Introduction
Sericin protein from the cocoon of silkworm
(Bombyx mori) is regarded as the resistant protein against
digestion together with buckwheat and soybean proteins (1–3).
Sericin is a type of protein created by silkworms in the production
of silk. Silk emitted by the silkworm consists of two main
proteins, sericin and fibroin, with fibroin being the structural
center of the silk, and sericin being the sticky material
surrounding it. Notably, during the production of silk cloth, the
sericin protein is discarded.
In a previous study, we reported the dyeing property
of silkworm cocoon (4,5). Lipid oxidation on vegetable oil and
frying oil using the indicators peroxide value (POV), carbonyl
value (COV) and anisidin value (AnV) has been previously identified
(6,7). Additionally, recent studies have
focused on the antioxidative activity of seafood using oxygen
radical absorbance capacity (ORAC), hydroxyl radical averting
capacity (HORAC) and electron spin resonance (ESR) methods
(8,9).
By contrast, Dash et al showed that sericin
protein from silkworm inhibited UVB-induced apoptosis in human skin
keratinocytes (10), and that the
antioxidant potential of the protein against hydrogen
peroxide-induced oxidative stress in skin fibroblasts using the
indicators of catalase, lactate dehydrogenase and malondialdehyde
activities (11). Zhaorigetu et
al demonstrated that sericin protein exerts a suppressant
effect on skin cancer (12) and
colon cancer (13), while Li et
al demonstrated a protective effect of sericin and sericin
peptide against alcohol-induced liver and gastric injuries in mice
(14,15). Aramwit et al (16) and Ohnishi et al (17) identified a cell-protective effect of
sericin on collagen and nitric oxide, and cryopreserved rat islets,
respectively. Additionally, Manosroi et al (18) and Jin-Bo et al (19) identified the antioxidant and
tyrosinase inhibition activity of sericin protein extracted from
silkworm.
Efforts have been made to determine whether the
utilization of sericin proteins extracted from cocoon of silkworm
(Bombyx mori) may be beneficial for human consumption. One
of the physico-chemical properties of sericin is its antioxidant
activity (20). Cocoon shells vary
in color, i.e., white, green, yellow and red. The color components
coexist and accumulate in the sericin layer of cocoons. Among
different colored cocoons, yellow-green cocoon shells contain
various flavonol compounds with antioxidant activity (21). In this study, we investigated the
antioxidant activities of different types of sericin proteins
extracted from the shell of the cocoon, designated hereafter as
white sericin protein and yellow-green sericin protein, using the
1,1-Diphenyl-2-picrylhydrazyl (DPPH), chemiluminescence, ORAC and
ESR methods. Furthermore, we examined whether or not the
antioxidant activity of bread was elevated by the addition of
sericin protein.
Materials and methods
Samples
White sericin and yellow-green sericin powder was
used. Sericin powder was also added to bread, while additive-free
bread served as the control. White sericin powder was purchased
from Kashiro Sangyo Co. Ltd. (Shiga, Japan) and yellow-green
sericin powder was purchased from Shiono-ya (Kyoto, Japan). Bread
was used as an example of processed food to which sericin powder
was added.
Preparation of the bread
Ingredients including wheat flour (Camellia, Nissin
Flour Milling, Tokyo, Japan), sericin, dry yeast (Super Camellia,
Nissin Foods Group, Tokyo, Japan) and butter (Yotsuba Milk Products
Co., Ltd., Hokkaido, Japan) were placed in a bread machine
(National Panasonic, SD-BM101, Osaka, Japan) (Table I). Bread was made in the preset
standard course (4 h).
 | Table IBasic bread formulations. |
Table I
Basic bread formulations.
| Ingredient | Amount (g) |
|---|
| Wheat flour | 200 |
| Sericin | 8 |
| Sugar | 13.6 |
| Salt | 4 |
| Dry yeast | 2.2 |
| Butter | 8 |
| Water (tap
water)a | 144 |
Sample preparation
To measure the antioxidant activity, each sample (5
g) was homogenized with 5 ml water using a mortar for 2 min. Then,
15 ml of water was added and the mixture was homogenized for 1 min.
The sample solutions were centrifuged at 15,000 × g for 15 min. The
supernatant was removed and the sample was frozen at −30°C as the
stock sample solutions.
