Introduction
Neuropathic pain, caused by lesions to the
somatosensory nervous system, in either the peripheral or the
central components, is a major cause of distress in patients. The
currently available analgesic drugs used for the treatment of
chronic pain are not particularly effective in such cases, in part
due to our incomplete understanding of the mechanisms underlying
the development and maintenance of neuropathic pain. Substantial
evidence has established that neuroinflammation, involving the
activation of glia and the release of inflammatory mediators,
contributes to neuropathic pain (1–3).
Following nerve injury, inflammatory mediators are released from
primary afferent terminals into the spinal cord and glial cells
(microglia and astrocytes) in the spinal dorsal horn are activated
by several inflammatory molecules. Glial activation leads to
pro-inflammatory responses with pathological effects, such as
neuronal hyperexcitability, neurotoxicity and chronic inflammation,
resulting in neuropathic pain (4).
Naringenin and its glycoside naringin are flavonoids
abundant in grapefruit and other citrus fruits and juices (5). Naringin is metabolised to naringenin
(4′,5,7-trihydroxyflavanone), which readily crosses the blood brain
barrier (6). Previous studies
demonstrated that naringenin and naringin attenuate the release of
lipopolysaccharide-induced pro-inflammatory mediators in
vitro (7–9) and in vivo (10). It was suggested that naringenin and
naringin exert anti-inflammatory and neuroprotective effects and
may be of therapeutic value in various neurodegenerative diseases.
Naringin was previously reported to improve long-term learning and
memory ability in a mouse model of Alzheimer’s disease (11). Furthermore, naringin was shown to
reduce cognitive deficits in kainic acid-induced status epilepticus
models (12) and 3-nitropropionic
acid-induced Huntington’s disease models (13). In addition, treatment with naringin
was found to inhibit diabetes-induced neuropathic pain (14). However, the potential effects of
naringenin on nerve injury-induced neuropathic pain have not been
investigated. Using a rat model of neuropathic pain following L5
spinal nerve ligation (SNL), the present study was undertaken to
investigate the role of naringenin administered intrathecally in
pain behaviors, pro-inflammatory cytokine and chemokine production,
as well as glial activation.
Materials and methods
Experimental animals
Male Sprague-Dawley rats, weighing 220–250 g, were
housed in a temperature-controlled room in plastic cages with free
access to food and water at 22–25°C on a 12-h light/dark cycle. All
the experimental protocols described in this study were approved by
the regulations stipulated by Guangdong Medical College Animal Care
Committee, which follows the protocol outlined in the Guide for the
Care and Use of Laboratory Animals published by the US National
Institute of Health (NIH publication no. 85–23, revised in
1985).
SNL neuropathic pain model
Peripheral nerve injury was produced by SNL
according to a previously described method (15). Briefly, under chloral hydrate
anesthesia (300 mg/kg, intraperitoneally; Sigma-Aldrich, St. Louis,
MO, USA), the dorsal vertebral column was surgically exposed from
L4 to L6. The paraspinal muscles were separated from the spinal
processes at the L4–L6 level and the L5 transverse process was
carefully removed. The right L5 spinal nerves were exposed and
tightly ligated distal to the dorsal root ganglion using 6–0 silk
thread. The same procedure was followed in the sham-operated group,
without ligation of the L5 spinal nerve.
Drug administration
Under chloral hydrate anesthesia (300 mg/kg,
intraperitoneally), the rats were implanted with a PE-10
intrathecal catheter (BD Biosciences, Bedford, MA, USA) in the
lumbar enlargement (close to the L4–L5 segments) for intrathecal
drug administration. Following a 7-day recovery, the catheter
placement was verified by observing transient hind paw paralysis
induced by intrathecal injection of lidocaine (2%, 5 μl). Animals
that failed to show any paralysis were excluded from the
experiments. Naringenin (Sigma-Aldrich) was dissolved in 5%
dimethyl sulfoxide and then diluted with 0.9% (w/v) saline
solution. The animals were divided into 5 groups for
administration: the sham-vehicle group (10 μl normal saline was
injected into sham-operated rats) and SNL rats receiving treatment
with naringenin (50, 100 and 200 mg/kg) or vehicle (10 μl normal
saline) once daily, from 3 days prior to surgery to 7 days after
the surgery (n=6 rats per treatment group).
