1. Introduction
Complex diseases, such as malignant tumors and
diabetes, are a common occurrence and represent a major public
health concern. Despite the significant advances in cancer
treatment, the overall cancer-related mortality is ~90%, due to
late-stage diagnosis and failure to optimize treatment. Therefore,
effective biomarkers for cancer diagnosis are urgently needed.
Cancers such as lung, colon and breast cancer are
frequently diagnosed at a late stage. Despite intensified efforts
focused on improving the survival of cancer patients, only a
moderate improvement is generally achieved. Failure of early
diagnosis often leads to low treatment efficiency and poor
prognosis; thus, the identification of a signal characterizing the
early stages of formation and progression of cancer may reduce the
incidence of this disease (1).
Early findings of clinical studies indicated that early detection
may offer a variety of novel efficient and cost-effective
opportunities for cancer treatment (2).
The mechanisms underlying cancer development are
complicated and cancer was originally perceived as a genetic
disease. However, it was previously demonstrated that the
initiation and progression of cancer involve epigenetic
abnormalities, such as DNA methylation, histone modifications,
nucleosome positioning and aberrant expression of non-coding RNAs,
specifically microRNAs (3–7). The term ‘epigenetics’ was originally
used by Waddington in 1939 to describe the causal interactions
between genes and phenotype, including alterations in chromatin
structure and their effects on gene function. DNA methylation is
currently deemed to be one of the essential epigenetic mechanisms
(8). Failure of proper maintenance
of epigenetic marks may result in inappropriate activation or
inhibition of various signaling pathways, leading to disease
states, such as cancer. Aberrant DNA hypermethylation often occurs
in the promoter regions of specific transcription factors that are
involved in the formation and progression of malignant tumors.
Accordingly, DNA methylation may be a biomarker of early cancer
detection. In this study, we briefly reviewed the correlation
between DNA methylation and early disease diagnosis.
2. Epigenetics and DNA methylation
Epigenomes comprise all genome-wide chromatin
modifications, including covalent modification of DNA via
methylation, histone modifications and regulation of small RNAs.
Unlike DNA alterations that may constitute an inheritable
mechanism, epigenetics represent a labile and dynamic mechanism for
the regulation of cell-specific gene expression patterns,
adaptation of response to environmental factors and mediation of
disease development. It was demonstrated that epigenomic
variability leads to susceptibility to disease, even among
individuals with identical genetic information (9). Epigenomes are potentially significant
for the comprehensive understanding of cell development, tissue
differentiation and disease biogenesis. In general, the process of
epigenetics mainly refers to chromatin structure alterations that
comprise DNA methylation, histone modifications and RNA
interference. DNA methylation and histone modifications may
modulate the interaction of cis-acting elements with
trans-acting factors and gene expression. Various epigenetic
experiments, involving genomic imprinting (10), manner of monoallelic expression
(11) and high-throughput
sequencing technology (12), are
currently available and commonly used to gain insight into this
phenomenon.
DNA methylation is a common epigenetic modification
involving the methylation of 5′-cytosine residues by
methyltransferases and is often detected in the dinucleotides of
CpG sequences. Approximately 70–80% of the CpG dinucleotides
undergo methylation and are referred to as ‘CpG islands’, which are
abundant in the human genome (13).
Methylation is often localized in promoter regions and occasionally
in transcriptional regulatory regions in mammals, plants and even
prokaryotes (14,15). A number of studies indicated that
DNA methylation is crucial in the regulation of transcriptional
silencing and transcription potential (16,17).
Aberrant methylation of promoters in eukaryotic cells may lead to
silencing of important genes, such as tumor suppressor genes,
affecting their related transcriptional pathways and ultimately
leading to the development of disease.
3. Process of DNA methylation
DNA methylation may be classified as hyper- and
hypomethylation, according to increased and decreased levels of
genomic modification, respectively. Hypermethylation is an
epigenetic alteration often leading to gene-inactivating deletions
and translocations. Hypermethylated cells may exhibit a phenotype
of drug-resistance (18) or
malignant proliferation. Wu et al (19) demonstrated that 16 genes, including
BMP4, POU4F3 and GDNF, are frequently
hypermethylated in tumors. The other alteration is hypomethylation
(reduced level or loss of methylation compared to that in normal
cells). Chromosome remodeling or reactivation of transposable
elements was generated via demethylation of exons and introns or
repetitive DNA sequences. An increased number of DNA
hypomethylation loci in CpGs was shown to increase chromosomal
instability and oncogene activation. For example, the
hypomethylation of LINE-1 and Alu retrotransposons is frequently
associated with lung cancer (20),
the hypomethylation of the mesothelin promoter may contribute to
the development of malignant mesothelioma (21) and DNA hypermethylation was
previously described in peritoneal mesothelioma (22). DNA hypomethylation per se may
induce complex disease. However, a single specific mechanism
associating DNA hypomethylation with cancer has not yet been
identified.