Chemicals
DPPH, tris (hydroxymethyl) aminomethane (Tris),
2,2′-Azobis (2-amidinopropane) dihydrochloride (AAPH), potassium
dihydrogenphosphate (KH2PO4), dipotassium
hydrogenphosphate (K2HPO4), ethanol (99.5%),
methanol (HPLC grade), hydrochloric acid, sodium
dihydrogenphosphate (NaH2PO4), disodium
hydrogenphosphate (Na2HPO4) and sodium
tetraborate were purchased from Wako Pure Chemical Industries, Ltd.
(Osaka, Japan). Fluorescein sodium salt was purchased from
Sigma-Aldrich Japan (Tokyo, Japan), and
6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox)
was purchased from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan).
Luminol and cytochrome c from horse heart were obtained from
Nacalai Tesque (Kyoto, Japan).
Measurement of DPPH radical scavenging
activity
Measurement of DPPH radical scavenging activity for
all fractions was performed using the method by Yamaguchi et
al (22). Briefly, an aliquot
of antioxidant solution (200 μl) was mixed with 100 mM Tris-HCl
buffer (pH 7.4, 800 μl) and added to 1 ml of 500 μM DPPH in EtOH.
The mixture was agitated vigorously, left to stand for 20 min at
room temperature in the dark, and then subjected to reverse-phase
high performance liquid chromatography (HPLC) analysis. The HPLC
equipment consisted of a Shimadzu LC-6A pump, a Rheodyne injector
fitted with a 20 μl loop and a Shimadzu SPD-6AV UV-VIS detector set
at 517 nm (0.064 AUFS). Analyses were conducted in a TSKgel
Octyl-80Ts column (4.6×250 mm, Tosoh, Tokyo, Japan) at room
temperature with a mobile phase of MeOH/H2O (7:3, v/v)
at a flow rate of 0.8 ml/min. The DPPH radical-scavenging activity
was evaluated from the difference in peak area decrease of the DPPH
radical detected at 517 nm between a blank and a sample. Trolox was
used as a control standard. The data were expressed as mmol of
Trolox equivalent per 100 ml of each sericin sample.
Chemiluminescence experiment
procedure
The chemiluminescence method has been described in
detail previously (23). Briefly,
the AAPH (40 mM) reagent was dissolved in 100 mM phosphate buffer
(pH 7.0). The sericin sample solutions were also diluted to the
appropriate concentration using the same buffer. The AAPH solution
was heated at 37°C for 2 min to generate peroxyl radicals. The AAPH
solution (0.2 ml) was then mixed with 0.2 ml phosphate buffer as
the control or with 0.2 ml diluted sericin sample, and the solution
mixture was then heated at 37°C for 2 min. For exposure to heating,
a water bath (Thermo Max TM-1, AS ONE Corporation, Osaka, Japan)
was used. The temperature was maintained within ±3°C. Immediately
after heating, 0.2 ml luminol solution was added to the mixture for
chemiluminescence measurement. For the luminol solution, luminol
(0.113 mM) and cytochrome c (0.004 mM) were dissolved in a
mixture of 100 mM sodium tetraborate buffer (pH 9.28), water and
methanol (volume ratio, 9:1:30). Final concentrations of AAPH,
luminol and cytochrome c were 13.333, 0.038 and 0.001 mM,
respectively. Chemiluminescence intensity was measured using a
photon counter Lumitester C-110 (Kikkoman Co., Tokyo, Japan), where
one relative light unit (RLU) represented ~43 photons/sec.
Calculation of the IC50 of
peroxyl radical scavenging
As an indicator of antioxidative capacity, the
inhibition of chemiluminescence intensity was measured by the
change of RLU value. The lower the RLU value the more inhibition of
chemiluminescence intensity. The value of IC50 was
defined as the concentration of the sericin sample that reduced the
RLU value of the phosphate buffer (control) to half. First, the
antioxidative value was calculated using the formula: (log Io/I) ×
100, where Io is the RLU value of the phosphate buffer as the
control, and I is the RLU value of each concentration of the
sericin sample. When the value of this formula was 30.103, the I
value corresponded to half-inhibition.
