Introduction
The physical factors of the human environment
include temperature, humidity and noise. Living permanently in a
high ambient temperature affects the daily life and work
environment. More importantly, a high humidity and noisy
environment cause damage to the body (1). Among these physical environmental
stresses, temperature is the most significant ecological condition.
As temperature is a factor that is widespread in the majority of
environments, there is a lack of space or time stability (2). High ambient temperature beyond the
scope of the thermal neutral zone has been considered to be the
deadliest pressure source in rodents, as it triggers a wide variety
of biological responses, and results in neurological, endocrine
disorders and immune dysfunctions (3). Under the action of stress, a number of
hormones are excreted to counteract the adverse effect on organism
function. The stress hormones (catecholamines, glucocorticoid and
growth hormone) play a critical role in regulating the circulation
and nutritional requirement of potentially harmful situations, via
the ‘flight or fight’ reaction (4).
Lycium barbarum belongs to the plant family
Solanaceae. The red berries, also known as wolfberry or gou
qi zi, are a well-known traditional Chinese medicine. Currently,
the red berries have been extensively used to manufacture various
types of healthy foods and products, including medicinal beverages
and healthy soups (5). Lycium
barbarum has a high level of polysaccharides, which is analogously
40% by dry mass, and therefore, studies have been devoted to the
liquid fraction of the berries and the lycium barbarum
polysaccharides (LBPs) (6). It has
been previously reported that LBPs act against the influences of
aging and oxidation (7,8,9),
adjust the immune function (10,11),
increase the exercise tolerance capacity, relieve fatigue and
aging, and express antioxidant activity (12–14).
Treatment with LBP prevented the rise of blood glucose in
hypertensive rats, guarded against liver damage caused by
alcohol-induced oxidative stress in rats (15–17),
and reduced the side-effects of chemotherapy and radiotherapy in
the treatment of cancer (18–20).
Our previous study showed that LBP clearly inhibited oxidative
stress, weakened the contractile response to noradrenaline, and it
also enhanced the expression of endothelial nitric oxide synthase
and heat-shock protein 70 (HSP70) in rats undergoing exhaustive
exercise (21).
The objectives of the present study were: i) to
investigate the effects of high ambient temperature on neuropeptide
Y (NPY) gene expression in the hypothalamus and the concentration
of corticotropin-releasing hormone (CRH), cortisol (Cor), HSP70 and
epinephrine (EPI) in the plasma of rats; and ii) to examine the
alleviating effects of LBP intervention under high ambient
temperatures on these physiological parameters in Sprague-Dawley
(SD) rats.
Materials and methods
Animals and heat exposure schedule
Five-week-old male SD rats, weighing 150–180 g, were
purchased from the Laboratory Animal Center of Ningxia Medical
University (Yinchuan, China). The rats were placed individually in
plastic cages and were maintained on a 12-h light/dark cycle
(lights on between 08:00 and 20:00 h) at a normothermic temperature
(24.0±1°C), with access to food and water at liberty. The
experimental procedures were approved by the Animal Ethics
Committee of Ningxia Medical University and in accordance with the
guidelines of the Council of the Physiological Society of China
(?). Following a week-long adaptive phase, 24 rats were randomly
divided into the control (CN), high ambient temperature (HT) and HT
+ LBP (HTL) groups. The temperature inside the climatic chamber was
raised to 32.0±0.2°C (relative humidity, 60±5%) in the HT and HTL
groups, whereas the rats in the CN group were maintained at a
temperature of 24.0±1°C throughout the study. In the HTL group, the
rats were treated with LBP by gavage [200 mg/kg body weight
(b.w.)], whereas the rats in the HT and CN groups were treated with
normal saline by gavage (1 ml/100 g b.w.). The treatments lasted
for 14 days. The dose of LBP was chosen according to prophase
experiments (21) and it was safe,
effective and non-toxic in rats. In the course of the experiment,
cages were cleaned and food and water were replaced at a random
time every 2 or 3 days.
Tissue sampling and organ
preparation
Following heat exposure, each rat was anesthetized
by intraperitoneal injection of urethane (1.5 g/kg). Blood was
extracted from the abdominal vein with a syringe, which was washed
by heparin sodium solution prior to use. Subsequently, the blood
was centrifuged at 3,500 × g for 15 min to obtain plasma. The
plasma was stored at −80°C until analysis.