Mechanical and thermal sensitivity
measurement
Mechanical allodynia was assessed using von Frey
filaments (Stoelting, Kiel, WI, USA) by experimenters who were
blinded to group assignment. The ipsilateral hind paw was pressed
with one of a series of von Frey filaments with gradually
increasing stiffness (2, 4, 6, 8, 10, 15 and 20 g) applied to the
plantar surface for 5–6 seconds for each filament. A positive paw
withdrawal response was recorded if the animal briskly lifted the
hind paw. The interval between trials was ≥5 min. For each trial,
the same hind limb was stimulated 10 times by a single von Frey
filament prior to stimulation by the next larger filament. The
minimal value that resulted in ≥6 responses to 10 stimulations was
recorded. To assess thermal sensitivity, paw withdrawal latencies
to a radiant heat stimulus were measured using the paw flick test
apparatus (IITC Life Science, Woodland Hills, CA, USA). The thermal
withdrawal latency was recorded as the threshold of thermal
sensitivity. Each hind paw was tested 5 times at 5-min
intervals.
Tissue harvest
Following completion of the behavioral tests on day
14 postoperatively, the rats were deeply anesthetized and then
perfused intracardially with 250 ml cold saline. The ipsilateral
L4–L5 spinal cord tissue rostral to the injury site was removed,
dissected while on cold ice, removed quickly and placed in liquid
nitrogen for subsequent assays.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Glial fibrillary acidic protein (GFAP) and
macrophage antigen-1 (Mac-1) were used as markers of astrocytic and
microglial activation, respectively. The rats were deeply
anesthetized with chloral hydrate (300 mg/kg) and perfused
transcardially with phosphate-buffered saline (pH 7.4) followed by
4% paraformaldehyde. Total RNA from the L5 spinal segments was
extracted with TRIzol (Gibco-BRL Life Technologies Inc., Grand
Island, NY, USA). Complementary DNA (cDNA) was synthesized with
Oligo(dT) 12–18 using SuperScript®III Reverse
Transcriptase for qRT-PCR (Invitrogen Life Technologies, Carlsbad,
CA, USA). The sense primer for GFAP was 5′-GCTGGAGCA
AGACAAACATTC-3′ and the antisense primer was 5′-CCC
TACCCACTCCTACATCGT-3′. The sense primer for Mac-1 was
5′-TGGCTGCTACTACCGTCCCT-3′ and the antisense primer was
5′-CAGGTTGGAGCCGAATAGGT-3′. The sense primer for GAPDH was
5′-CCCCCAATG TATCCGTTGTG-3′ and the antisense primer was
5′-TAGCCCAGGATGCCCTTTAGT-3′. Equal amounts of RNA (1 μg) were used
to prepare cDNA using SYBR® Premix Ex Taq™ (Takara Bio,
Inc., Tokyo, Japan) and analysed with a qRT-PCR detection system
(Applied Biosystems, Foster City, CA, USA). All the values were
normalized to GAPDH expression.
ELISA
The spinal production of tumor necrosis factor-α
(TNF-α), interleukin-1β (IL-1β) and monocyte chemoattractant
protein-1 (MCP-1) was quantified by ELISA kits for rats according
to the manufacturer’s instructions (BioSource International,
Camarillo, CA, USA for IL-1β; R&D Systems, Minneapolis, MN, USA
for TNF-α; and BioLegend, San Diego, CA, USA for MCP-1). The values
were expressed as pg/mg protein.
Statistical analysis
The values are presented as means ± standard error
of the mean (SEM). For behavioral data, the comparisons were
performed using repeated measures of analysis of variance (ANOVA).