Preparation of hydrophilic-oxygen radical
absorbance capacity (H-ORAC) reaction solution
H-ORAC reaction solution was prepared as previously
described (9). Briefly, phosphate
buffer was used as the assay (control) buffer and was prepared by
combining 75 mM K2HPO4 and 75 mM
KH2PO4 to a final volume of 75 μmol (pH 7.0).
The AAPH reagent was dissolved in the buffer at a concentration of
31.7 mM. Fluorescein working solution was prepared at a
concentration of 94.4 nM by dissolving fluorescein sodium salt in
the buffer. Trolox standard solutions were prepared at
concentrations of 100, 50, 25, 12.5 and 6.25 μM by dissolving
Trolox in the buffer.
Measurement of H-ORAC
The ORAC value was obtained by measuring the
elimination capacity of peroxyl radicals generated by the AAPH
reagent and by measuring the time lapse degradation of fluorescein
(i.e., the rate of decrease in the intensity of fluorescence). The
ORAC assay was performed using a 96-well Mithras LB940 multimode
microplate reader (Berthold Technologies GmbH & Co. KG, Bad
Wildbad, Germany) as previously described (9). Briefly, 20 μl of each sample buffer
(obtained by appropriate dilution with the assay buffer) and
various concentrations of trolox standard solution (for
concentration of a standard curve) or blank buffer (as a control)
were placed in the individual wells of a 96-well transparent
microplate (Sanplatec Corp., Osaka, Japan). Fluorescein working
solution (200 μl) was added and the wells were agitated at 37°C for
10 min. Subsequently, 75 μl of AAPH solution was added to each of
the wells to initiate the reaction. The total volume of each
reaction solution was 295 μl. The fluorescence intensity [485 nm
(excitation)/535 nm (emission)] was then measured every 2 min over
90 min at pH 7.4 and 37°C. As the reaction progressed, fluorescein
was consumed and the fluorescence intensity was decreased. The
inhibition of fluorescence decay was considered to indicate the
presence of an antioxidant.
Typical ORAC assay kinetic curves in the presence of
various concentrations of trolox were determined. ORAC values were
then measured. The area under the kinetic curve (AUC) of the
standards and samples was calculated as: AUC = (0.5 + f10
min/f8 min + f12 min/f8 min
+ f14 min/f8 min + ··· + f90
min/f8 min) × 2, where fx min is the
fluorescence reading at cycle × min (23).
The standard regression line was obtained by
plotting the trolox concentrations against the net
AUCtrolox of each concentration: Net
AUCtrolox = AUCtrolox −
AUCcontrol, and Net AUCsample =
AUCsample − AUCcontrol; where
AUCtrolox is the AUC in the presence of trolox;
AUCcontrol is the AUC with blank control and
AUCsample is the AUC with sample buffer. The horizontal
axis is the net AUCtrolox and the vertical axis is the
concentration of trolox. The equation Y = ax + b was derived from
the above data and the values for a and b were obtained.
The final ORAC values of the samples were calculated
using the equation: ORAC value (μmol trolox equivalent/100 g) = [a
× (net AUCsample)] × 100/[sample], where [sample] is the
diluted concentration ratio of the sample. Data were presented
using Microsoft Excel.
Electron spin resonance analysis
ESR was conducted as previously described (8). Briefly, hydroxyl radical generation
was first examined by the DMPO method and iron (II) sulfate with or
without the fish sauce samples. The addition of 8.8 mM
H2O2 (50 μl) to the reaction mixture (320 μl)
was used to initiate Fenton’s reaction as depicted in the chemical
equation: Fe2+ + H2O2 →
Fe3+ + ·OH + OH−. After l min of hydroxyl
radical generation, spin adduct DMPO-OH· was measured using the ESR
spectrometer (JES-FR30: JEOL Ltd., Tokyo, Japan). ESR measurement
conditions were: output, 4 mW (9.4 GHz); magnetic field, 342.790±5
mT; modulation amplitude, 0.079 mT; time constant, 0.1 sec;
sweeping time, l min; amplification ratio, 32–125.
Calculation of the IC50 of
hydroxyl radical scavenging
IC50 values were defined as the
concentration of each sericin sample that reduced the control peak
height ratio of ESR (generation of hydroxyl radical) by half. The
antioxidative value was calculated using the formula: (log Io/I) ×
100, where Io is the ESR peak height ratio as the control, and I is
the ESR peak height ratio as a sample. Thus, the IC50
value was the concentration of samples at Io/I = 1/2, calculated
from the antioxidant results of ESR obtained in the
experiments.
