Introduction
Anatomical liver resection is currently the
preferred treatment for liver cancer. The procedure involves total
resection of the nidus, preserves a large amount of normal liver
tissue and minimizes blood loss (1,2). A
diverse number of instruments and devices are currently available
for use in liver resection, each exhibiting advantages and
disadvantages: Cut-Ultrasound Aspiration (CUSA), High-Frequency
Electrosurgical Knife, Ultrasound Cutter, Microwave Tissue
Coagulator, Helix Hydro-jet, Tissue Link, Argon Scalpel and Radio
Frequency Cutter, are the most commonly used. With the recent
development of medical microwave coagulation, along with improved
techniques and skill acquisition, hepatectomy may be performed more
efficiently in patients with liver disease (3,4). In
the present study, we collected and retrospectively analyzed the
clinical data from 128 patients with liver disease who were
surgically treated with anatomical hepatectomy and either microwave
coagulation therapy (n=66) or standard partial hepatectomy (n=62)
between January, 2009 and June, 2012 at the TangDu Hospital
Affiliated to the Fourth Military Medical University (Xi’an,
China). The aim of the study was to compare the efficacy and safety
of microwave coagulation to that of conventional liver resection in
patients with liver disease.
Materials and methods
Patients, groups and etiology
This study was approved by the Ethics Committee of
the TangDu Hospital. Informed consent was obtained from all the
recruited subjects. A total of 332 patients were treated with
hepatectomy in our hospital between January, 2009 and June, 2012.
Following the exclusion of patients with irregular hepatectomy
(n=150), 32 patients were treated with microwave ablation,
radiofrequency ablation and/or transcatheter arterial
chemoembolization prior to liver resection. A second hepatectomy
was performed in 13 patients. Five patients without liver cirrhosis
and four patients developed acute liver failure postoperatively. A
total of 128 patients met the inclusion criteria and were divided
into two groups based on whether the microwave tissue coagulator
technique was used intraoperatively (Table I). The microwave tissue coagulator
group (microwave group) included 66 patients and the conventional
group included 62 patients treated by anatomical liver resection
with conventional surgical instruments. The recruited patients
included 107 men and 21 women, with an average age of 50.3±10.0
years (range, 25–77 years). The cause of liver disease was
hepatitis B-related cirrhosis in 119 patients (93%), hepatic
cirrhosis with hepatitis C and B simultaneously or hepatitis C in
seven patients (5.5%) and alcoholic cirrhosis in two patients
(1.5%). All the patients underwent selective surgery in a
preoperative optimal medical condition. A total of 8% of the cases
classified as Child-Turcotte-Pugh (CTP) B, ameliorated to CTP A
following pharmacotherapy. The indocyanine green retention rate at
15 min was <12% in 89.2% of the patients.
 | Table ICouinaud classificationa of resected liver segments in patients
treated with microwave coagulation or conventional methods. |
Table I
Couinaud classificationa of resected liver segments in patients
treated with microwave coagulation or conventional methods.