For other data, comparisons were performed using ANOVA followed by
Bonferroni tests. Data were analyzed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
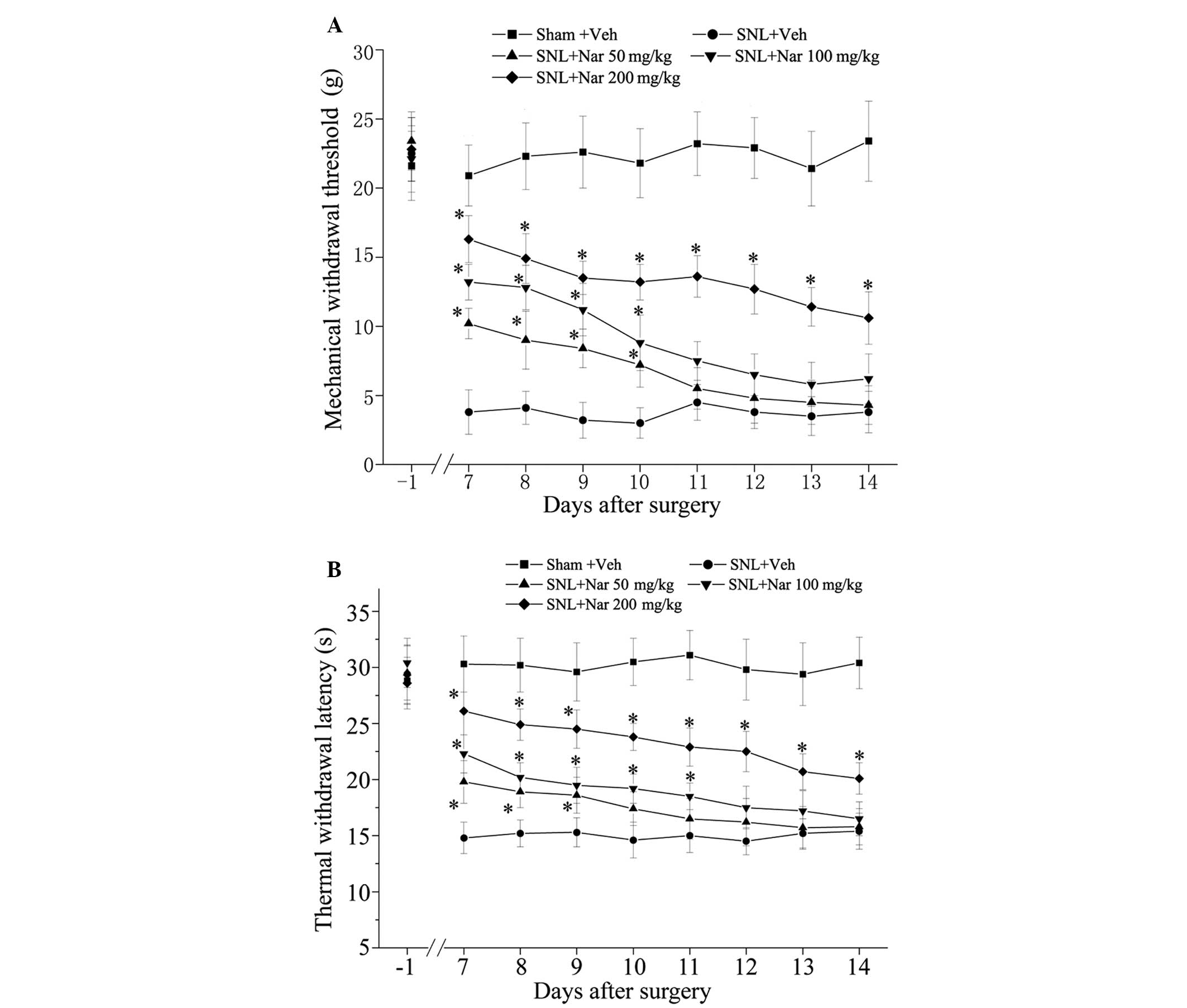
Naringenin dose-dependently attenuates
SNL-induced mechanical allodynia and thermal hypersensitivity
To determine whether naringenin prevents the
development of mechanical allodynia and thermal hypersensitivity,
different doses of naringenin (50, 100 and 200 mg/kg) were
administered intrathecally for 11 days, from 3 days prior to
surgery to 7 days after surgery and behavioral tests were
performed. We observed that the SNL injury resulted in prominent
mechanical allodynia and thermal hypersensitivity, as shown in the
SNL + Veh group. Repeated intrathecal administration of naringenin
produced a dose-dependent reduction of SNL-induced mechanical
hyperalgesia and thermal hypersensitivity, as shown in Fig. 1. Furthermore, the analgesic effects
of 200 mg/kg naringenin were prominent.
