Introduction
Chemotherapy is an indispensable component of the
comprehensive treatment of gastric cancer. Cisplatin (CDDP) is a
widely used chemotherapeutic drug for gastric cancer; however, the
resistance of gastric cancer cells to CDDP reduces its therapeutic
efficacy. Therefore, there is a need for CDDP-sensitizing agents to
enhance the effect of CDDP in the treatment of gastric cancer.
Survivin is a member of the inhibitor of apoptosis
protein family (1) that inhibits
cell apoptosis and division. The survivin gene is overexpressed in
gastric cancer cells (2), which may
be one of the main reasons for the resistance to CDDP. It was
previously demonstrated that aspirin may enhance the sensitivity of
HT-29 human colon cancer cells to 5-FU and a combination of aspirin
and 5-FU induces apoptosis of HT-29 cells in a time- and
concentration-dependent manner (3).
In the present study, we investigated whether aspirin plus CDDP
exhibited enhanced toxicity against SGC7901/CDDP cells.
Materials and methods
SGC7901 and SGC7901/CDDP cell
cultures
SGC7901 cells were obtained from the Cell Bank of
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in RPMI-1640 medium (Invitrogen Life Technologies,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 100
U/ml penicillin (North China Pharmaceutical Group Corporation,
Hebei, China) and 100 μg/ml streptomycin (North China
Pharmaceutical Group Corporation) in 75-cm2 flasks at
37°C in a humidified atmosphere of 5% CO2 and 95% air.
The pH value of the medium was adjusted to 7.2 with sterile 5.6%
NaHCO3 liquid and the medium was changed every 2–3 days.
The cells were subcultured when 80% confluence was reached.
As the SGC7901 cells grew to 80% confluence,
RPMI-1640 medium with 100 ng/ml CDDP (Qilu Pharmaceutical Co.,
Ltd., Jinan, China) was added and the medium was changed every 2–3
days. When 80% confluence was reached, the cells were subcultured
with RPMI-1640 medium to maintain good cell adhesion. As the cells
became adherent to the bottom of the cell culture flasks, RPMI-1640
medium with 200 ng/ml CDDP was added and the culture medium was
changed every 2–3 days. The method was repeated with CDDP
concentrations of 500, 700 and 1,000 ng/ml, until SGC7901/CDDP
cells were obtained. The growth and reproduction of SGC7901/CDDP
cells were maintained with RPMI-1640 medium with 1,000 ng/ml
CDDP.
The SGC7901/CDDP cells were divided into 4 groups
and treated with i) CDDP (10 μg/ml), ii) aspirin (3 mmol/l; Sigma,
St. Louis, MO, USA), iii) aspirin (3 mmol/l) plus CDDP (10 μg/ml)
and iv) physiological saline. Following incubation for 24 h, the
cells were collected with 0.25% trypsin and used in assays
measuring viability, apoptosis, survivin mRNA and survivin protein
expression.
MTT cell proliferation assay
The SGC7901/CDDP cells were cultured at a density of
5×104/ml in 96-well plates with 200 μl/well. After
adhering overnight at 37°C in a humidified 5% CO2 and
95% air atmosphere, the culture solution was aspirated and CDDP (10
μg/ml), aspirin (3 mmol/l), aspirin (3 mmol/l) plus CDDP (10 μg/ml)
and physiological saline were added to the respective groups. After
24 h, 20 μl 0.5% MTT solution was added to each well and incubated
for 4 h; the culture solution was discarded and 200 μl
dimethylsulphoxide was added to each well to dissolve the MTT
formazan crystals for 5 min. The absorbance at 490 nm was
determined by a multi-detection microplate reader (Sunrise™, Tecan
Ltd., Austria). Cell viability was calculated using the following
formula: viability=absorbance of the test group-blank/absorbance of
the normal group-blank × 100%.
Flow cytometry
The SGC7901/CDDP cells were centrifuged for 10 min
at 1,500 × g and the supernatant was discarded. The cells were
washed twice with 4°C phosphate-buffered saline (PBS) solution and
resuspended in PBS solution at a concentration of
1.0×106/l. A total of 100 μl solution was collected in
5-ml culture tubes and 5 μl propidium iodide (BD Pharmingen, San
Diego, CA, USA) were added. The cells were gently vortexed and
incubated for 15 min at room temperature in the dark. A total of
400 μl of 1X binding buffer was added to each tube. Analysis was
performed with an EPICS-XL II flow cytometer (Beckman Coulter,
Inc., Miami, FL, USA).