The rats were executed by cervical dislocation, and
the whole brain and hypothalamus were immediately removed and
placed into liquid nitrogen. Frozen samples were reserved at −80°C
until further analysis.
RNA extraction and quantitative
polymerase chain reaction (qPCR)
RNA was extracted from hypothalamic tissue (n=8 in
each group for the NPY mRNA assay), using an Axygen RNA kit (Axygen
Biosciences, Union City, CA, USA) according to the manufacturer’s
instructions. Total RNA concentrations were measured by
spectrophotometry at 260 nm. The purity of the RNA samples
(260/280) was: 1.65≤purity≤2.00. cDNA was synthesized using a
reverse transcription kit (TransGen Biotech Co., Ltd., Beijing,
China) according to the manufacturer’s instructions. qPCR was
carried out using a Top Green qPCR SuperMix (TransGen Biotech Co.,
Ltd.). Primer sequences and the amplified fragment size are listed
in Table I [Sangon Biotech
(Shanghai) Co., Ltd., Songjiang, Shanghai, China]. The specificity
of the PCR amplified products was authenticated by analysis of a
melting curve and agarose gel electrophoresis. The PCR conditions
were as follows: 94°C for 5 min (initial activation) followed by 36
cycles of amplification at 94°C for 30 sec for denaturation; 62°C
for 30 sec for annealing; and 72°C for 30 sec for extension. At the
end of each reaction, melting curve analyses were performed to
ensure the specificity of each reaction. The 2−ΔΔCt
method was used to calculate and correct the values of mRNA. In
each run, the control sample was an internal standard, followed by
standardization to the β-actin mRNA level. In the original
experiment, the amplification efficiencies of NPY and β-actin were
measured, and all amplification efficiencies were comparable. In
each sample, the ΔCt value was established by measuring the
difference between the Ct of NPY and β-actin as follows: ΔCt = Ct
(NPY) − Ct (β-actin). Subsequently, the ΔΔCt of each sample was
calculated by deducting the ΔCt value of the control: ΔΔCt = ΔCt
(sample) − ΔCt (control). Each sample of RNA was tested three
independent times and the average was used for the value in each
sample.
 | Table IGenBank login code, primer sequences
and forecast size of the amplification products. |
Table I
GenBank login code, primer sequences
and forecast size of the amplification products.
| Gene | GenBank | Forward primer | Reverse primer | bp |
|---|
| NPY | NM_012614 |
GCTCTGCGACACTACATCAATC |
GCATTTTCTGTGCTTTCTCTCA | 107 |
| β-actin | NM_031144 |
CACCCGCGAGTACAACCTTC |
CCCATACCCACCATCACACC | 207 |
Measurements of plasma CRH, Cor, HSP70
and EPI concentrations
The plasma concentrations of CRH, Cor, HSP70 and EPI
were detected by an ELISA kit (Cusabio Biotech Co., Ltd., Wuhan,
Hubei, China), and all procedures were performed according to the
manufacturer’s instructions (n=8 in each group). Samples were
arranged in replicates and the average value of each sample was
used for analysis.