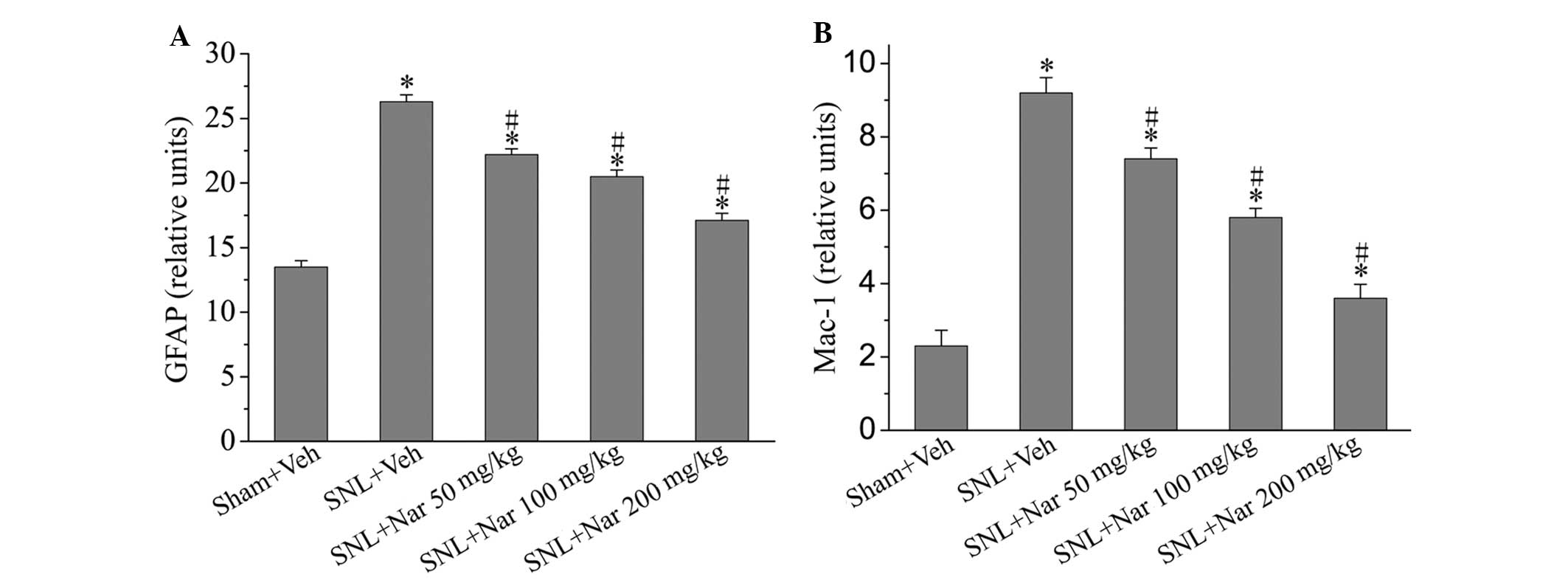
Effects of naringenin on glial activation
markers
We investigated GFAP and Mac-1 expression on day 14
after surgery in various groups to identify whether the analgesic
effects of naringenin were accompanied by inhibition of glial
activation. Compared to the sham group, the expression of GFAP and
Mac-1 was significantly increased in the SNL + Veh group. Following
treatment with naringenin, glial GFAP and Mac-1 expression levels
were significantly decreased compared to those in the SNL + Veh
group, although they remained higher compared to those in the sham
rats. In particular, 200 mg/kg naringenin exerted significant
inhibitory effects (Fig 2).