RNA extraction and semiquantitative
RT-PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies) and cDNA was reverse-transcribed
according to the manufacture’s instructions. The 447-bp survivin
DNA fragment was amplified using two primers synthesized by
Invitrogen Life Technologies, 5′-GCATGGGTGCCCCGACGTTG-3′ and
5′-GCTCCGGCCAGAGGCCTCAA-3′. The PCR reaction was performed in a
total volume of 20 μl containing 2 μl 10X PCR buffer, 0.8 μl
MgCl2, 1.0 μl dNTPs, 0.2 μl of each primer, 2.0 μl cDNA
and 1.0 μl Taq DNA polymerase. The amplification conditions were as
follows: denaturation at 94°C for 30 sec, annealing at 55°C for 60
sec and elongation at 72°C for 60 sec for 30 cycles. The 241-bp
β-actin fragment was amplified using two primers synthesized by
Invitrogen Life Technologies, 5′-TAAAGACCTCTATGCCAACACAGT-3′ and
5′-CACCATGGAGGGGCCGGACTCTTC-3′. The PCR reaction was performed in a
total volume of 20 μl containing 2 μl 10X PCR buffer, 1.6 μl
MgCl2, 1.0 μl dNTPs, 0.2 μl of each primer, 2.0 μl cDNA
and 1.0 μl Taq DNA polymerase. The amplification conditions were as
follows: denaturation at 94°C for 30 sec, annealing at 58°C for 40
sec and elongation at 72°C for 40 sec for 28 cycles. The PCR
products were separated on 1% agarose gels containing ethidium
bromide. The gel images were digitally recorded and analyzed by
computer-assisted image analyzer, Lab-work 4.5 analysis software
(Ultra Violet Products, Upland, CA, USA).
Western blotting
The SGC7901/CDDP cells were washed twice with 4°C
PBS. Following the addition of RIPA buffer (Invitrogen Life
Technologies), the cells were lysed on ice for 30 min and then
clarified by centrifugation at 10,000 × g for 10 min at 4°C. The
supernatants were used to assay protein concentration. A total of
25 μg protein were loaded, separated by polyacrylamide gel
electrophoresis and transferred onto PVDF membranes. The PVDF
membranes were incubated with 5% fat-free powder milk in 500 mmol/l
NaCl, 20 mmol/l Tris-HCL (pH 7.5) and 0.5% PBS-Tween-20 for 2 h at
room temperature, followed by incubation for 24 h with the
appropriate dilutions of primary antibody at 4°C in a refrigerator:
1:2,000 anti-human survivin antibodies (R&D Systems,
Minneapolis, MN, USA) and 1:500 β-actin (Wuhan Boster Biological
Technology, Ltd., Wuhan, China). Following washing with
Tris-buffered saline and Tween-20, the PVDF membranes were
incubated with 1:3,000 peroxidase-conjugated rabbit anti-goat
sencondary antibodies (Wuhan Boster Biological Technology) for 2 h
at room temperature. The proteins were visualized using
chemiluminescent peroxidase substrate (Pierce Biotechnology, Inc.,
Rockford, IL, USA) and the blots were quantified and analyzed by
computer-assisted image analyzer, Lab-work 4.5 analysis
software.
