Introduction
Immunoglobulin G (IgG) possesses complex-type
biantennary N-linked oligosaccharides at asparagine 297 of
the Cγ2 domain of the Fc fragment (1). Some of these oligosaccharides have
bisecting N-acetylglucosamine (GlcNAc), core-fucose,
galactose and sialic acid residues (2,3).
Patients with rheumatoid arthritis (4) and other chronic inflammatory diseases,
such as systemic lupus erythematosus, Sjogren’s syndrome and
tuberculosis (5,6), exhibit elevated serum levels of
agalactosyl IgG, an IgG oligosaccharide that lacks the terminal
galactose. We recently reported that serum agalactosyl IgG levels
may be a novel diagnostic marker for the activity and clinical
course of inflammatory bowel disease (IBD) (7) and developed a method to determine
agalactosyl IgG using a lectin-antibody ELISA (8). Furthermore, we demonstrated the
pathophysiological role of agalactosyl IgG in IBD using a mouse
model of experimental colitis that is deficient in
β-1,4-galactosyltransferase (9).
Those experiments indicated that the increase in agalactosyl IgG
levels in patients with IBD may be associated with the host’s
defense against inflammation, rather than the etiology of IBD.
We previously evaluated the levels of agalactosyl
IgG by measuring the ratio of agalactosylated to fucosylated IgG
oligosaccharides (G0F/G2F) (7) and
demonstrated that G0F/G2F is a marker of IBD clinical activity and
prognosis of recurrence. However, some patients with Crohn’s
disease do not exhibit elevated agalactosyl IgG levels, despite
severe disease activity, suggesting that genetic factors may
dictate IgG galactosylation. Furthermore, the level of IgG
agalactosylation was shown to increase with age (10) and may be regulated by a variety of
environmental factors, including food and infection; therefore, the
relative effect of genetic and environmental factors has not been
clearly determined. To determine the effect of genetic factors on
the agalactosylation of IgG, we investigated the correlations of
G0F/G2F and other biochemical data within pairs of monozygotic and
dizygotic twins who underwent simultaneous medical check-ups.
Materials and methods
Subjects
The characteristics of the participants are
summarized in Table I. Sixteen
monozygotic twin pairs (14 males and 18 females, aged 40.8±19.3
years) and 13 dizygotic twin pairs (10 males and 16 females, aged
42.5±16.9 years) who underwent simultaneous medical check-ups as
pairs between 1984 and 1994 were enrolled in this study. All the
participants were healthy. Written informed consent was obtained
from each subject and the study protocol was approved by the Ethics
Committee of Osaka University. We also randomly selected unrelated
pairs from this pool of participants and a total of 145 unrelated
pairs were analyzed to serve as controls for genetic
association.
 | Table ISubject participant characteristics
(means ± SD). |
Table I
Subject participant characteristics
(means ± SD).
| Characteristics | Monozygotic
twins | Dizygotic twins |
|---|
| Pairs (n) | 16 | 13 |
| Male/female | 14/18 | 10/16 |
| Age (years) | 40.8±19.3 | 42.5±16.9 |
|
γ-glutamyltranspeptidase (IU/l) | 25.4±25.7 | 22.8±35.4 |
| Alanine
aminotransferase (IU/l) | 16.8±9.01 | 14.8±9.30 |
| White blood
cells/μl | 5,909±1,819 | 5,276±1,505 |
| G0F/G2F ratio | 1.10±0.68 | 1.07±0.55 |
IgG purification
Serum IgG was purified using protein G sepharose
(Amersham Pharmacia Biotech, Buckinghamshire, UK). Briefly, serum
diluted 1:1 with phosphate-buffered saline (PBS) was loaded onto a
protein G sepharose column. The column was subsequently washed with
a minimum of 10 column volumes of PBS, followed by the same volume
of 10 mM ammonium bicarbonate. Column-bound IgG was eluted using
0.1% trifluoroacetic acid.