Statistics
The values are provided as mean ± standard
deviation. Statistical comparisons were made by one-way analysis of
variance. The statistical analyses were performed with SPSS version
16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
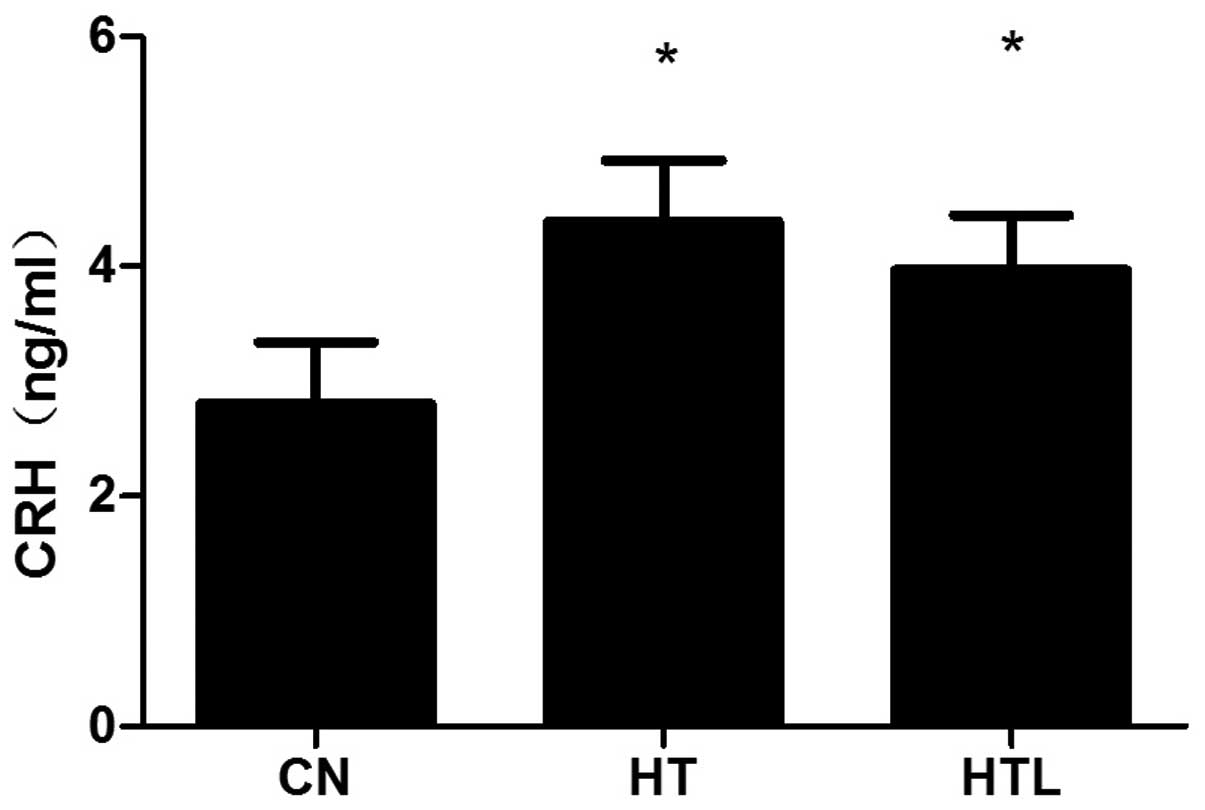
Plasma CRH concentrations
In the plasma, the concentration of CRH
significantly increased in the HT (P<0.05) and HTL (P<0.05)
groups compared to the CN group. The plasma CRH level in the HTL
group was not significant compared with that of the HT group
(P>0.05) (Fig. 1).
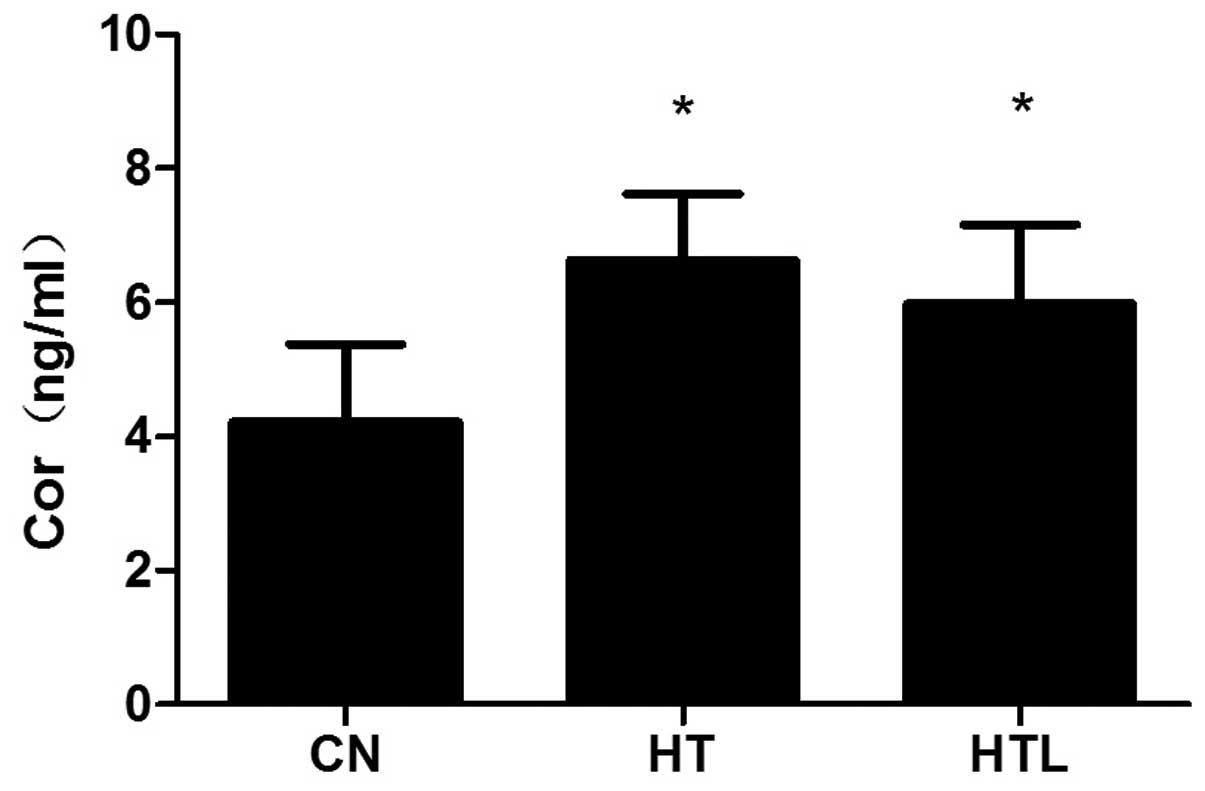
Plasma Cor concentrations