4. Detection methods of disease-associated
DNA methylation
Complicated diseases have been associated with the
methylation status. Previous studies on cardiovascular and
neurodegenerative diseases, by Stenvinkel et al (23) and Obeid et al (24), respectively, reported that DNA
methylation is involved in major human pathologies. Investigating
the potential and practical methods of DNA methylation is essential
for determining whether there is an association between aberrant
DNA methylation within CpG-rich sequences and cancer.
Several different methods have been proposed for DNA
methylation analysis and some of the advantages and disadvantages
of the different approaches, particularly concentrating on
genome-scale DNA methylation, were outlined based on enzymes
combined with PCR methods. Conducting a DNA methylation analysis
requires a highly precise and accurate determination of the
methylation status. Several methods based on PCR have been
developed to evaluate the methylation level of genes. Bisulphite
treatment and PCR amplification are used for locus-specific
detection. For example, a quantitative methylation-specific PCR
assay was developed for high-throughput analysis and a real-time
assay for individual methylated targets. Assays of other useful
techniques may be applied to genes with 5-methylcytosine (m5C). The
distribution of m5C within DNA is unique and may be used for
genome-scale methylation analysis (25). For example, restriction landmark
genome scanning was the first DNA methylation profiling technique
that was widely used in identifying methylated loci in species or
in a tissue-specific manner. Chromatin immunoprecipitation, based
on microarray or next-generation sequencing, used antibodies or
methyl-binding proteins for massive methylated DNA profiling. These
powerful approaches provide accurate, reproducible and sensitive
data in comprehensive methylation epigenomic and genomic
typing.
5. DNA methylation as a biomarker for early
cancer diagnosis
As a specific pattern of gene expression in mammals,
DNA methylation is essential for tissue development. Abnormal DNA
methylation commonly disrupts molecular signaling mechanisms and
leads to the development of various diseases, such as cancer. DNA
methylation was the first epigenetic alteration to be identified in
cancer (26). Therefore, it is
considered to be a hallmark of cancer, it is detected in several
types of cancer cells, including colon, breast, ovarian and
cervical cancer cells and is associated with alterations in
specific gene expression.
DNA methylation and cancer
The DNA methylation status is correlated with cancer
and the methylation level is inversely correlated with mRNA
expression levels. Jin et al (27) reported that the risk of Barrett’s
esophagus (BE) progressing into esophageal adenocarcinoma is 30- to
125-fold higher compared to the general population. Methylation
assays were performed in 195 subjects with BE to evaluate the
methylation levels and frequencies of individual genes, including
p16, RUNX3, HPP1, NELL1, TAC1,
SST, AKAP12 and CDH13 and five of these genes
(NELL1, TAC1, SST, AKAP12 and
CDH13) were found to harbour methylated sites (27). In breast cancer patients, the
patterns of methylation of the ESR1 and 14-3-3-σ
promoters were significantly different compared to healthy controls
(28). Glockner et al
(29) reported that methylation of
the tissue factor pathway inhibitor 2 gene was frequently detected
in 171 samples from human colorectal cancers. Therefore, the
aberrant methylation or hypermethylation of these promoters led to
the dysregulated expression of cancer-related genes, facilitating
the development of malignant tumors. In addition to the methylation
of cancer-related genes, the genome-wide m5C levels of leukocyte
DNA are also independently associated with breast cancer. Choi
et al (30) compared 176
breast cancer cases with 173 healthy controls and demonstrated that
the m5C levels were significantly lower in breast cancer.