Statistical analysis
Data are expressed as means ± SD of at least three
independent experiments. One-way ANOVA was used to compare three or
more groups. All the analyses were performed with SPSS software,
version 19.0 (IBM SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
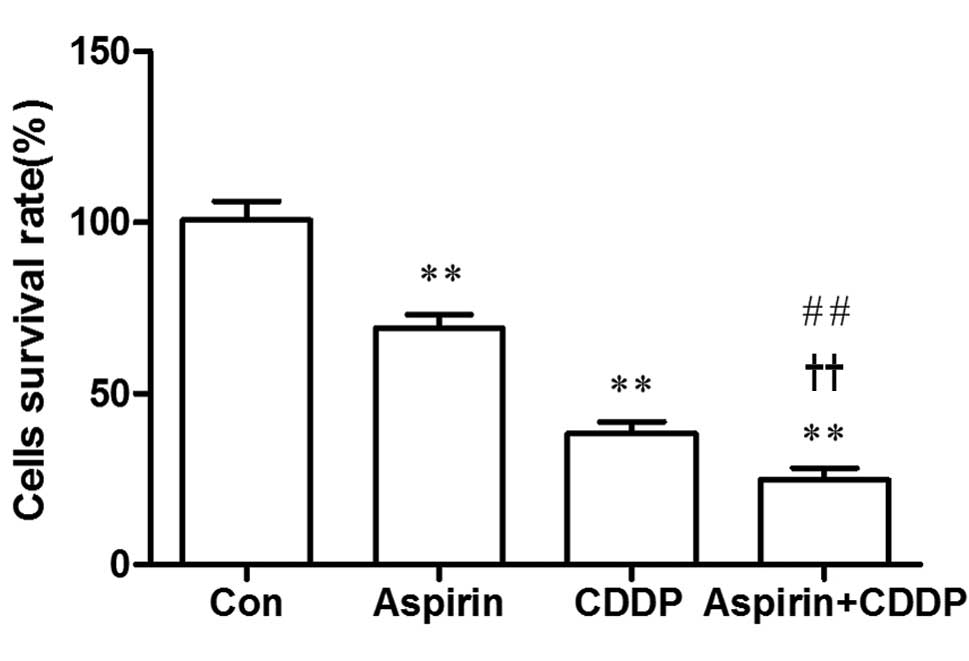
Cell viability
Compared to the control group, the survival rate of
the SGC7901/CDDP cells in the CDDP, aspirin and aspirin plus CDDP
groups were lower and the difference was statistically significant
(P<0.01). Cell growth was significantly inhibited in the aspirin
plus CDDP group and the survival rate of cells in the aspirin plus
CDDP was lower compared to that in the CDDP and in the aspirin
groups. The difference was statistically significant (P<0.01)
(Fig. 1).
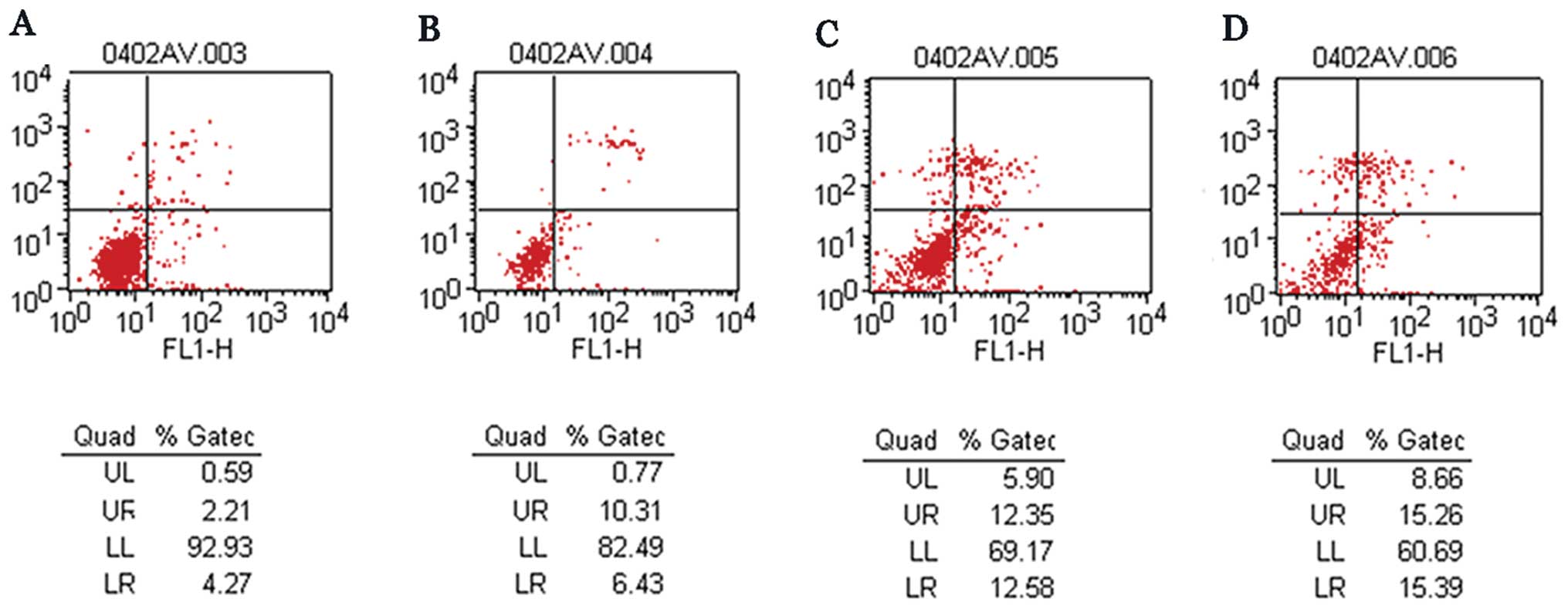
Cell apoptosis
The apoptotic rate in the aspirin, CDDP, aspirin
plus CDDP and control groups was 16.74, 24.93, 30.65 and 6.48%,
respectively. Furthermore, the apoptotic rate in the aspirin plus
CDDP group was significantly higher compared to that in the other
groups (Fig. 2).