Plasma Cor levels were significantly increased in
the HT and HTL rat groups that were exposed to the high ambient
temperature (P<0.05), compared to the rats in the CN group.
However, there were no clear differences in the plasma Cor levels
between the HT and HTL groups (P>0.05) (Fig. 2).
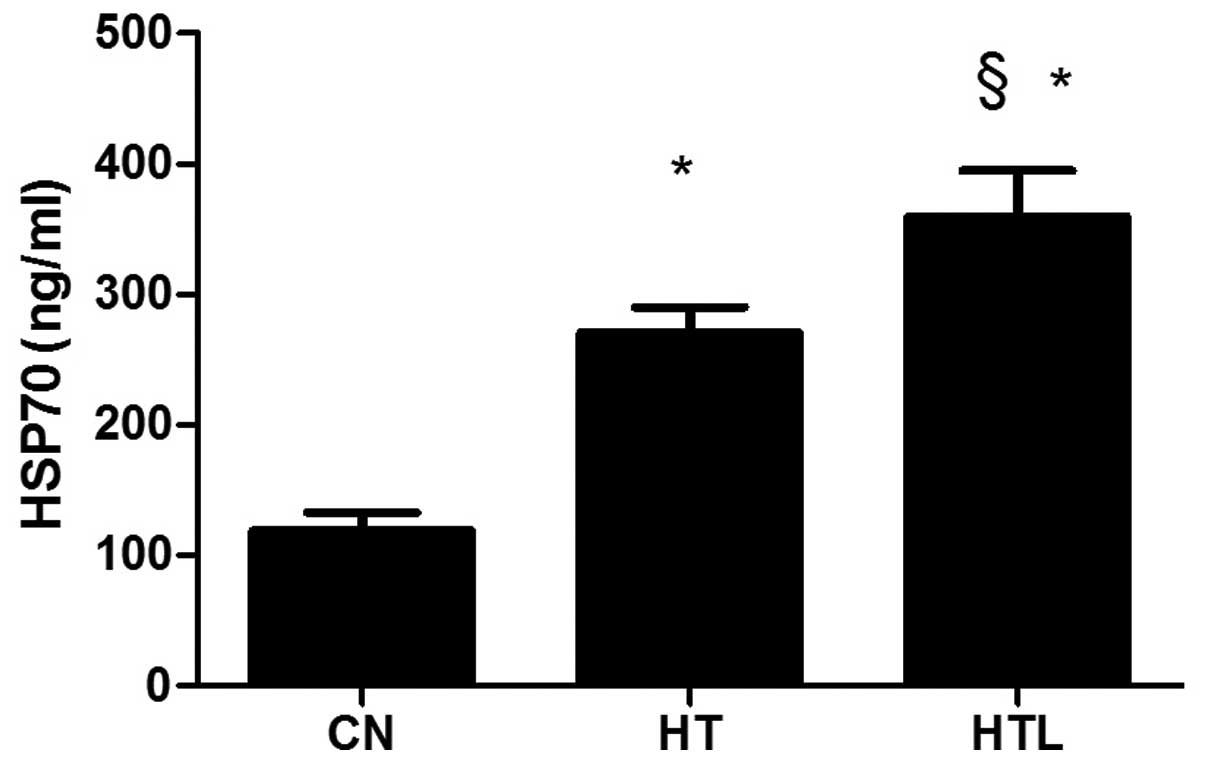
Plasma HSP70 concentrations
After 14 days of heat exposure, the plasma level of
HSP70 in the HT group was significantly (P<0.05) higher compared
with the CN group, whereas the plasma HSP70 level in the HTL group
was markedly (P<0.05) higher compared with the HT group
(Fig. 3).