Analysis of IgG oligosaccharides
The pyridylaminated N-linked oligosaccharide of IgG
was analyzed using reverse-phase high-performance liquid
chromatography (HPLC). N-linked oligosaccharides were
released from serum IgG and labeled with 2-aminopyridine as
previously described (7). Briefly,
N-linked oligosaccharides were released from purified IgG
samples following overnight incubation with 0.5 mU glycopeptidase F
(Takara Bio, Inc., Sigma, Japan) at 37°C. The oligosaccharides were
then incubated with 0.5 mM ammonium acetate (pH 4.0) for 30 min,
lyophilized and labeled with 2-aminopyridine using GlycoTag (Takara
Bio, Inc.) according to the manufacturer’s instructions. Excess
reagent was removed with a cellulose cartridge glycan preparation
kit (Takara Bio, Inc.) and the oligosaccharides were incubated with
2 M acetic acid at 80°C for 2 h to remove sialic acids. The
pyridylamino (PA)-oligosaccharides from IgG were analyzed with
reverse-phase HPLC (Hitachi High-Technologies Corporation, Tokyo,
Japan) using a LaChrom Ultra C18 (2-μm) column (Hitachi
High-Technologies Corporation) with 10 mM sodium phosphate (pH 4.4,
solvent A) and 10 mM sodium phosphate plus 0.5% 1-butanol (solvent
B) at a flow rate of 0.5 ml/min at 40°C. The glycans were separated
with a gradient of 0–50% solvent B for 30 min, followed by 50%
solvent B for 10 min. The PA-oligosaccharides were detected using a
fluorescence detector (LaChrom Elite, Hitachi) at wavelengths of
320 nm for excitation and 400 nm for emission.
Statistical analysis
The patient characteristics are presented as mean ±
SD. The Spearman’s rank correlation coefficient was used to assess
the correlation of continuous variables within each pair. P<0.05