DNA methylation associated with cancer
diagnosis
The identification of patients with organ-confined
carcinoma is key to early-stage diagnosis of cancer. Common
organ-confined cancers, such as lung, hepatic, breast, cervical,
colorectal and genitourinary tract cancers, result in patient death
due to the lack of effective clinical diagnosis. In these cancers,
the methylation of the promoter regions of tumor suppressor genes,
such as CDH1, APC, MGMT, RASSF1A,
GSTP1, p16 and RAR-β2, affect the activity of
tumor suppressor genes, typically leading to transcriptional
silencing. A number of important genes that undergo silencing
interfere with important cancer-related cell pathways. Thus, the
aberrant methylation of the promoters of cancer-related genes may
be deemed as a potential biomarker contributing to early cancer
detection and prediction of prognosis.
A series of novel DNA methylation biomarkers in the
plasma and stool were developed for various detection purposes.
Vimentin is transcriptionally silent in normal epithelia and
aberrant vimentin expression has been used as a cancer marker in
fecal DNA testing (31). In 2005,
vimentin was licensed by the US Food and Drug Administration (FDA)
for colorectal cancer (CRC) diagnosis. Another CRC-related gene,
SEPT9, is commonly detected in the plasma of patients with
primary CRC and was submitted to the FDA for marketing application
in 2010 as a molecular marker for early clinical stage CRC.
SEPT9 as a DNA methylation biomarker was also associated
with breast cancer; Gonzalez et al (32) indicated that increased SEPT9
expression may contribute to the pathogenesis of certain types of
breast cancer. Another potential biomarker for breast cancer is the
methylation of PITX2. The evaluation of the PITX2
methylation status among different breast cancer patient
populations successfully increased the outcome prediction
performance. Harbeck et al (33) investigated PITX2 methylation
in 399 breast cancer specimens and identified low-risk patients
with hormone receptor-positive and node-negative disease. Hartmann
et al (34) also analyzed
the DNA methylation levels of PITX2 in 241 breast cancer
specimens and concluded that methylation of PITX2 was
correlated with clinical outcome. Hrasovec et al (35) reported that the alterations of CpG
sites in TMEM25 were correlated with CRC, with TMEM25
hypermethylation possibly playing a significant role in altering
the expression of this gene in CRC.
6. Conclusion
The elucidation of the mechanisms that underlie DNA
methylation changes in cancer cells may help identify a number of
cancer-specific methylation markers, assisted by promising
detection methods, ultimately resulting in optimized clinical
applications. The realization that DNA methylation may be involved
in human malignancies and is ubiquitous in human diseases is likely
to promote the development of novel diagnostic, preventive and
therapeutic strategies.
The association between methylation and heredity is
currently an emerging research topic. The presence of modified m5C
affecting the phenotype of the offspring may be parentally
inherited. Carone et al (36) indicated that parental diet may
affect cholesterol and lipid metabolism in the offspring, defined a
model system to study the environmental reprogramming of the
heritable epigenome and concluded that epigenetic information may
be inherited and represents environmental information. In another
aspect, epigenetic information is one of the main areas of interest
for the development of non-invasive prenatal diagnosis.
Papageorgiou et al (37)
reported the presence of epigenetic differences between placental
and peripheral blood and identified a large number of previously
unreported fetal epigenetic molecular markers that have the
potential of being developed into targets for non-invasive prenatal
diagnosis. Zhao et al (38)
considered hypermethylated RASSF1A to be an epigenetic
marker for the detection of fetal DNA in maternal plasma.
Epigenetic information passed from parent to offspring and DNA
methylation from gametes was shown to be predominantly maternal
(39). Similar mechanisms
maintaining the imprinted and non-imprinted methylation passed on
from the mother represent an interesting focus of investigation and
may be clinically applied for non-invasive prenatal diagnosis. Wang
et al (40) recently
reported that the hypermethylation status of the testis derived
transcript gene promoter may represent a valuable prognostic marker
for glioblastoma.