| Classification | Microwave group
(n=66) | Conventional group
(n=62) |
|---|
| I | 0 | 2 |
| II | 1 | 0 |
| III | 3 | 2 |
| IV | 2 | 3 |
| V | 5 | 2 |
| VI | 4 | 7 |
| VII | 6 | 4 |
| VIII | 3 | 6 |
| II+III | 7 | 4 |
| III+IVb | 0 | 1 |
| IVb+V | 4 | 1 |
| V+VI | 7 | 9 |
| V+VIII | 1 | 2 |
| VI+VII | 8 | 5 |
| VII+VIII | 5 | 3 |
| I+IV+V | 0 | 1 |
| II+III+IV | 3 | 4 |
| IVb+V+VI | 1 | 0 |
| V+VI+VII | 2 | 0 |
| V+VI+VII+VIII | 4 | 6 |
Microwave tissue coagulator and surgical
technique
The surgical procedures were performed by the same
operating team. Microwave tissue coagulation consisted of two parts
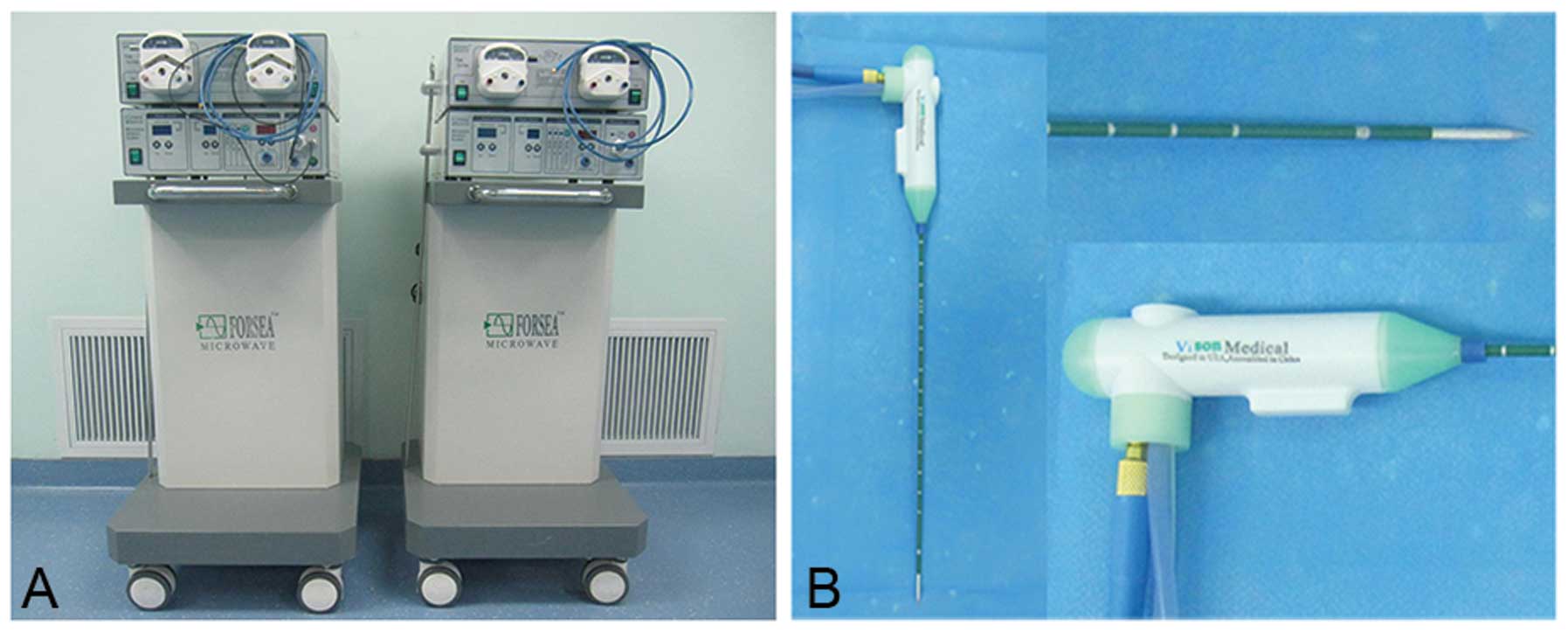
(Fig. 1): the first used the FORSEA
MTC-3C microwave ablation system (Qinghai Microwave Electronic
Institute, Nanjing, China), with an output power of 2,450±50 MHz
continuous wave and a range of 0–100 W (80–100 W was used
intraoperatively, with construction of the coagulation belt at 80
and 100 W for hematischesis) that can coagulate a vascular area of
5 mm in diameter. The second part utilized the Cool-Circle
microwave applicator or antenna (actual needle length of 180 mm