was considered to indicate a statistically significant
difference.
Results
IgG oligosaccharide profiles
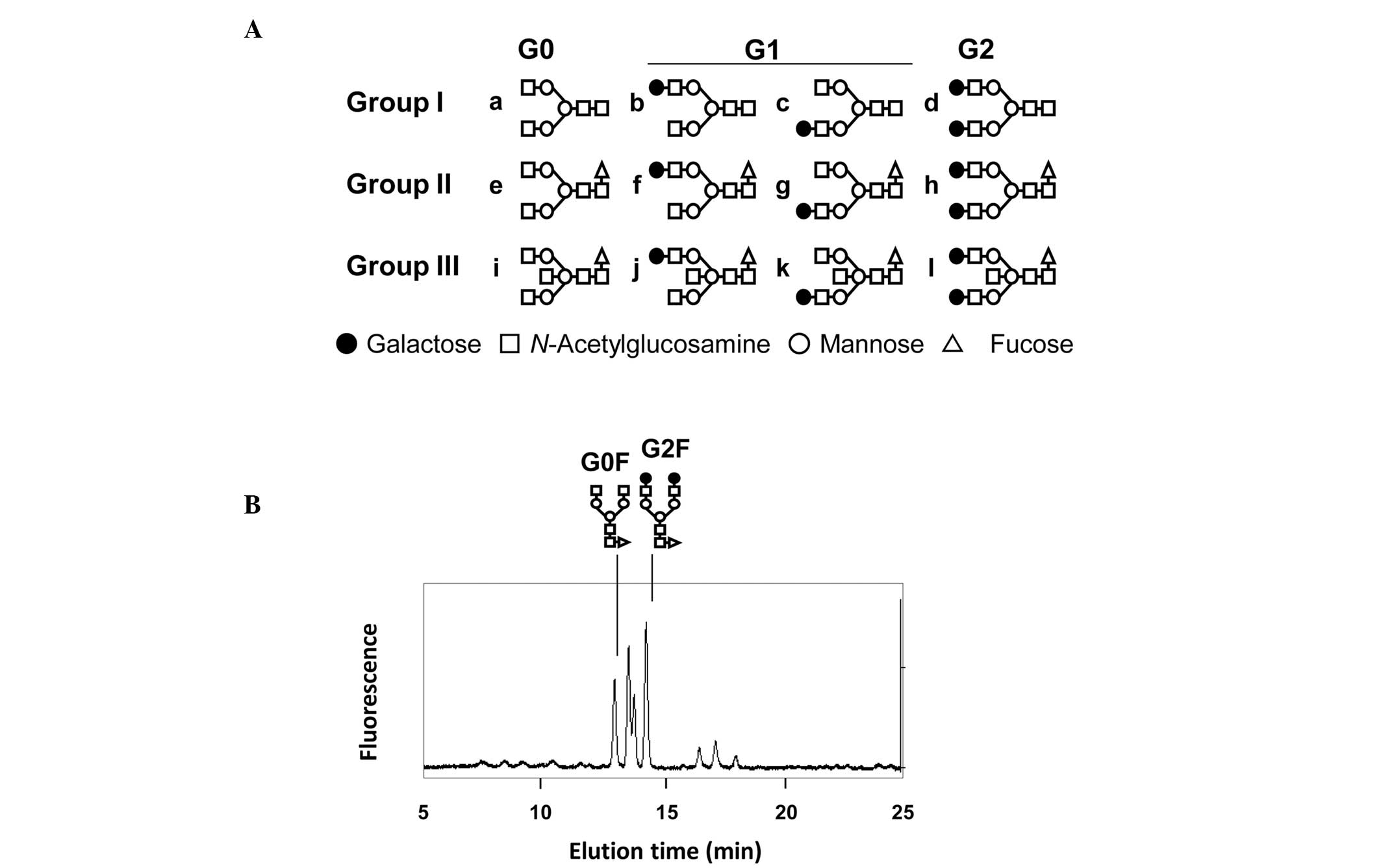
The normal oligosaccharide structures of neutral
human IgG contain 12 major structural variants (Fig. 1A). We analyzed the profiles of IgG
neutral oligosaccharides using HPLC in combination with fluorescent
labeling of oligosaccharides. In our previous study (7), the G0F/G2F ratio was described as the
ratio of the peak height of G0 (agalactosylated IgG) to G2
(fucosylated IgG oligosaccharide group II) (Fig. 1B). Since the majority of IgG
oligosaccharides belong to group II, the G0F/G2F ratio represents
the total agalactosylation of IgG.
G0F/G2F ratio
We measured the G0F/G2F ratio of IgG
oligosaccharides in 32 monozygotic and 26 dizygotic twin pairs. The
G0F/G2F ratio was not found to be significantly correlated within
monozygotic twin (R=0.215935), dizygotic twin (R=−0.21377), or
unrelated pairs (R=−0.0369) (Fig.
2A–C).
Correlations of different markers within
pairs
The correlations in serum γ-glutamyltranspeptidase
(γGTP) levels were higher within monozygotic twin (R=0.955181)
compared to those within dizygotic twin (R=0.21293) and unrelated
pairs (R=0.00177) (Fig. 3A).
Alanine aminotransferase levels (R=0.525267 for monozygotic,
R=0.460332 for dizygotic and R=0.001406 for unrelated pairs) and
white blood cell (WBC) count (R=0.524062 for monozygotic,
R=0.295489 for dizygotic and R=−0.002164 for unrelated pairs) did
not exhibit a strong correlation within twin pairs, although both
were found to be significant in monozygotic twin pairs (P=0.0367
and P=0.0372, respectively) (Fig.
3B–C).