The modified DNA m5C that extensively occurs within
the genome was extensively investigated over the last few decades;
however, numerous putative methylated RNAs have been identified and
characterized, regarding location, mechanism of formation and
cellular function (41). Additional
characterization of specific RNA methyltransferase enzymes and the
association between DNA and RNA methylation is yet to be
investigated (42). In eukaryotes,
the majority of m5C methyltransferases are predicted to be nuclear
or nucleolar proteins, which corresponds well to their functions in
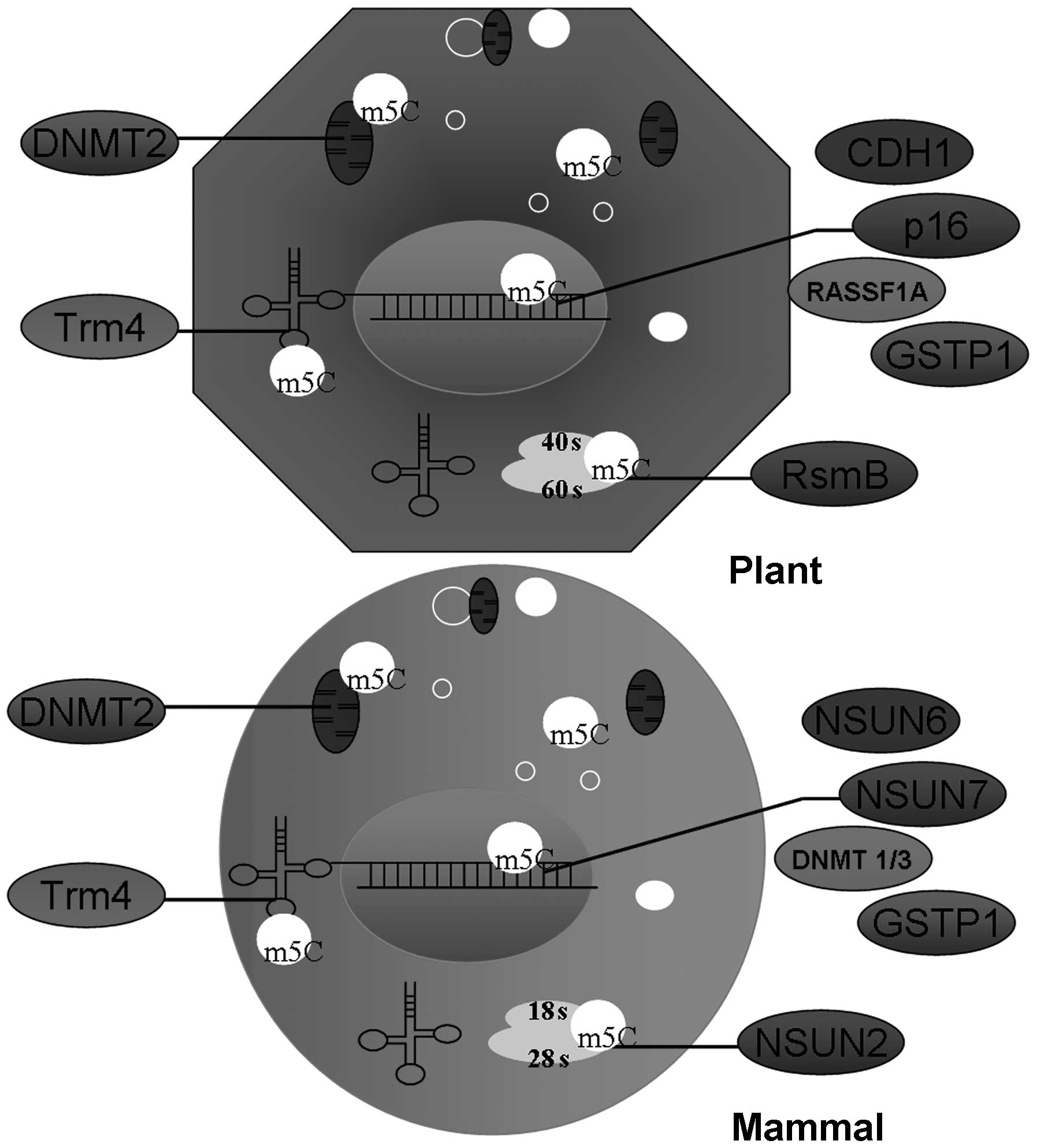
tRNA and rRNA processing (Fig. 1)
(43). The expression of the human
Trm4 (hTrm4) cDNA in yeast was identified, the first human gene
encoding tRNA methylase responsible for the methylation of hTrm4
(44). Various regulatory RNAs,
coding RNAs and newly discovered RNAs obtained based on
high-throughput sequencing techniques may help elucidate the
mechanisms of epigenetics (45).
References
|
1
|
Heyn H, Mendez-Gonzalez J and Esteller M:
Epigenetic profiling joins personalized cancer medicine. Expert Rev
Mol Diagn. 13:473–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith RA, Brooks D, Cokkinides V, Saslow D
and Brawley OW: Cancer screening in the United States, 2013: a
review of current American Cancer Society guidelines, current
issues in cancer screening, and new guidance on cervical cancer
screening and lung cancer screening. CA Cancer J Clin. 63:88–105.