with an external diameter of 14 G, with a needle tip releasing
power), which can ablate a coagulation zone to an optimal
yellow-white color after 10–20 sec, up to a diameter of 10 mm, with
a stitch length of 15–20 mm. No radiation was applied outside a
range of 3–5 cm. Hepatic segmental resection according to the
Couinaud criteria (5) was performed
following identification of the large segmental vessels with
B-ultrasound delineation. For large tumors compressing liver
tissue, the identification of the segments was not clear and the
anatomical boundaries were marked by injection of methylthioninium
chloride under B-ultrasound guidance. There was no control of the
porta hepatis in the microwave group during the procedure; however,
53 cases were controlled from the conventional group (left or right
according to the Glisson system). The liver segment occupied by the
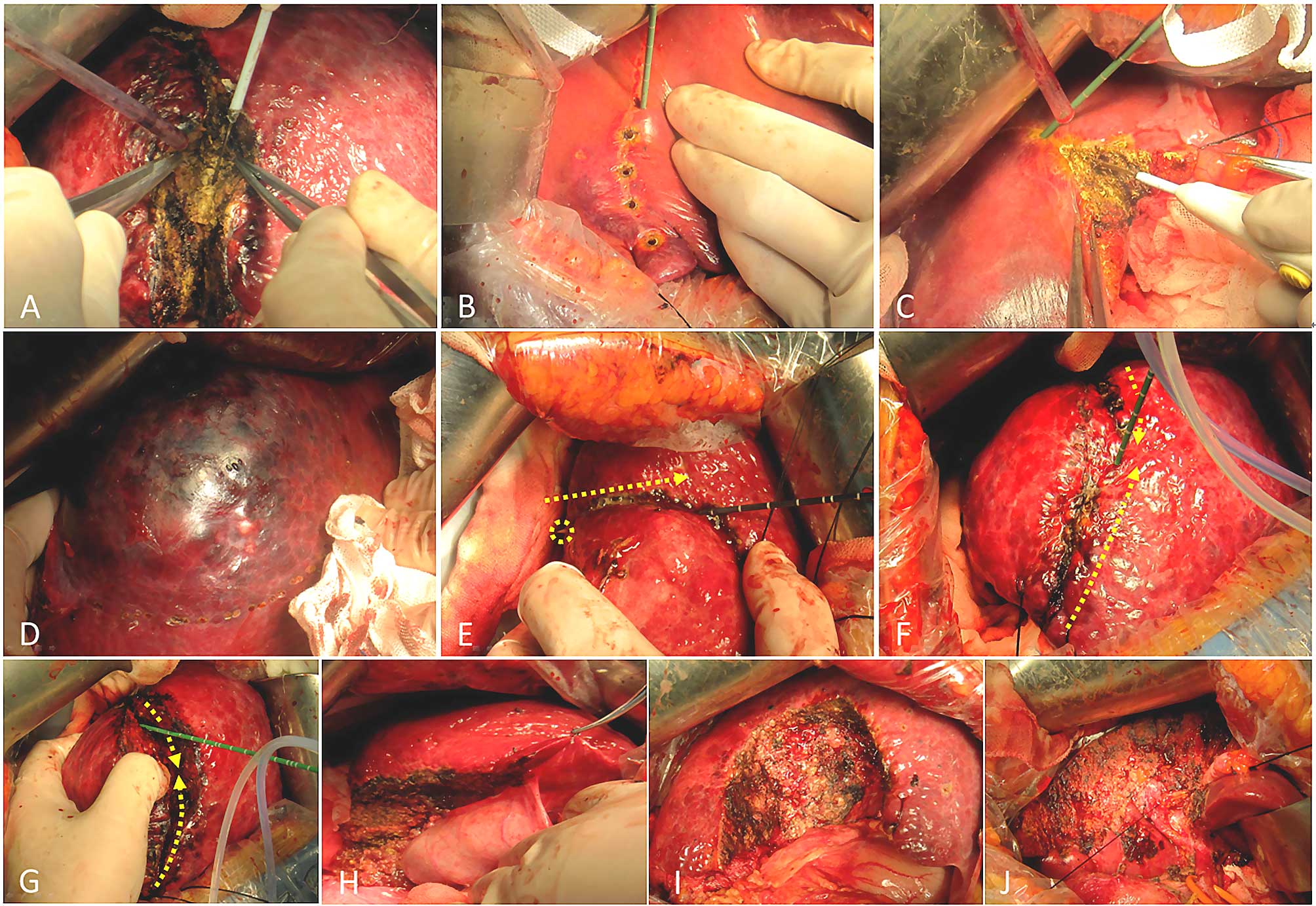
tumor was removed through the midline of the coagulated area by an
electro-scalpel or after the initial coagulative puncta, the tissue
was dissected whilst cauterizing the next puncta (Fig. 2).