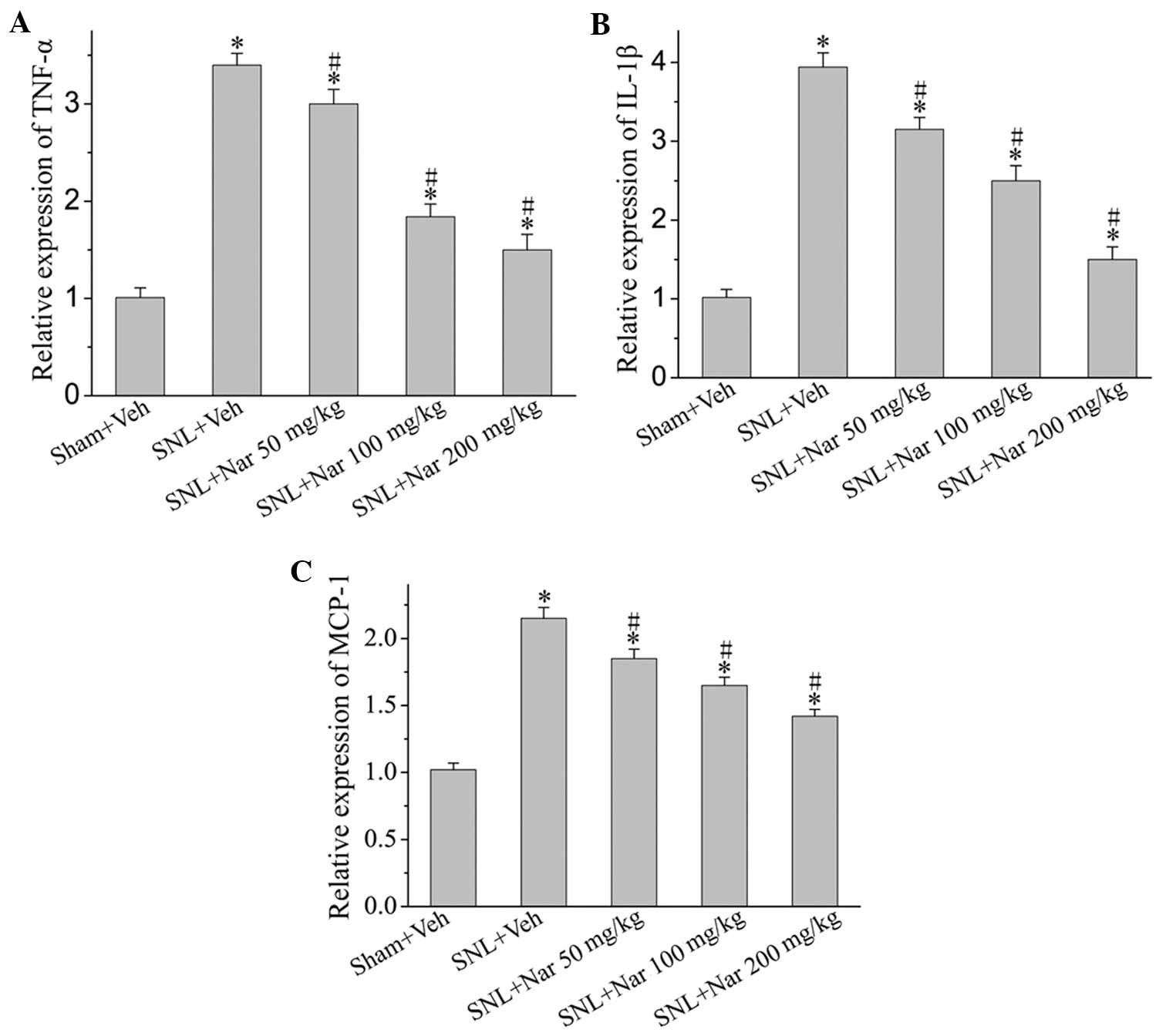
Effects of naringenin on SNL-induced
elevated pro-inflammatory cytokine and chemokine expression
SNL-induced neuropathic pain was associated with a
marked increase in the protein levels of pro-inflammatory cytokines
and chemokines, such as TNF-α, IL-1β and MCP-1. However,
intrathecal naringenin administration achieved a significant
decrease in the release of these pro-inflammatory cytokines and
chemokines in a dose-dependent manner (Fig 3).