Statistical analysis
The Fisher’s exact test, Student’s t-test and
correlation analysis were performed using Microsoft excel.
Results and Discussion
Measurement of radical scavenging
activity by the DPPH-HPLC method
Measured results are shown in Table II. DPPH is a stable,
artificial-free radical. Yamaguchi et al reported that the
DPPH radical scavenging activities of onion, broccoli, and burdock
were 104, 890, and 3685 μmol Trolox eq./100 g, respectively
(24,25). When compared with these values,
yellow-green and white sericin proteins were 23.7 and 18.2 mmol
Trolox eq./100 g, respectively, indicating high DPPH radical
scavenging activities. The value of yellow-green sericin was
significantly higher than that of white sericin. Higher DPPH
radical scavenging activity was presumably caused by the additional
effect of flavonoid pigment contained in yellow-green sericin.
Thus, sericin proteins may demonstrate a high DPPH radical
scavenging activity.
 | Table IIAntioxidant activity of sericin
measured by the DPPH-HPLC method. |
Table II
Antioxidant activity of sericin
measured by the DPPH-HPLC method.
| Sample | Radical-scavenging
activity)* (mmol Trolox eq./100
g) |
|---|
| White sericin | 18.2±0.7a |
| Yellow-green
sericin | 23.7±0.4a |
| Bread (no
sericin) | 3.1±0.5 |
| Bread (added to
sericin powder) | 3.3±1.4 |
Measurement of peroxyl radical scavenging
activity by chemiluminescence method
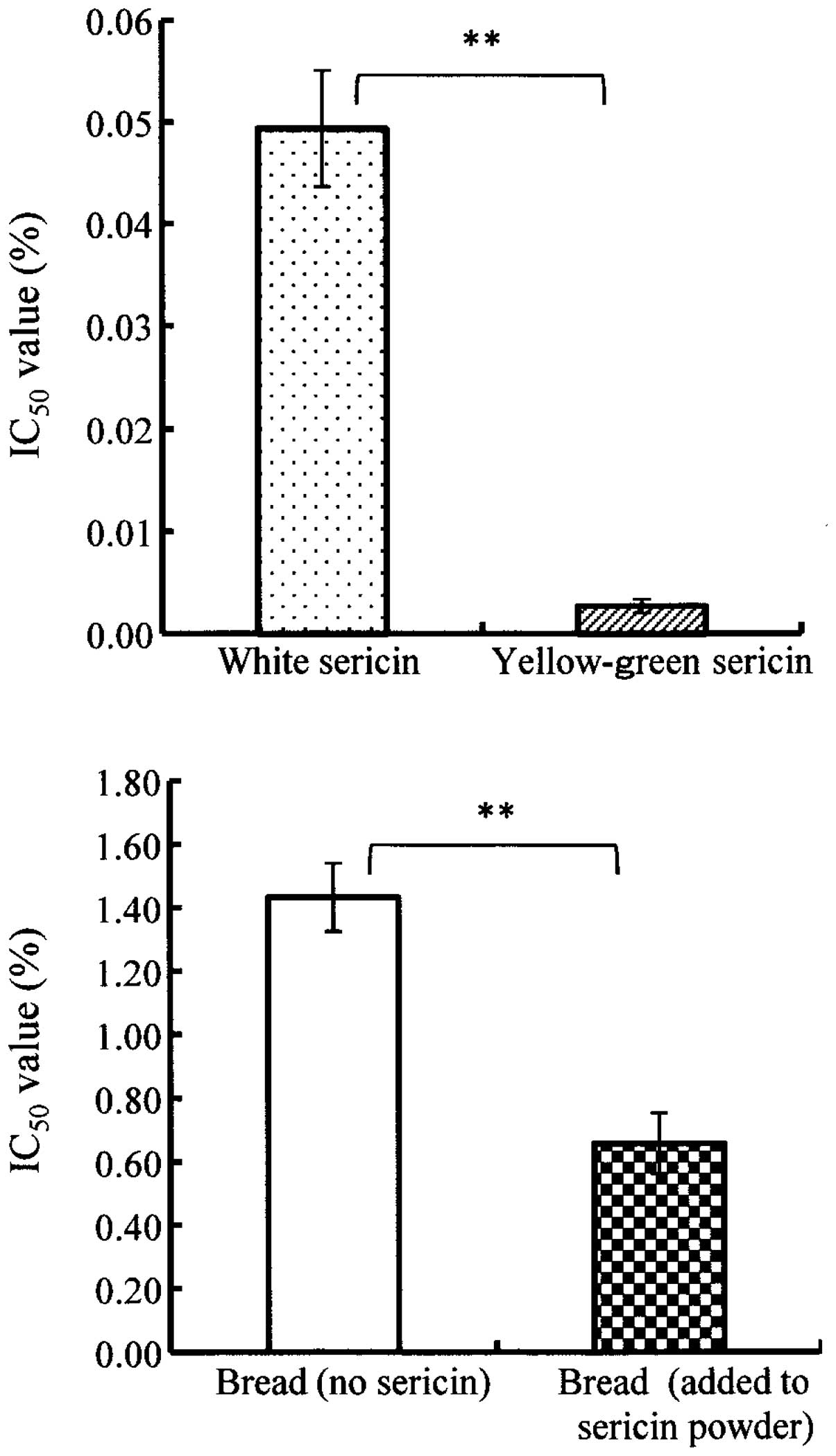
Measured results are shown in Fig. 1. The yellow-green and white sericin
proteins indicated high peroxyl radical scavenging activities.
Nagao et al reported that the IC50 value of the
peroxyl radical scavenging activity was 0.1% in kale, which is
known to exhibit a high antioxidative property (water-extracted
sample) (26). Specifically, the
antioxidant activity of yellow-green sericin protein < 0.01% of
the IC50 value was significantly higher than that of the
white sericin protein of ~0.05% of IC50 value
(p<0.01). In the same manner as the DPPH radical scavenging
activity, it is considered that the higher peroxyl radical
scavenging activity was caused by additional effect of flavonoid
pigment contained in yellow-green sericin. In addition, bread with
sericin powder had significantly high peroxyl radical scavenging
activity when compared with bread without sericin powder
