benign ovarian epithelial tumors was found to be 43.75,
26.32 and 3.84%, respectively. The CpG islands in the WWOX gene in
normal ovarian tissues were completely unmethylated (Table I). The rate of CpG island
methylation in epithelial ovarian cancer tissues was higher than
that of the remaining three ovarian tissue types, and these
differences were found to be statistically significant (P<0.01).
Additionally, the methylation rate of borderline epithelial ovarian
tumors was higher than that of benign ovarian epithelial tumors and
normal ovarian tissue, respectively (Fisher’s exact test;
P<0.05).
 | Table IMethylation status of WWOX gene
promoter CpG island in different ovarian tissue types. |
Table I
Methylation status of WWOX gene
promoter CpG island in different ovarian tissue types.
| Tissue type | Positive no. | Negative no. | Methylation rate
(%) | Test method | P-value |
|---|
| Epithelial ovarian
cancer | 21 | 27 | 43.75a |
χ2=28.243 | <0.01 |
| Borderline epithelial
ovarian tumor | 5 | 13 | 27.78b | Fisher’s exact
test | |
| Benign ovarian
epithelial tumor | 1 | 25 | 3.84b | Fisher’s exact
test | |
| Normal ovarian
tissue | 0 | 33 | 0.00b | Fisher’s exact