Statistical analysis
Multiple factors were used in the comparative
analysis, including age, gender, preoperative alanine
aminotransferase (ALT) levels, hepatectomy classification
(classified into three groups: resection of 1, 2, or ≥3 segments),
duration of liver dissection, intraoperative blood loss,
intraoperative erythrocyte transfusion volume, ALT levels on the
1st, 3rd and 5th postoperative days, postoperative drainage volume
from the abdominal cavity on the 2nd, 5th and 7th days,
postoperative liver function recovery time (return to CTP A),
postoperative cumulative time of fever and postoperative
complications (biliary fistula and hydrothorax). The measurement
data are expressed as the means ± standard deviation following
statistical analysis with the t-test. Enumeration data were
analyzed with the χ2 test for RxC tables or the Fisher’s
exact test if beyond the χ2 test. SPSS software, version
13.0 for Windows (SPSS, Inc., Chicago, IL, USA) was used for
analyses and the results were considered significant for levels of
α=0.05.
Results
Postoperative outcome and histological
examination
There was no reported perioperative mortality. The
average hepatic portal occlusion time was 13.7±5.2 min in the
conventional group with closure of the section of remnant liver in
41 cases; however, no sections were closed in the microwave group.
Following histological examination, 115 of the 128 cases were
diagnosed with hepatocellular carcinoma, six with combined
hepatocellular and intrahepatic cholangiocarcinoma, three with
intrahepatic cholangiocellular carcinoma, two with clear cell
hepatocellular carcinoma and two with colon cancer liver
metastasis.
Postoperative complications
Five patients developed a postoperative biliary
fistula and three had a large hemorrhage (controlled with
medication in two patients and further surgery in one case with
bleeding from the right inferior phrenic artery, no one bleeding
from sections of the liver remnants). There were no patients with
evidence of hyperpyrexia and the mean cumulative time of reported
fever in the microwave group was 0.9±0.8 days, with the majority of
patients developing a low-grade fever on the 2nd and 3rd
postoperative day (52.1% of all cases with fever) in the microwave
group. The mean cumulative time of reported fever was 1.1±0.9 days
in the conventional group (P=0.196) (Table II).
 | Table IIOutcomes of anatomical hepatectomy in
the microwave coagulation and the conventional groups. |
Table II
Outcomes of anatomical hepatectomy in
the microwave coagulation and the conventional groups.