(p<0.01). Improvement of peroxyl radical scavenging activity was
observed by the addition of sericin (~4% of bread powder).
Measurement of peroxyl radical
eliminating activity by the ORAC method
As shown in Table
III, the peroxyl radical eliminating activity was measured by
the ORAC method. It has been reported by Yamaguchi et al
(25) that peroxyl radical
eliminating activities of burdock, ginger, and komatsuna (Japanese
mustard spinach) were 7.5, 4.8, and 12.3 mmol Trolox eq./100 g,
respectively, and the activity of kamaboko was also reported by
Harada et al (9) as 0.166
mmol Trolox eq./100 g. Particularly, the value of yellow-green
sericin protein (29.3 mmol Trolox eq./100 g) was significantly
higher than that of the white sericin protein (10.0 mmol Trolox
eq./100 g) (p<0.05). Similar to the DPPH and peroxyl radical
scavenging activities, higher peroxyl radical eliminating activity
was presumably the result of flavonoid pigment contained in
yellow-green sericin protein.
 | Table IIIAntioxidant activity of sericin
measured by the ORAC method. |
Table III
Antioxidant activity of sericin
measured by the ORAC method.
| Sample | ORAC value* (mmol Trolox eq./100 g) |
|---|
| White sericin | 10.0±0.4a |
| Yellow-green
sericin | 29.3±4.0a |
| Bread (no
sericin) | 0.1±0.0a |
| Bread (added to
sericin powder) | 0.4±0.0a |
Measurement of hydroxyl radical
scavenging activity by the ESR method
As shown in Fig. 2,
the hydroxyl radical scavenging activity was measured by the ESR
method. The yellow-green and white sericin proteins indicated high
antioxidant activity wherein the activity of white sericin protein
was higher than that of yellow-green sericin protein although there
was no significant difference. By using all the above-mentioned
methods, yellow-green sericin indicated higher values. Based on
these results, it is assumed that the antioxidant capacity of
flavonoid pigment may not be involved in the elimination of
hydroxyl radicals. Additionally, bread with sericin powder had
significantly high hydroxyl radical scavenging activity when
compared with bread without sericin. As for the hydroxyl radical
scavenging activity, the improvement of hydroxyl radical scavenging
activity was observed by the addition of sericin (~4 % of bread
powder) (Fig. 2).