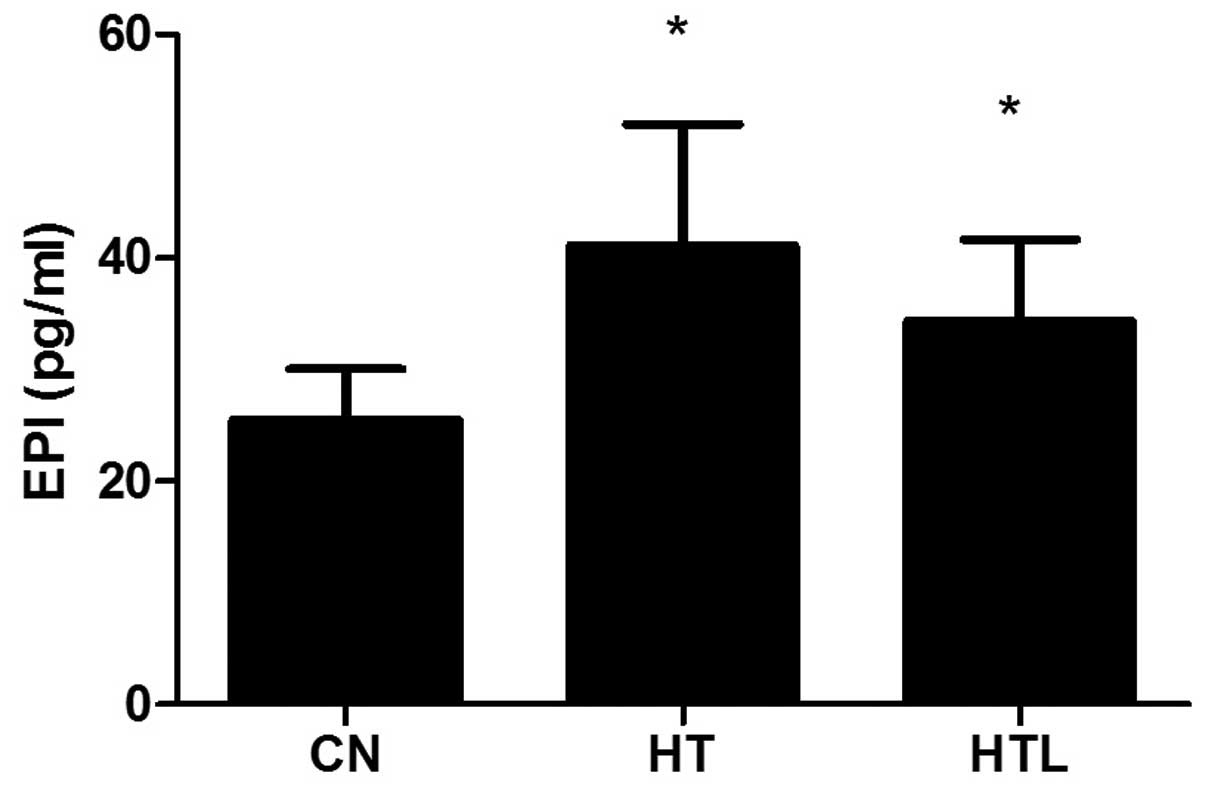
Plasma EPI concentrations
After 14 days of heat exposure, the plasma EPI level
in the HT (P<0.05) or HTL (P<0.05) group was significantly
higher compared with the CN group. The EPI level in the plasma of
the HTL group was not significantly different compared with the HT
group (P>0.05) (Fig. 4).