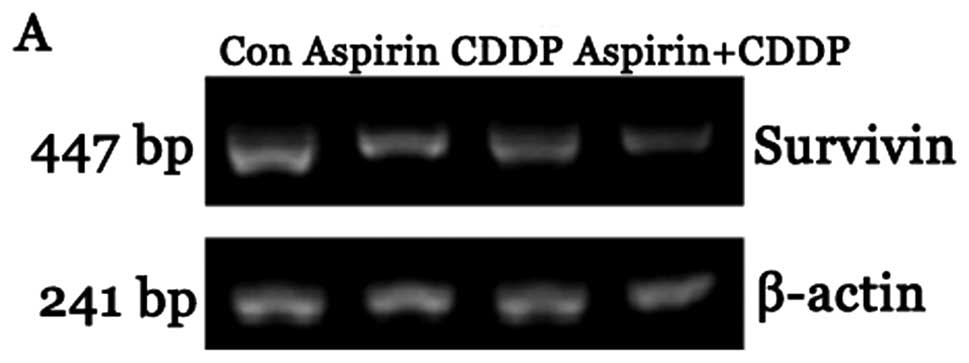
Survivin mRNA expression
The expression of survivin mRNA in the CDDP and
aspirin plus CDDP groups was significantly reduced compared to that
in the control group. The difference was statistically significant
(P<0.05). Furthermore, the expression of survivin mRNA in the
aspirin plus CDDP was significantly reduced compared to that in the
aspirin and CDDP alone groups. The difference was statistically
significant (P<0.05) (Fig.
3).
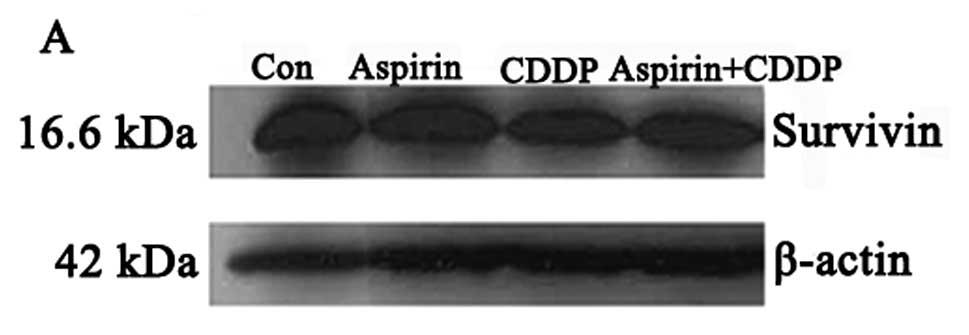
Survivin protein expression
The expression of survivin protein in the CDDP and
aspirin plus CDDP groups was significantly reduced compared to that
in the control group. The difference was statistically significant
(P<0.05). Furthermore, the expression of survivin protein in the
aspirin plus CDDP group was significantly reduced compared to that
in the aspirin and CDDP alone groups. The difference was
statistically significant (P<0.05) (Fig. 4).
Discussion
Gastric cancer is associated with high morbidity and
mortality. Approximately 60% of gastric cancer patients in western
countries present with advanced-stage disease (4), which is also the case for gastric
cancer patients in China (5).