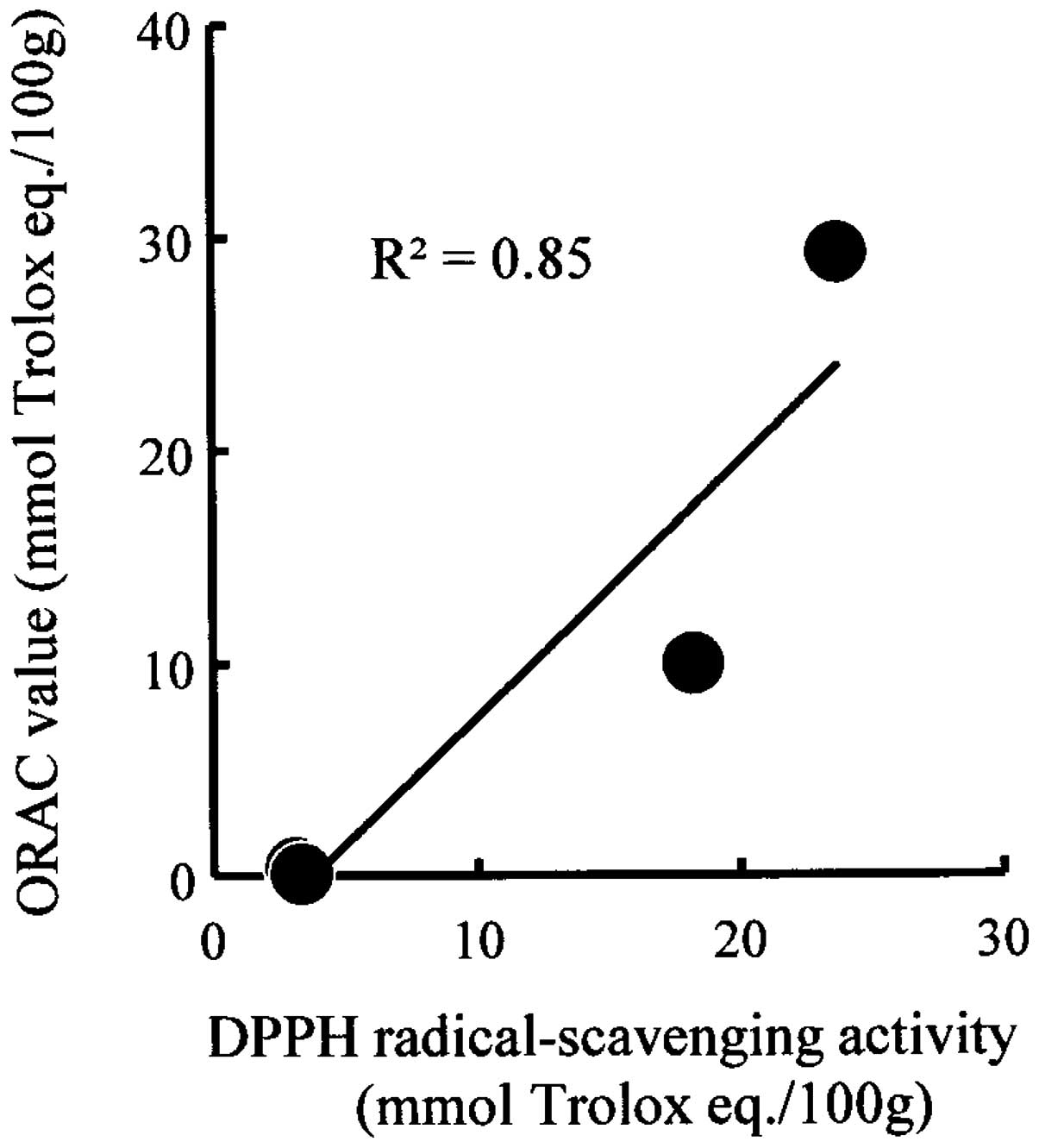
Correlation between DPPH radical
scavenging activity and ORAC value
Based on the values obtained from the four types of
samples, the correlation coefficient between the DPPH radical
scavenging activity and ORAC value was calculated (Fig. 3). As shown in Fig. 3, a positive correlation of
R2=0.85 was observed. Conflicting reports have concluded
that DPPH and ORAC methods have no correlation (27) or that the methods are correlated
(28). By contrast, a positive
correlation was observed in the case of sericin proteins and bread
to which sericin powder was added.