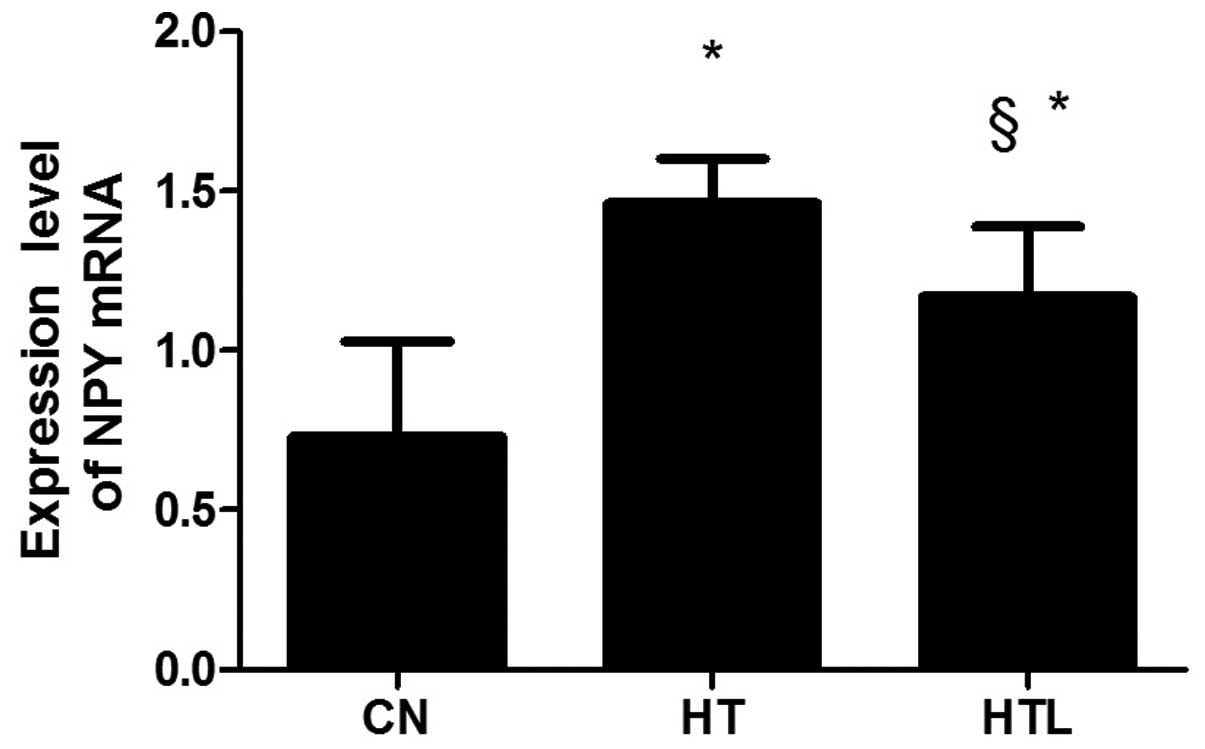
NPY mRNA expression
The mRNA expression level of NPY was significantly
downregulated in the HT group compared with the CN group
(P<0.05). The expression of NPY mRNA markedly increased in the
HTL group compared with the HT group (P<0.05). However, there
were no significant differences in the levels of NPY mRNA between
the CN and HTL groups (P>0.05) (Fig.
5).
Discussion
In the present study, the primary purpose was to
investigate the effects of high ambient temperature on the
physiological parameters in SD rats. Our previous study showed that
the mean abdominal temperature (Tab) in the light and dark phases
of day in the HT and HTL groups was slightly higher than those of
the CN group, but Tab did not differ between these groups (22). High ambient temperature has a
negative impact on the performance of the neuroendocrine system.