Surgery and chemotherapy are currently the mainstay of treatment
for patients with advanced gastric cancer. CDDP is one of the most
widely used chemotherapeutic agents for the treatment of gastric
cancer. However, resistance to CDDP is a major cause of ineffective
treatment; therefore, there is a need for CDDP-sensitizing agents
to improve the effects of chemotherapy.
Non-steroidal anti-inflammatory drugs (NSAIDs) are
widely used in clinical practice due to their antipyretic,
analgesic, anti-inflammatory and antirheumatic properties. The
antitumor effect of NSAIDs has been extensively investigated
(6–8). Li et al (9) demonstrated that regular NSAID
administration may reduce the incidence of colon cancer by 50% and
also reduce the incidence of esophageal and gastric cancer. The
apoptosis of cancer cells induced by aspirin may be the mechanism
through which aspirin interferes with esophageal carcinogenesis and
may be indicative of the potential of NSAIDs as chemopreventive
agents in esophageal cancer.
NCX-4016 (a derivative of aspirin containing a nitro
group that releases nitric oxide in a sustained fashion for several
hours in cells and in vivo) combined with CDDP was shown to
sensitize drug-resistant strains of human ovarian cancer cells to
CDDP. Furthermore, the inhibitory effect of CDDP plus NCX-4016 on
drug-resistant strains of human ovarian cancer cells was
significantly higher compared to that of CDDP and NCX-4016 alone,
indicating that NCX-4016 may enhance the sensitivity of
drug-resistant strains of human ovarian cancer cells to CDDP and
may specifically eliminate CDDP-refractory cancer cells in patients
with recurrent ovarian cancer (10). Kumar and Singh (11) suggested that pre-exposure of tumor
cells to aspirin may lower the concentration of CDDP required to
exert its cytotoxic effects. This finding may help design novel
antitumor protocols with reduced doses of CDDP. Those studies
indicate a novel method for overcoming CDDP resistance in the
treatment of patients with gastric cancer.
Nakamura et al (12) reported a negative correlation
between survivin expression in gastric cancer cells and the
survival time of patients with gastric cancer receiving CDDP
chemotherapy. Those results indicated that survivin may be pivotal
in the development of gastric cancer and resistance to CDDP and,
therefore, controlling the expression of the survivin gene may be
useful in the chemotherapy of gastric cancer, a hypothesis also
supported by other studies (13,14).
The abovementioned results indicated that the survivin gene is
closely associated with resistance to CDDP in the chemotherapy of
gastric cancer.
In our experiments, the results of the MTT assay
demonstrated that the cell survival rate in the aspirin plus CDDP
group was significantly reduced. The flow cytometry test results
revealed that the apoptotic rate in the aspirin plus CDDP group was
significantly higher compared to that in the other groups. In
addition, the RT-PCR and western blotting results revealed that the
expression of survivin mRNA and protein were significantly reduced
in the aspirin plus CDDP group. Taken together, these experimental
results indicate that aspirin plus CDDP may exhibit significantly
enhanced toxicity against SGC7901/CDDP cells compared to aspirin or
CDDP alone, possibly through reducing survivin expression and
inducing the apoptosis of SGC7901/CDDP cells.
Related research demonstrated that NSAIDs may induce
the apoptosis of tumor cells through a COX-2 non-dependent pathway
and exert antitumor effects (15,16).
Shao et al (17) suggested
that the inhibition of NFκB activity is a plausible mechanism for
apoptosis induced by the wild-type p53 gene transfer in human colon
cancer cells and that anti-NFκB reagent aspirin may render these
cells more susceptible to apoptosis. Adachi et al (18) suggested that increased ROS
generation is one of the key mechanisms underlying the
NSAID-mediated anticancer effects on various types of cancer cells.
Oh et al (19) reported that
the enhancement of mitochondrial permeability transition-dependent
apoptosis by salicylates may be the mechanism underlying the
protective effect of aspirin and other NSAIDs against colon, lung
and breast cancers. Pathi et al (20) also suggested that the anticancer
activity of aspirin may be due to its salicylate metabolite.
Our results were obtained by aspirin (3 mmol/l) plus
CDDP (10 μg/ml) acting on SGC7901/CDDP cells for 24 h. However,
further investigation is required to determine whether the
increased toxicity is dose- and time-dependent and whether there
are additional mechanisms underlying the increased toxicity
exhibited by aspirin plus CDDP against SGC7901/CDDP cells.