| Factors | Microwave group
(n=66) | Conventional group
(n=62) | t/χ2 | P-value |
|---|
| Age, years | 50.7±9.9 | 49.9±10.2 | 0.403 | 0.688 |
| Gender, male | 52 (78.8%) | 55 (88.7%) | 2.295 | 0.130 |
| Preoperative ALT
levels, U/l | 20±7.2 | 18±6.8 | 1.613 | 0.109 |
| Intraoperative status
of liver dissection |
| Hepatectomy
classification | - | - | 0.863 | 0.649 |
| Resection of 1
segement | 24 (36.4%) | 26 (41.9%) | - | - |
| Resection of 2
segements | 32 (48.5%) | 25 (40.3%) | - | - |
| Duration, min | 61±31 | 48±25 | 2.603 | 0.010 |
| Hemorrhage, ml | 245±102 | 355±171 | −2.052 | 0.042 |
| Erythrocyte
transfusion, min | 150±67 | 215±112 | −4.012 | <0.001 |
| Postoperative ALT
levels, U/l |
| Day 1 | 458±387 | 408±302 | 0.811 | 0.419 |
| Day 3 | 296±212 | 358±274 | −1.435 | 0.154 |
| Day 5 | 108±71 | 236±204 | −4.794 | <0.001 |
| Drainage volume of
the abdominal cavity, ml |
| Day 2 | 110±88 | 135±117 | −1.371 | 0.173 |
| Day 5 | 25±19 | 30±24 | −1.312 | 0.192 |
| Day 7 | 16±13 | 20±15 | −1.615 | 0.109 |
| Liver function
recovery time (days) | 4.3±3.8 | 5.1±4.2 | −1.132 | 0.260 |
| Cumulative time of
fever (days) | 0.9±0.8 | 1.1±0.9 | −1.299 | 0.196 |
| Complications |
| Biliary fistula | 2 (3.0%) | 3 (4.8%) | a | 0.673 |
| Hydrothorax | 16 (24.2%) | 18 (29.0%) | 0.376 | 0.540 |
Comparison between the microwave
coagulation and conventional liver resection groups
The univariate analysis revealed that age, gender,
preoperative ALT levels, hepatectomy classification, ALT levels on
the 1st and 3rd postoperative day, postoperative drainage volume of
the abdominal cavity, postoperative liver function recovery time
(recovery to CTP B or A), postoperative cumulative time of fever,
biliary fistula and hydrothorax were not significantly different
between the two groups (P>0.05). However, there were significant
differences in the duration of liver dissection, intraoperative
blood loss, intraoperative erythrocyte transfusion volume and ALT
levels on the 5th postoperative day (P<0.05) between the two
groups: the microwave group required a longer operative time, but
was associated with less intraoperative blood loss and reduced
blood transfusion requirements compared to the conventional group
(Table II).
Discussion
The current procedure for liver surgery is based on
the principle described by Couinaud (5), stating that the liver is divided into
eight functional segments. Accordingly, each segment possesses its
own portal vein, hepatic artery and biliary tract, with potential
interspace between two adjacent segments. Anatomical hepatectomy
involves resection of a segment, sector, hemiliver or trisector
(II+III+IV+V+VIII±I, or IV+V+VI+VII+VIII±I) (5). For patients with cirrhosis and liver
cancer, anatomical hepatectomy combines radical excision of the
nidus with low levels of blood loss and the highest possible
preservation of liver tissue. Recent technological advances have
allowed surgeons to use novel instruments and devices for liver
resection, including CUSA, High-Frequency Electrosurgical Knife,
Ultrasound Cutter and Microwave Tissue Coagulator.
The application of microwave fields in hepatic
surgery was first used in the 1970s. Successive punctures with a
monopolar antenna create a continuous zone allowing hepatic
dissection in the midline with reduced blood loss. The resonance of
hydrogen and polar material from a microwave field heat tissue via
mutual friction between hydrogen and hydrogen, polar material and
its counterpart and hydrogen and polar material; subsequently, the
antenna produces protein coagulation (6).
Intraoperative hemorrhage is one of the most
significant factors associated with prognosis and the complications
of liver surgery (7,8); therefore, a number of surgeons prefer
the microwave coagulator for controlling hematischesis (9,10). The
present study demonstrated that the duration of liver dissection,
intraoperative blood loss and intraoperative erythrocyte
transfusion volume were significantly different between the groups
treated with microwave coagulation and conventional techniques
(P<0.05). As the microwave coagulator is theoretically capable
of coagulating vessels of 5 mm in diameter, hepatectomy with
mini-hemorrhagic spots following dissection of the coagulation area
may result in less transfixion or coagulation of duct stumps during
liver transection, thus simplifying the procedure. Therefore,
surgeons are able to concentrate on the manipulation of the large
vessels, reducing the overall blood loss. Generally, with more
mini-hemorrhagic spots occurring in the conventionally treated
group, while the porta hepatis is controlled, the surgeons may
perform liver dissection and manipulate hemorrhagic spots at a
faster rate, resulting in a relatively poor hematischesis
control.