2013. View Article : Google Scholar
|
|
3
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dawson MA and Kouzarides T: Cancer
epigenetics: from mechanism to therapy. Cell. 150:12–27. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aran D and Hellman A: DNA methylation of
transcriptional enhancers and cancer predisposition. Cell.
154:11–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Esteller M: Cancer epigenomics: DNA
methylomes and histone-modification maps. Nat Rev Genet. 8:286–298.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tolstorukov MY, Sansam CG, Lu P, et al:
Swi/Snf chromatin remodeling/tumor suppressor complex establishes
nucleosome occupancy at target promoters. Proc Natl Acad Sci USA.
110:10165–10170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pereira MA, Kramer PM, Conran PB, et al:
Effect of chloroform on dichloroacetic acid and trichloroacetic
acid-induced hypomethylation and expression of the c-myc gene and
on their promotion of liver and kidney tumors in mice.
Carcinogenesis. 22:1511–1519. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fraga MF, Ballestar E, Paz MF, et al:
Epigenetic differences arise during the lifetime of monozygotic
twins. Proc Natl Acad Sci USA. 102:10604–10609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reik W, Collick A, Norris ML, et al:
Genomic imprinting determines methylation of parental alleles in
transgenic mice. Nature. 328:248–251. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Onyango P, Jiang S, Uejima H, et al:
Monoallelic expression and methylation of imprinted genes in human
and mouse embryonic germ cell lineages. Proc Natl Acad Sci USA.
99:10599–10604. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tost J, Dunker J and Gut IG: Analysis and
quantification of multiple methylation variable positions in CpG
islands by pyrosequencing. Biotechniques. 35:152–156.
2003.PubMed/NCBI
|
|
13
|
Craig JM and Bickmore WA: The distribution
of CpG islands in mammalian chromosomes. Nat Genet. 7:376–382.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gutierrez JC, Callejas S, Borniquel S, et
al: DNA methylation in ciliates: implications in differentiation
processes. Int Microbiol. 3:139–146. 2000.PubMed/NCBI
|
|
15
|
Guintivano J, Arad M, Gould TD, et al:
Antenatal prediction of postpartum depression with blood DNA
methylation biomarkers. Mol Psychiatry. May 21–2013.(Epub ahead of
print). View Article : Google Scholar
|
|
16
|
Di Ruscio A, Ebralidze AK, Benoukraf T, et
al: DNMT1-interacting RNAs block gene-specific DNA methylation.
Nature. 503:371–376. 2013.PubMed/NCBI
|
|
17
|
Xie W, Schultz MD, Lister R, et al:
Epigenomic analysis of multilineage differentiation of human
embryonic stem cells. Cell. 153:1134–1148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang X, Monitto CL, Demokan S, et al:
Identification of hypermethylated genes associated with cisplatin
resistance in human cancers. Cancer Res. 70:2870–2879. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu X, Rauch TA, Zhong X, et al: CpG island
hypermethylation in human astrocytomas. Cancer Res. 70:2718–2727.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Daskalos A, Nikolaidis G, Xinarianos G,
Savvari P, Cassidy A, Zakopoulou R, Kotsinas A, Gorgoulis V, Field
JK and Liloglou T: Hypomethylation of retrotransposable elements
correlates with genomic instability in non-small cell lung cancer.
Int J Cancer. 124:81–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan K, Kajino K, Momose S, et al:
Mesothelin (MSLN) promoter is hypomethylated in malignant
mesothelioma, but its expression is not associated with methylation
status of the promoter. Hum Pathol. 41:1330–1338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hama R, Watanabe Y, Shinada K, et al:
Characterization of DNA hypermethylation in two cases of peritoneal
mesothelioma. Tumour Biol. 33:2031–2040. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stenvinkel P, Karimi M, Johansson S, et
al: Impact of inflammation on epigenetic DNA methylation - a novel
risk factor for cardiovascular disease? J Intern Med. 261:488–499.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Obeid R, Schadt A, Dillmann U, et al:
Methylation status and neurodegenerative markers in Parkinson
disease. Clin Chem. 55:1852–1860. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laird PW: Principles and challenges of
genome-wide DNA methylation analysis. Nat Rev Genet. 11:191–203.