Discussion
In the present study, we investigated the analgesic
effect of naringenin on the development of neuropathic pain and the
possible underlying mechanisms. Our data demonstrated that repeated
intrathecal administration of naringenin was able to
dose-dependently attenuate mechanical allodynia and thermal
hyperalgesia induced by SNL. Moreover, naringenin significantly
inhibited peripheral neuropathy-induced spinal astrocytic and
microglial activation. Furthermore, the synthesis of
pro-inflammatory mediators in the spinal cord of neuropathic rats
was suppressed by naringenin. These results suggested that
naringenin prevents the development of neuropathic pain, possibly
through inhibition of neuroinflammation.
Neuroinflammation is crucial in the pathogenesis of
neuropathic pain (2). In the
present study, the astrocytes marked by GFAP and microglia marked
by Mac-1 were highly activated following SNL, which was consistent
with the findings of previous studies (16–18).
However, the astrocyte and microglial activation was suppressed by
naringenin and this suppressive effect appeared to be parallel to
the effect of naringenin on mechanical allodynia and thermal
hyperalgesia. The release of multiple pro-inflammatory mediators
from the activated glia (19) leads
to a long-term alteration of synaptic transmission in the central
nervous system and plays a key role in the development of
neuropathic pain (20). In this
study, naringenin suppressed the increase in the spinal TNF-α,
IL-1β and MCP-1 levels in rats following SNL, which was consistent
with the effects of naringenin on mechanical allodynia and thermal
hyperalgesia.
Naringin and naringenin exert a potent
anti-inflammatory effect and have been used successfully in the
treatment of inflammatory diseases, such as Alzheimer’s disease
(11). In the present study, we
observed that repeated administration of naringenin was able to
alleviate neuropathic pain in a dose-dependent manner. Furthermore,
the anti-allodynia effect of naringenin (200 mg/kg) remained
significant 7 days after discontinuation of the treatment. These
data suggest that intrathecal treatment with naringenin exerts a
strong antinociceptive effect. Once this effect is achieved, it may
last over a long period of time. This characteristic indicates that
naringenin may be suitable for the long-term treatment of chronic
pain. Moreover, naringenin penetrates the blood brain barrier
easily. Therefore, naringenin may be a useful drug in the treatment
of neuropathic pain.
Acknowledgements
This study was supported by grants from the Doctoral
Program of Guangdong Medical College, China (no. XB1021) and the
Breeding Project of the Education Department of Guangdong Province,
China (no. LYM10084).
References
|
1
|
Jha MK, Jeon S and Suk K: Glia as a link
between neuroinflammation and neuropathic pain. Immune Netw.
12:41–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kiguchi N, Kobayashi Y and Kishioka S:
Chemokines and cytokines in neuroinflammation leading to
neuropathic pain. Curr Opin Pharmacol. 12:55–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ren K and Dubner R: Interactions between
the immune and nervous systems in pain. Nat Med. 16:1267–1276.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Milligan ED and Watkins LR: Pathological
and protective roles of glia in chronic pain. Nat Rev Neurosci.