Following opening of the porta hepatis, the
increased transfixion or occlusion of duct stumps requires a
certain degree of hematischesis. The key reasons for blood loss in
patients treated with the microwave coagulator are occlusion of
hepatic vein branches and disconnection of the lateral branches
around the liver in patients with cirrhosis.
With a larger coagulation area, large vessel
branches of vascular deep tissue become exposed following
dissection of the yellow-white belt, causing cirrhotic liver tissue
to be more firm during dissection, with fragile vascular
structures; therefore, gentle manipulation and dissection is
required, with extra caution during the manipulation of the duct
stumps, possibly leading to surgeon fatigue in the conventional
group.
This study demonstrated that the duration of liver
dissection was significantly less in the conventional group
compared to that in the microwave group. In the conventional group,
surgeons performed liver resection more rapidly to shorten the
duration of interruption of the porta hepatis flow during control.
However, this was offset due to the longer time spent on
coagulation.
Following the completion of the liver dissection in
the conventional group, the surgeons spent more time treating
hemorrhagic spots in liver sections. B-ultrasound was used to
locate large vessels and the anatomical boundaries of resection, as
well as to check the entire liver and eliminate any micro-nidus
and/or suspected nidus. Using the microwave coagulator, this
procedure may be performed with more finesse. Previous studies
reported that the application of microwave coagulation in
unresectable liver cancer may be performed safely, with a
significant overall benefit (11,12).
During surgery, we observed the following: i) liver
tissue is more fragile compared to the large ducts near the porta
hepatis, while resistance may be felt when the microwave antenna
punctures the duct wall; ii) the antenna tip should be isolated
with gauze or other tools to prevent side effects; iii) at the
fringe of the liver, which is free of large vascular branches, the
tissue should be penetrated a little, then coagulated, followed by
antenna withdrawal and continuous coagulation; the superficial zone
should be ablated from the fringe to the middle, until a successive
coagulative zone is completed, whereas the deep belt is created by
the same procedure; iv) for the hematischesis of the liver
sections, the antenna should be inserted into the section near the
hemorrhagic spots, but not directly penetrate the spots; and v)
although microwave coagulation is theoretically capable of blocking
a 5-mm duct, for ducts <3 mm in diameter, coagulation was
selected, whereas for ducts >3 mm in diameter, ligation and
transfixion are considered to be the optimal methods.
The quality of hepatectomy is advanced based on
proficient skills on new instruments and tools; however, this may
be achieved only after mastering conventional techniques and
thoroughly comprehending liver anatomy.
In our study, the ALT levels on the 5th
postoperative day were significantly different between the two
groups, possibly due to the impairment of transfixed tissue
following closure of remnant liver segments in the conventionally
treated group. Microwave coagulation may simplify liver surgery
techniques by avoiding hepatic portal occlusion and section
closing.
Following anatomical hepatectomy, the CTP score may
be affected by several factors, including large hemorrhage, poor
compensation of liver function and a reduction in the amount of
normal tissue. However, the liver function recovery time was not
significantly different between the two groups, which is possibly
due to the procedure affecting liver function, postoperative
pharmacological management and nutritional support.
The use of microwave technology in liver surgery has
been associated with only a few complications (13,14).
In our study, postoperative fever was not significantly different
between the two groups and it was only a low-grade fever, easily
manageable with medical treatment. Compared to other tools used in
liver surgery, the histopathological examination of the resected
specimens from the microwave group revealed more prominent edge
necrosis (6). Therefore, caution is
advised when inserting the microwave needle, particularly when
closing large vascular structures. The bile duct is the largest
structure of the Glisson system. Our study revealed no significant
differences between the two groups in terms of biliary fistula
formation or large hemorrhages. The histopathological examination
of liver cross-sections revealed a superior efficacy of the
microwave coagulation technique for duct stump closure.