Antioxidant property of sericin
proteins
Antioxidative mechanisms of sericin proteins, as
well as the scavenging and eliminating components remain to be
elucidated. Kato et al hypothesized that the scavenging
function may be provided by the chelating effect of hydroxyl groups
of hydroxyamino acids (serine and threonine) that are abundantly
contained in sericin (20). Sericin
has clearly demonstrated antioxidant capacities against multiple
radicals through the measurement of radical scavenging
(eliminating) activities by four different methods in this study.
Thus, sericin proteins may be efficiently utilized as beneficial
for human consumption.
Acknowledgements
The authors are grateful to Associated Professor
Hiroshi Sakai (Department of Materials Chemistry and
Bioengineering, Oyama National College of Technology) for his kind
instructions, advice and discussion.
References
|
1
|
Sasaki M, Yamada H and Kato N: A resistant
protein, sericin improves atropine-induced constipation in rats.
Food Sci Technol Res. 6:280–283. 2000. View Article : Google Scholar
|
|
2
|
Kato N, Kayashita J and Sasaki M:
Physiological functions of buckwheat protein and sericin as
resistant proteins. J Jpn Soc Nutr Food Sci. 53:71–75. 2000.(In
Japanese).
|
|
3
|
Higaki N, Sato K, Suda H, Suzuka T, Komori
T, Saeki T, Nakamura Y, Ohtsuki K, Iwami K and Kanamoto R: Evidence
for the existence of soybean resistant protein that captures bile
acid and stimulates its fecal excretion. Biosci Biotechnol Biochem.
70:2844–2852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sakai H, Ohsawa K, Takechi T, Hattori Y,
Mizutani C and Yamazaki T: Dyeing property of silkworm cocoon. J
Textile Eng. 55:67–71. 2009. View Article : Google Scholar
|
|
5
|
Sakai H, Ohsawa K, Takechi T, Hattori Y,
Mizutani C and Yamazaki T: Dyeing of silkworm cocoon using acid
dye. J Textile Eng. 55:125–128. 2009. View Article : Google Scholar
|
|
6
|
Takechi T, Takamura H and Matoba T:
Application of colorimetric method for determination of lipid
peroxides in foods. Food Sci Technol Res. 10:460–463. 2004.
View Article : Google Scholar
|
|
7
|
Research group on frying-cooking group,
Kinki branch office, The Japan Society of Cookery Science.
Characteristics of frying oil reaching its usable life with a
flavor score of 3. J Cookery Sci Jpn. 43:38–43. 2010.(In
Japanese).
|
|
8
|
Harada K, Maeda T, Hasegawa Y, Tokunaga T,
Tamura Y and Koizumi T: Antioxidant activity of fish sauces
including puffer (Lagocephalus wheeleri) fish sauce measured
by the oxygen radical absorbance capacity method. Mol Med Rep.
3:663–668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harada K, Wada R, Yaguchi S, Maeda T, Date
R, Tokunaga T, Kazumura K, Shimada K, Matsumoto M, Wako T,
Yamaguchi N and Shigyo M: Supplementation with Japanese bunching
onion (Allium fistulosum L.) expressing a single alien
chromosome from shallot increases the antioxidant activity of
Kamaboko fish jelly paste in vitro. Biomed Rep. 1:355–358.
2013.PubMed/NCBI
|
|
10
|
Dash R, Mandal M, Ghosh SK and Kundu SC:
Silk sericin protein of tropical tasar silkworm inhibits
UVB-induced apoptosis in human skin keratinocytes. Mol Cell
Biochem. 311:111–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dash R, Acharya C, Bindu PC and Kundu SC:
Antioxidant potential of silk protein sericin against hydrogen
peroxide-induced oxidative stress in skin fibroblasts. BMB Rep.
31:236–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhaorigetu S, Yanaka N, Sasaki M, Watanabe
H and Kato N: Silk protein, sericin, suppresses DMBA-TPA-induced
mouse skin tumorigenesis by reducing oxidative stress, inflammatory
responses and endogenous tumor promoter TNF-α. Oncol Rep.