References
|
1
|
Johnson ME and Howerth EW: Survivin: a
bifunctional inhibitor of apoptosis protein. Vet Pathol.
41:599–607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ashktorab H, Dawkins FW, Mohamed R, et al:
Apoptosis induced by aspirin and 5-fluorouracil in human colonic
adenocarcinoma cells. Dig Dis Sci. 50:1025–1032. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin DM, Bray F, Ferlay J, et al: Global
cancer statistics 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
5
|
He J, Gu D, Wu X, et al: Major causes of
death among men and women in China. N Engl J Med. 353:1124–1134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levy GN: Prostaglandin H synthases,
nonsteroidal anti-inflammatory drugs and colon cancer. FASEB J.
11:234–247. 1997.PubMed/NCBI
|
|
7
|
Yang VW, Shields JM, Hamilton SR, et al:
Size-dependent increase in prostanoid levels in adenomas of
patients with familial adenomatous polyposis. Cancer Res.
58:1750–1753. 1998.PubMed/NCBI
|
|
8
|
Marks F and Furstenberger G: Cancer
chemoprevention through interruption of multistage carcinogenesis.
The lessons learnt by comparing mouse skin carcinogenesis and human
large bowel cancer. Eur J Cancer. 36:314–329. 2000. View Article : Google Scholar
|
|
9
|
Li M, Lotan R, Levin B, et al: Aspirin
induction of apoptosis in esophageal cancer: a potential for
chemoprevention. Cancer Epidemiol Biomarkers Prev. 9:545–549.
2000.PubMed/NCBI
|
|
10
|
Bratasz A, Weir NM, Parinandi NL, et al:
Reversal to cisplatin sensitivity in recurrent human ovarian cancer
cells by NCX-4016, a nitro derivative of aspirin. Proc Natl Acad
Sci USA. 103:3914–3919. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar A and Singh SM: Priming effect of
aspirin for tumor cells to augment cytotoxic action of cisplatin
against tumor cells: implication of altered constitution of tumor
microenvironment, expression of cell cycle, apoptosis, and survival
regulatory molecules. Mol Cell Biochem. 371:43–54. 2012. View Article : Google Scholar
|
|
12
|
Nakamura M, Tsuji N, Asanuma K, et al:
Survivin as a predictor of cis-diamminedichloroplatinum
sensitivity in gastric cancer patients. Cancer Sci. 95:44–51.
2004.
|
|
13
|
Daly C, Wong V, Burova E, et al:
Angiopoietin-1 modulates endothelial cell function and gene
expression via the transcription factor FKHR (FOXO1). Genes Dev.
18:1060–1071. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wall NR, O’Connor DS, Plescia J, et al:
Suppression of survivin phosphorylation on Thr34 by flavopiridol
enhances tumor cell apoptosis. Cancer Res. 63:230–235.
2003.PubMed/NCBI
|
|
15
|
Ding H, Han C, Zhu J, et al: Celecoxib
derivatives induce apoptosis via the disruption of mitochondrial
membrane potential and activation of caspase 9. Int J Cancer.
113:803–810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jendrossek V, Handrick R and Belka C:
Celecoxib activates a novel mitochondrial apoptosis signaling
pathway. FASEB J. 17:1547–1549. 2003.PubMed/NCBI
|
|
17
|
Shao J, Fujiwara T, Kadowaki Y, et al:
Overexpression of the wild-type p53 gene inhibits NF-kappaB
activity and synergizes with aspirin to induce apoptosis in human
colon cancer cells. Oncogene. 19:726–736. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adachi M, Sakamoto H, Kawamura R, et al:
Nonsteroidal anti-inflammatory drugs and oxidative stress in cancer
cells. Histol Histopathol. 22:437–442. 2007.PubMed/NCBI
|
|
19
|
Oh KW, Qian T, Brenner DA, et al:
Salicylate enhances necrosis and apoptosis mediated by the
mitochondrial permeability transition. Toxicol Sci. 73:44–52. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pathi S, Jutooru I, Chadalapaka G, et al:
Aspirin inhibits colon cancer cell and tumor growth and
downregulates specificity protein (Sp) transcription factors. PLoS
One. 7:e482082012. View Article : Google Scholar : PubMed/NCBI
|