In conclusion, we demonstrated that microwave tissue
coagulation for anatomical hepatectomy is efficacious and safe for
the ablation of any micro-nidus and/or potentially malignant nidus
under B-ultrasound guidance. With appropriate training and skills,
the quality of hepatectomy may be improved with microwave
coagulation. However, there are certain disadvantages to microwave
coagulation: the limited use of this technique outside of research
centers and the requirement for technological improvements, with
more rapid coagulation and less destruction of adjacent tissues.
The limitations of this study lie with its retrospective nature.
Further investigation is required in a prospective study, with
careful matching of the two groups for the type of resection
performed.
Acknowledgements
The authors would like to thank the device team,
including Li Zang, Yin Duan, Zhi-peng Yu and Lu Wang for the
photograph collection and maintenance of the equipment. This study
was supported by a grant from the National Natural Science
Foundation of China (no. 81172287).
References
|
1
|
Yin DL, Jiang HC, Liang YJ, Meng XZ, Wang
JB, Zheng TS and Liu LX: Precise hepatectomy guided by minimally
invasive surgery: a novel strategy for liver resection.
Hepatogastroenterology. 59:1951–1959. 2012.PubMed/NCBI
|
|
2
|
Guglielmi A, Ruzzenente A, Conci S,
Valdegamberi A and Iacono C: How much remnant is enough in liver
resection? Dig Surg. 29:6–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Percivale A, Griseri G, Gastaldo A,
Benasso M and Pellicci R: Microwave assisted liver resection:
clinical feasibility study and preliminary results. Minerva Chir.
67:415–420. 2012.PubMed/NCBI
|
|
4
|
Inokuchi R, Seki T, Ikeda K, et al:
Percutaneous microwave coagulation therapy for hepatocellular
carcinoma: Increased coagulation diameter using a new electrode and
microwave generator. Oncol Rep. 24:621–627. 2010.
|
|
5
|
Couinaud C: Liver anatomy: portal (and
suprahepatic) or biliary segmentation. Dig Surg. 16:459–467. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabuse K: Basic knowledge of a microwave
tissue coagulator and its clinical applications. J Hepatobiliary
Pancreat Surg. 5:165–172. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kappa SF, Gorden DL, Davidson MA, Wright
JK and Guillamondegui OD: Intraoperative blood loss predicts
hemorrhage-related reoperation after orthotopic liver
transplantation. Am Surg. 76:969–973. 2010.
|
|
8
|
Yildirim IO, Salihoglu Z, Bolayirli MI,
Colakoglu N and Yuceyar L: Prospective evaluation of the factors
effective on morbidity and mortality of the patients having liver
resection surgeries. Hepatogastroenterology. 59:1928–1932.
2012.PubMed/NCBI
|
|
9
|
Imura S, Shimada M, Utsunomiya T, et al:
Ultrasound-guided microwave coagulation assists anatomical hepatic
resection. Surg Today. 42:35–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nanri M, Udo K, Kawasaki M, et al:
Microwave tissue coagulator induces renal apoptotic damage to
preserved normal renal tissue following partial nephrectomy. Clin
Exp Nephrol. 13:424–429. 2009. View Article : Google Scholar
|
|
11
|
Itoh S, Ikeda Y, Kawanaka H, et al:
Efficacy of surgical microwave therapy in patients with
unresectable hepatocellular carcinoma. Ann Surg Oncol.
18:3650–3656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martin RC, Scoggins CR and McMasters KM:
Safety and efficacy of microwave ablation of hepatic tumors: a
prospective review of a 5-year experience. Ann Surg Oncol.
17:171–178. 2010.PubMed/NCBI
|
|
13
|
Livraghi T, Meloni F, Solbiati L and Zanus
G; Collaborative Italian Group using AMICA system. Complications of
microwave ablation for liver tumors: results of a multicenter
study. Cardiovasc Intervent Radiol. 35:868–874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Veltri A, Gazzera C, Rotondella C,
Camerano F, Busso M and Gandini G: Image-guided microwave ablation
of hepatic tumours: preliminary experience. Radiol Med.
117:378–392. 2012. View Article : Google Scholar : PubMed/NCBI
|