10:537–543. 2003.PubMed/NCBI
|
|
13
|
Zhaorigetu S, Sasaki M, Watanabe H and
Kato N: Supplemental silk protein, sericin, suppresses colon
tumorigenesis in 1,2-dimethylhydrazine-treated mice by reducing
oxidative stress and cell proliferation. Biosci Biotechnol Biochem.
65:2181–2186. 2001. View Article : Google Scholar
|
|
14
|
Li YG, Ji DF, Chen S and Hu GY: Protective
effects of sericin protein on alcohol-mediated liver damage in
mice. Alcohol Alcohol. 43:246–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li YG, Ji DF, Lin TB, Zhong S, Hu GY and
Chen S: Protective effect of sericin peptide against
alcohol-induced gastric injury in mice. Chin Med J (Engl).
121:2083–2087. 2008.PubMed/NCBI
|
|
16
|
Aramwit P, Kanokpanont S, De-Eknamkul W,
Kamei K and Srichana T: The effect of sericin with variable
amino-acid content from different silk strains on the production of
collagen and nitric oxide. J Biomater Sci Poly Ed. 20:1295–1306.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohnishi K, Murakami M, Morikawa M and
Yamaguchi A: Effect of the silk protein sericin on cryopreserved
rat islets. J Hepatobiliary Pancreat Sci. 19:354–360. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manosroi A, Boonpisuttinant K, Winitchai
S, Manosroi W and Manosroi J: Free radical scavenging and
tyrosinase inhibition activity of oils and sericin extracted from
Thai native silkworm (Bombyx mori). Pharm Biol. 48:855–860.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan J-B, Wu L-P, Chen L-S, Mao X-Y and Ren
F-Z: Antioxidant activities of silk sericin from silkworm Bombyx
mori. J Food Biochem. 33:74–88. 2009. View Article : Google Scholar
|
|
20
|
Kato N, Sato S, Yamanaka A, Yamada H, Fuwa
N and Nomura M: Silk protein, sericin, inhibits lipid peroxidation
and tyrosinase activity. Biosci Biotechnol Biochem. 62:145–147.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takechi T, Maekawa Z and Sugimura Y: Use
of sericin as an ingredient of salad dressing. Food Sci Technol
Res. 17:493–497. 2011. View Article : Google Scholar
|
|
22
|
Yamaguchi T, Takamura H, Matoba T and
Terao J: HPLC method for evaluation of the free radical-scavenging
activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci
Biotechnol Biochem. 62:1201–1204. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harada K, Okano C, Kadoguchi H, Okubo Y,
Ando M, Kitao S and Tamura Y: Peroxyl radical scavenging capability
of fish sauces measured by the chemiluminescence method. Int J Mol
Med. 12:621–625. 2003.PubMed/NCBI
|
|
24
|
Yamaguchi T, Oda Y, Katsuda M, Inakuma T,
Ishiguro Y, Kanazawa K, Takamura H and Matoba T: Changes in
radical-scavenging activity of vegetables during different thermal
cooking processes. J Cookery Sci Jpn. 40:127–137. 2007.
|
|
25
|
Yamaguchi T, Hara H, Nishimoto T, Matoba T
and Takamura H: Evaluation of the proximate components and
antioxidant activity of Yamatoyasai, the traditional vegetables of
Nara. J Cookery Sci Jpn. 45:197–203. 2012.(In Japanese).
|
|
26
|
Nagao K, Hisamatsu Y, Awatsuhara R, Endo N
and Harada K: Chinese cuisine menu design with improved antioxidant
activity. J Cookery Sci Jpn. 46:324–334. 2013.(In Japanese).
|
|
27
|
Shoji N: Validation of functional activity
of paprika with antioxidant activity assay ‘ORAC’. Tohoku Agric
Res. 62:219–220. 2009.(In Japanese).
|
|
28
|
Yamada J, Akahori Y, Matsuda H, Hasegawa
Y, Maeda T and Harada K: Correlation between the DPPH radical
scavenging activity and ORAC for dried bonito and other stock
types. J Cookery Sci Jpn. 43:201–205. 2010.(In Japanese).
|