2010. View
Article : Google Scholar
|
|
26
|
Riggs AD and Jones PA: 5-methylcytosine,
gene regulation, and cancer. Adv Cancer Res. 40:1–30. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin Z, Cheng Y, Gu W, et al: A
multicenter, double-blinded validation study of methylation
biomarkers for progression prediction in Barrett’s esophagus.
Cancer Res. 69:4112–4115. 2009.PubMed/NCBI
|
|
28
|
Martinez-Galan J, Torres B, Del Moral R,
et al: Quantitative detection of methylated ESR1 and 14-3-3-sigma
gene promoters in serum as candidate biomarkers for diagnosis of
breast cancer and evaluation of treatment efficacy. Cancer Biol
Ther. 7:958–965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Glockner SC, Dhir M, Yi JM, et al:
Methylation of TFPI2 in stool DNA: a potential novel biomarker for
the detection of colorectal cancer. Cancer Res. 69:4691–4699. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi JY, James SR, Link PA, et al:
Association between global DNA hypomethylation in leukocytes and
risk of breast cancer. Carcinogenesis. 30:1889–1897. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen WD, Han ZJ, Skoletsky J, et al:
Detection in fecal DNA of colon cancer-specific methylation of the
nonexpressed vimentin gene. J Natl Cancer Inst. 97:1124–1132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gonzalez ME, Peterson EA, Privette LM, et
al: High SEPT9_v1 expression in human breast cancer cells is
associated with oncogenic phenotypes. Cancer Res. 67:8554–8564.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harbeck N, Nimmrich I, Hartmann A, et al:
Multicenter study using paraffin-embedded tumor tissue testing
PITX2 DNA methylation as a marker for outcome prediction in
tamoxifen-treated, node-negative breast cancer patients. J Clin
Oncol. 26:5036–5042. 2008. View Article : Google Scholar
|
|
34
|
Hartmann O, Spyratos F, Harbeck N, et al:
DNA methylation markers predict outcome in node-positive, estrogen
receptor-positive breast cancer with adjuvant anthracycline-based
chemotherapy. Clin Cancer Res. 15:315–323. 2009. View Article : Google Scholar
|
|
35
|
Hrasovec S, Hauptman N, Glavac D, et al:
TMEM25 is a candidate biomarker methylated and down-regulated in
colorectal cancer. Dis Markers. 34:93–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carone BR, Fauquier L, Habib N, et al:
Paternally induced transgenerational environmental reprogramming of
metabolic gene expression in mammals. Cell. 143:1084–1096. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Papageorgiou EA, Fiegler H, Rakyan V, et
al: Sites of differential DNA methylation between placenta and
peripheral blood: molecular markers for noninvasive prenatal
diagnosis of aneuploidies. Am J Pathol. 174:1609–1618. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao F, Wang J, Liu R, et al:
Quantification and application of the placental epigenetic
signature of the RASSF1A gene in maternal plasma. Prenat Diagn.
30:778–782. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Muers M: Methylation from mother. Nat Rev
Genet. 12:62011. View
Article : Google Scholar
|
|
40
|
Wang LJ, Bai Y, Bao ZS, et al:
Hypermethylation of testis derived transcript gene promoter
significantly correlates with worse outcomes in glioblastoma
patients. Chin Med J (Engl). 126:2062–2066. 2013.PubMed/NCBI
|
|
41
|
Zheng G, Dahl JA, Niu Y, et al: ALKBH5 is
a mammalian RNA demethylase that impacts RNA metabolism and mouse
fertility. Mol Cell. 49:18–29. 2013.PubMed/NCBI
|
|
42
|
Motorin Y, Lyko F and Helm M:
5-methylcytosine in RNA: detection, enzymatic formation and
biological functions. Nucleic Acids Res. 38:1415–1430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jurkowski TP and Jeltsch A: On the
evolutionary origin of eukaryotic DNA methyltransferases and Dnmt2.
PLoS One. 6:e281042011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brzezicha B, Schmidt M, Makalowska I, et
al: Identification of human tRNA:m5C methyltransferase catalysing
intron-dependent m5C formation in the first position of the
anticodon of the pre-tRNA Leu (CAA). Nucleic Acids Res.
34:6034–6043. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stevens M, Cheng JB, Li D, et al:
Estimating absolute methylation levels at single-CpG resolution
from methylation enrichment and restriction enzyme sequencing
methods. Genome Res. 23:1541–1553. 2013. View Article : Google Scholar
|