test | |
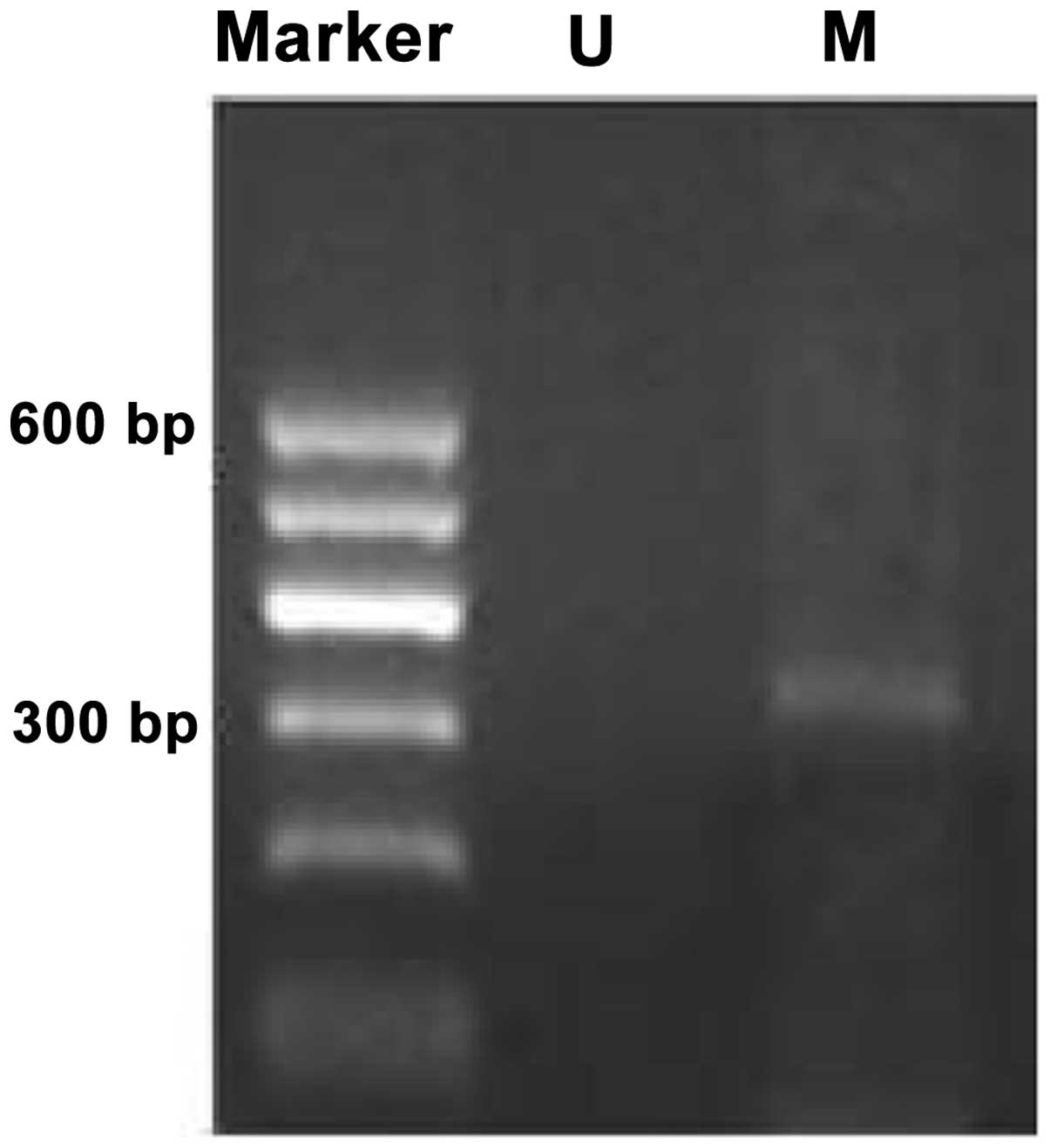
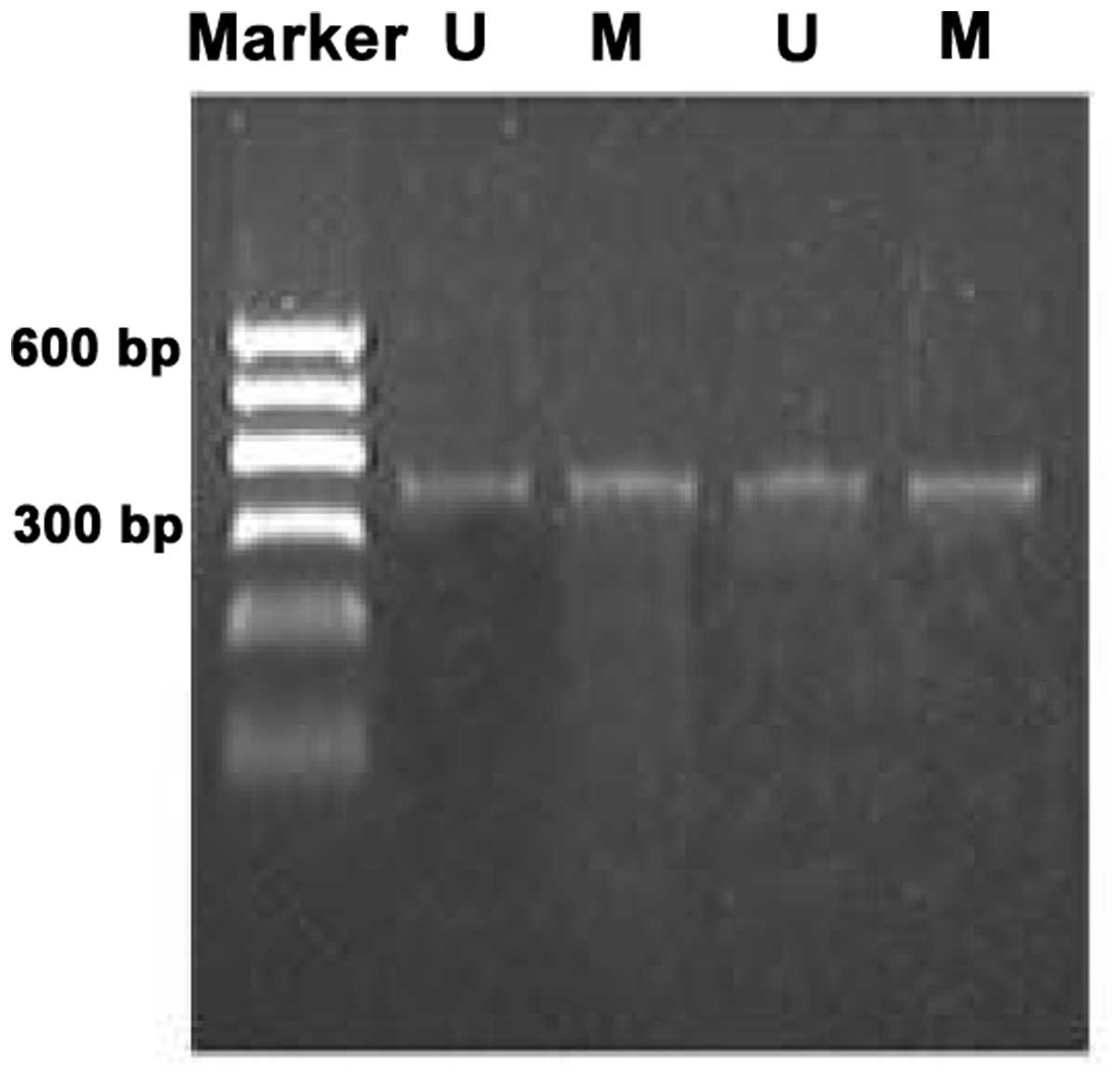
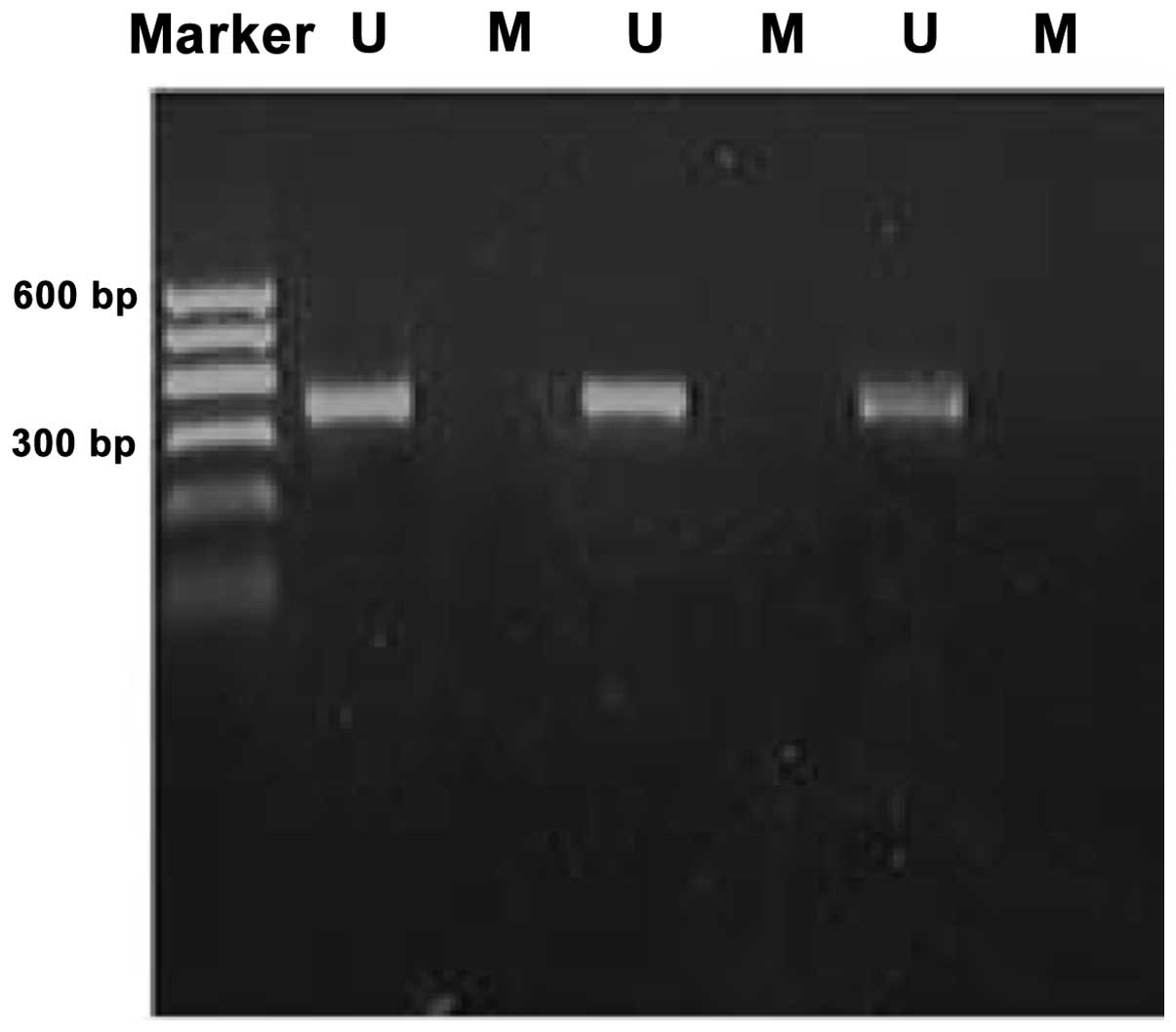
Methylation bands were identified in 21 of 48
epithelial ovarian cancer cases, of which 13 were fully methylated
(Fig. 1) and 8 were partly
methylated (Fig. 2). Five of 18
borderline epithelial ovarian tumor cases had methylation bands, of
which 3 were fully methylated and 2 were partly methylated. One
benign ovarian epithelial tumor case was completely methylated. In
normal ovarian tissues, only unmethylated bands were amplified
(Fig. 3).