10:23–36. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hare JT and Elliott DP: Grapefruit juice
and potential drug interactions. Consult Pharm. 18:466–472.
2003.PubMed/NCBI
|
|
6
|
Zbarsky V, Datla KP, Parkar S, Rai DK,
Aruoma OI and Dexter DT: Neuroprotective properties of the natural
phenolic antioxidants curcumin and naringenin but not quercetin and
fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res.
39:1119–1125. 2005.PubMed/NCBI
|
|
7
|
Kanno S, Shouji A, Tomizawa A, Hiura T,
Osanai Y, Ujibe M, Obara Y, Nakahata N and Ishikawa M: Inhibitory
effect of naringin on lipopolysaccharide (LPS)-induced endotoxin
shock in mice and nitric oxide production in RAW 264.7 macrophages.
Life Sci. 78:673–681. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bodet C, La VD, Epifano F and Grenier D:
Naringenin has anti-inflammatory properties in macrophage and ex
vivo human whole-blood models. J Periodontal Res. 43:400–407. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park HY, Kim GY and Choi YH: Naringenin
attenuates the release of pro-inflammatory mediators from
lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear
factor-κB and inhibiting mitogen-activated protein kinases. Int J
Mol Med. 30:204–210. 2012.PubMed/NCBI
|
|
10
|
Shiratori K, Ohgami K, Ilieva I, Jin XH,
Yoshida K, Kase S and Ohno S: The effects of naringin and
naringenin on endotoxin-induced uveitis in rats. J Ocul Pharmacol
Ther. 21:298–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang D, Gao K, Li X, Shen X, Zhang X, Ma
C, Qin C and Zhang L: Long-term naringin consumption reverses a
glucose uptake defect and improves cognitive deficits in a mouse
model of Alzheimer’s disease. Pharmacol Biochem Behav. 102:13–20.
2012.PubMed/NCBI
|
|
12
|
Golechha M, Chaudhry U, Bhatia J, Saluja D
and Arya DS: Naringin protects against kainic acid-induced status
epilepticus in rats: evidence for an antioxidant, anti-inflammatory
and neuroprotective intervention. Biol Pharm Bull. 34:360–365.
2011. View Article : Google Scholar
|
|
13
|
Kumar P and Kumar A: Protective effect of
hesperidin and naringin against 3-nitropropionic acid induced
Huntington’s like symptoms in rats: possible role of nitric oxide.
Behav Brain Res. 206:38–46. 2010.PubMed/NCBI
|
|
14
|
Kandhare AD, Raygude KS, Ghosh P, Ghule AE
and Bodhankar SL: Neuroprotective effect of naringin by modulation
of endogenous biomarkers in streptozotocin induced painful diabetic
neuropathy. Fitoterapia. 83:650–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SH and Chung JM: An experimental model
for peripheral neuropathy produced by segmental spinal nerve
ligation in the rat. Pain. 50:355–363. 1992. View Article : Google Scholar
|
|
16
|
Feng X, Zhang F, Dong R, Li W, Liu J, Zhao
X, Xue Q, Yu B and Xu J: Intrathecal administration of clonidine
attenuates spinal neuroimmune activation in a rat model of
neuropathic pain with existing hyperalgesia. Eur J Pharmacol.
614:38–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morgenweck J, Griggs RB, Donahue RR,
Zadina JE and Taylor BK: PPARgamma activation blocks development
and reduces established neuropathic pain in rats.
Neuropharmacology. 70:236–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Willcockson HH and Valtschanoff
JG: Influence of the vanilloid receptor TRPV1 on the activation of
spinal cord glia in mouse models of pain. Exp Neurol. 220:383–390.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reichling DB and Levine JD: Critical role
of nociceptor plasticity in chronic pain. Trends Neurosci.
32:611–618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schomberg D and Olson JK: Immune responses
of microglia in the spinal cord: contribution to pain states. Exp
Neurol. 234:262–270. 2012. View Article : Google Scholar : PubMed/NCBI
|