Discussion
The agalactosylation of IgG increases with age and
is associated with a number of inflammatory diseases. Although the
present study included a limited number of twin pairs, the results
clearly demonstrated that IgG agalactosylation was not
significantly affected by genetics. Of note, γGTP levels were found
to be significantly correlated in the 16 pairs of monozygotic twins
investigated. Since γGTP levels are often associated with alcohol
consumption, this finding suggests that taste and metabolism of
alcohol are associated with genetic factors. Although the WBC count
is known to vary under different conditions, it was similar between
the monozygotic twins in this study. Therefore, compared to WBC,
the agalactosylation of IgG appears to be less affected by genetic
and more by environmental factors. Furthermore, our studies
indicated that twin studies may not a suitable approach to
glycobiology investigations.
As the HPLC analysis of IgG oligosaccharides is
costly and time-consuming, high-throughput systems, such as ELISA,
are required to investigate large numbers of monozygotic/dizygotic
twins. Although the lectin-antibody ELISA that we recently
developed (8) may be a suitable
tool for large-scale analysis of IgG oligosaccharides, it is
difficult to evaluate the normal levels of IgG agalactosylation
using this method.
To summarize, although the ABO blood type is
completely regulated by genetic factors, our results indicated that
IgG oligosaccharides are more closely associated with environmental
factors and genetic factors do not play a significant role. There
are several reports available on the epigenetic regulation of
glycosyltransferase genes (8,11) and
further studies are required to investigate the epigenetic and
environmental factors affecting the agalactosylation of IgG.
Abbreviations:
|
IgG
|
immunoglobulin G
|
|
IBD
|
inflammatory bowel disease
|
References
|
1
|
Sox HC Jr and Hood L: Attachment of
carbohydrate to the variable region of myeloma immunoglobulin light
chains. Proc Natl Acad Sci USA. 66:975–982. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi N, Ishii I, Ishihara H, et al:
Comparative structural study of the N-linked oligosaccharides of
human normal and pathological immunoglobulin G. Biochemistry.
26:1137–1144. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mizuochi T, Taniguchi T, Shimizu A, et al:
Structural and numerical variations of the carbohydrate moiety of
immunoglobulin G. J Immunol. 129:2016–2020. 1982.PubMed/NCBI
|
|
4
|
Parekh RB, Dwek RA, Sutton BJ, et al:
Association of rheumatoid arthritis and primary osteoarthritis with
changes in the glycosylation pattern of total serum IgG. Nature.
316:452–457. 1985. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomana M, Schrohenloher RE, Koopman WJ, et
al: Abnormal glycosylation of serum IgG from patients with chronic
inflammatory diseases. Arthritis Rheum. 31:333–338. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bond A, Alavi A, Axford JS, et al: The
relationship between exposed galactose and N-acetylglucosamine
residues on IgG in rheumatoid arthritis (RA), juvenile chronic
arthritis (JCA) and Sjogren’s syndrome (SS). Clin Exp Immunol.
105:99–103. 1996.PubMed/NCBI
|
|
7
|
Shinzaki S, Iijima H, Nakagawa T, et al:
IgG oligosaccharide alterations are a novel diagnostic marker for
disease activity and the clinical course of inflammatory bowel
disease. Am J Gastroenterol. 103:1173–1181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shinzaki S, Kuroki E, Iijima H, et al:
Lectin-based immunoassay for aberrant IgG glycosylation as the
biomarker for Crohn’s disease. Inflamm Bowel Dis. 19:321–331.
2013.PubMed/NCBI
|
|
9
|
Shinzaki S, Iijima H, Fujii H, et al:
Altered oligosaccharide structures reduce colitis induction in mice
defective in β-1,4-galactosyltransferase. Gastroenterology.
142:1172–1182. 2012.PubMed/NCBI
|
|
10
|
Parekh R, Isenberg D, Rook G, et al: A
comparative analysis of disease-associated changes in the
galactosylation of serum IgG. J Autoimmun. 2:101–114. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawamura YI, Toyota M, Kawashima R, et al:
DNA hypermethylation contributes to incomplete synthesis of
carbohydrate determinants in gastrointestinal cancer.
Gastroenterology. 135:142–151. 2008. View Article : Google Scholar : PubMed/NCBI
|