Association between the methylation rate
of WWOX gene promoter CpG island and clinicopathological
characteristics of epithelial ovarian cancer
The rate of CpG island methylation in the WWOX gene
promoter region in late-stage (stage III and IV) epithelial ovarian
cancer tissues was higher than that of early-stage (stage I and II)
epithelial ovarian cancer tissues, and these differences were found
to be statistically significant (P<0.05; Table II). By contrast, no statistically
significant correlation was detected between the methylation rate
of the WWOX gene and clinicopathological characteristics such as
age, pathologies (serous epithelial and mucinous carcinoma),
pathological grading (I, II and III), ascites and lymph node
metastasis.
 | Table IIAssociation between the methylation
rate of WWOX gene promoter CpG islands and the clinicopathological
characteristics of epithelial ovarian cancer. |
Table II
Association between the methylation
rate of WWOX gene promoter CpG islands and the clinicopathological
characteristics of epithelial ovarian cancer.
| Clinicopathological
characteristics | Positive no. | Negative no. | Methylation rate
(%) | χ2
value | P-value |
|---|
| Age (years) | | | | | |
| >50 | 13 | 15 | 46.43 | 0.196 | 0.658 |
| ≤50 | 8 | 12 | 40.00 | | |
| Clinical stages | | | | | |
| I–II | 7 | 17 | 29.17 | 4.148 | 0.042 |
| III–IV | 14 | 10 | 58.33 | | |
| Pathological
grading | | | | | |
| G1 | 5 | 9 | 35.71 | | |
| G2 | 9 | 11 | 45.00 | 0.602 | |
| G3 | 7 | 7 | 50.00 | | 0.740 |
| Pathological
type | | | | | |
| Serous | 12 | 17 | 41.38 | 0.167 | |
| Mucinous | 9 | 10 | 47.37 | | 0.683 |
| Lymph node
metastasis | | | | | |
| Yes | 6 | 7 | 46.15 | 0.042 | |
| No | 15 | 20 | 42.86 | | 0.838 |
| Ascites | | | | | |
| Yes | 14 | 16 | 46.67 | 0.277 | |
| No | 7 | 11 | 38.89 | | 0.599 |
Discussion
Epithelial ovarian cancer is a common malignancy in
females. It is characterized by a complex etiology, incidence of
concealment and rapid progression. The mortality rate is the
highest of three malignant tumors in females (cervical, endometrial
and epithelial ovarian cancer), and it is a serious threat to
women’s health (8). The development
of epithelial ovarian cancer is a multi-factor and multi-step
process and is not only associated with genetic changes, but also
epigenetic changes. The role of epigenetic changes has become more
apparent. DNA methylation is an important epigenetic mechanism. In
recent years, it has been considered as the third mechanism which
inactivates tumor suppressor genes, as well as loss of
heterozygosity and gene mutation (9). A related study found that P16, ARHI,
FHIT and other tumor suppressor gene promoter CpG islands have
overmethylation in epithelial ovarian cancer (10–12).
Makarla et al(13) analyzed
the methylation status of the promoters of 8 tumor-related
suppressor genes in epithelial ovarian tumors, including RASSF1A
and P16, using the MSP method and found that all 8 genes had
methylation in invasive carcinoma, but only two genes were
methylated in benign ovarian tumor tissue. This indicates that the
abnormal methylation of genes in ovarian cancer is a common
phenomenon and suggests that abnormal DNA methylation may play an
important role in the development of ovarian carcinoma in
humans.
The WWOX gene is a newly identified tumor suppressor
gene. It was first isolated and identified with shotgun sequencing
by Bednarek et al(4) in 2000
and plays an important role in promoting cell apoptosis and
inhibiting cell proliferation, adhesion and transfer. Our previous
studies have shown that the expression of WWOX mRNA and WWOX
protein in epithelial ovarian cancer tissues and cell line were
clearly reduced (14,15). To further explore the correlation
between the methylation state of WWOX gene promoter CpG islands and
epithelial ovarian cancer, we used the MSP method to detect the
methylation of CpG islands of the WWOX gene in different types of
ovarian cancer. The findings confirmed that the WWOX gene was
methylated in ovarian epithelial cancer. Combined with previous
studies, we speculate that the aberrant methylation of CpG island
may lead to reduced or absent WWOX gene expression, which may be an
important mechanism for the occurrence of epithelial ovarian
cancer.
It is widely believed that the abnormal methylation
of DNA often occurs in the early stage of cancer. In our study, the
methylation rate of WWOX gene promoter CpG islands in borderline
epithelial ovarian tumors was higher than that of normal ovarian
tissue and ovarian benign epithelial tumor and in the one case of
benign ovarian epithelial tumor the region was completely
methylated. These are indications that molecular level changes may
already exist in ovarian epithelial cells before the appearance of
the cancer. The results also illustrate that abnormally methylated
DNA may be an early event in carcinogenesis involved in the
occurrence of epithelial ovarian cancer. In addition, the results
show that the rate of CpG island methylation in WWOX gene promoter
region in late-stage epithelial ovarian cancer tissues was higher
than that of early-stage epithelial ovarian cancer tissues. We
speculate that the reduction or absence of expression of the WWOX
gene caused by unusual promoter methylation may be closely
correlated with the clinical development of epithelial ovarian
cancer.
As an important epigenetic mechanism, DNA
methylation is currently the subject of intense research. This
study shows that the aberrant methylation state of WWOX gene CpG
islands is closely correlated with the occurrence and development
of epithelial ovarian cancer. In the future, detecting DNA
methylation of the WWOX gene may become a new technique to
determine the occurrence, development and prognosis of epithelial
ovarian cancer. In addition, our findings imply that aberrant DNA
methylation induced WWOX gene inactivation, which led to the
occurrence of epithelial ovarian cancer. However, whether
epithelial ovarian cancer progression also causes WWOX gene
methylation, or if there is a combination of the two mechanisms,
requires further study.
References
|
1
|
Li XQ, Guo YY and De W: DNA methylation
and microRNAs in cancer. World J Gastroenterol. 18:882–888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagarajan RP and Costello JF: Epigenetic
mechanisms in glioblastoma multiforme. Semin Cancer Biol.
19:188–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanai Y: Alterations of DNA methylation
and clinicopathological diversity of human cancers. Pathol Int.
58:544–558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bednarek AK, Laflin KJ, Daniel RL, et al:
WWOX, a novel WW domain-containing protein mapping to human
chromosome 16q23.3–24.1, a region frequently affected in breast
cancer. Cancer Res. 60:2140–2145. 2000.PubMed/NCBI
|
|
5
|
Iliopoulos D, Guler G, Han SY, et al:
Fragile genes as biomarkers: epigenetic control of WWOX and FHIT in
lung, breast and bladder cancer. Oncogene. 24:1625–1633. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qin HR, Iliopoulos D, Semba S, et al: A
role for the WWOX gene in prostate cancer. Cancer Res.
66:6477–6481. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan J, Zhang M and Zhang J:
Helicobacter pylori infection promotes methylation of WWOX
gene in human gastric cancer. Biochem Biophys Res Commun.
408:99–102. 2011. View Article : Google Scholar
|
|
8
|
Roett MA and Evans P: Ovarian cancer: an
overview. Am Fam Physician. 80:609–616. 2009.
|
|
9
|
Azhikina TL and Sverdlov ED: Study of
tissue-specific CpG methylation of DNA in extended genomic loci.
Biochemistry (Mosc). 70:596–603. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Surowiak P, Materna V, Maciejczyk A, et
al: Decreased expression of p16 in ovarian cancers represents an
unfavourable prognostic factor. Histol Histopathol. 23:531–538.
2008.PubMed/NCBI
|
|
11
|
Feng W, Marquez RT, Lu Z, et al: Imprinted
tumor suppressor genes ARHI and PEG3 are the most frequently
down-regulated in human ovarian cancers by loss of heterozygosity
and promoter methylation. Cancer. 112:1489–1502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Potapova A, Hoffman AM, Godwin AK, et al:
Promoter hyper-methylation of PALB2 susceptibility gene in
inherited and sporadic breast and ovarian cancer. Cancer Res.
68:998–1002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Makarla PB, Saboorian MH, Ashfaq R, et al:
Promoter hyper-methylation profile of ovarian epithelial neoplasms.
Clin Cancer Res. 11:5365–5369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan HC, Lu XY and Han QY: WWOX mRNA
expression in epithelial ovarian cancer and its clinical
significance. Acta Academiae Medicinae Xuzhou. 27:126–128. 2007.(In
Chinese).
|
|
15
|
Yan HC, Xue JQ, Lu XY, et al: Effects of
WWOX gene transfection on cell growth of epithelial ovarian cancer.
Zhonghua Fu Chan Ke Za Zhi. 43:361–365. 2008.(In Chinese).
|