The hypothalamic-pituitary-adrenal (HPA) axis is an important part
of the neuroendocrine system and its main action is to connect the
functions of the hypothalamus, pituitary gland and adrenal cortex
(23). The HPA axis, not only
regulates and controls the reaction to stress, but also has a
number of other functions, including body heat regulation, the
regulation of metabolism and immune function. The HPA axis plays a
key role in the process of stress response by regulating the
secretion of Cor. Cor is widely accepted as a standard for stress
response as secretion of Cor has been shown to increase when
animals are exposed to a stress state (24). The stress-induced activation of the
HPA axis is dependent on CRH, which is the primary regulator of
stress. CRH activates the HPA axis by inducing the release of the
adrenocorticotropic hormone (ACTH) from the pituitary gland, which
in turn stimulates the adrenocortical release of Cor (25).
In humans, CRH is the major hormone of the HPA axis.
CRH is a 41-amino-acid neuropeptide, which is produced and released
by the hypothalamus. When the body is exposed to a stressful
environment, CRH is released by the hypothalamus (26–29). A
study by Elias et al (30)
showed that the concentration of Cor and CRH was increased
immediately, following a long-time high-intensity exercise. Results
of the present study have shown that exposure to high ambient
temperature increased the level of CRH in plasma. When the
secretion of CRH increases, CRH stimulates the cells of the
anterior pituitary to synthesize and release the ACTH, which in
turn stimulates the cells of the adrenal cortex to synthesize and
release Cor (31,32). A previous human study has shown that
Cor is the end result of the HPA-axis activation (33). Cor can also be considered a reliable
indicator of the initial strength of nauseous or harmful events
(34). Megahed et al
(35) showed that in Upper Egypt,
the summer heat stress induced the increase of Cor in the serum of
Buffalo-Cows. The present study has confirmed that exposure to high
ambient temperature caused a significant increase in the Cor plasma
level. Therefore, the study has shown that high ambient temperature
exposure causes a clear activation of the HPA axis, manifested as
the increased secretion of CRH and Cor. Based on these data, there
was no significant difference between the HTL and HT groups in the
rat plasma levels of Cor and CRH. This finding may indicate that
LBP had no evident effect on the alleviation of the increased
plasma levels that were caused by heat stress.
HSPs are molecular chaperones, that play a crucial
role in protein transport, aggregation, degradation, folding and
unfolding (36). Furthermore, these
highly-conserved proteins belong to the stress-responsive protein
family, and play a key role in protecting cells against stress and
apoptosis (37). Hsp70 is one of
the largest and most conserved families that participate in
cytothesis and other protective mechanisms (38). In addition, various types of
environmental stresses and toxic chemical substances are able to
upregulate the expression of Hsp70 (39–41).
It is known that HSP play important physiological actions in
situations involving both pantosomatous and cellular stress. HSP
reacts quickly to environmental stress, and also protects cells
from it. Additionally, all manner of environmental stresses and
nocuous chemical materials induce the expression of HSP70. Yu and
Bao (42) reported that heat stress
induced an increase in HSP70 protein and mRNA levels in the heart
and liver of broiler chickens. Thus, in the present study, the
plasma level of HSP70 was detected in rats exposed to high ambient
temperature. The data show that the levels of HSP70 in the HT and
HTL groups were significantly increased compared to the CN group.
The data also show that the level of HSP70 was markedly increased
in LBP-treated animals compared to the HT group. In addition, the
upregulation of NPY mRNA expression was observed in the LBP-treated
rats. Therefore, HSP70 may participate in this upregulation by
protecting the cells from the deleterious effects of heat
stress.
A large-scale stress-antileptic physiological
reaction refers to a complicated neural endocrine, and the
interaction between the immune system to preserve internal
homeostasis and to respond to life-threatening unexpected events
(43). The first sign of the stress
response is the activation of the sympathetic nervous system. This
activation increases the release of catecholamines and the release
of co-located substances, including neuropeptides at the
sympathetic neuroeffector juncture into the blood. For a number of
years, it has been known that stress induces a cascade of adaptive
neuroendocrine responses, which generally includes the synthesis
and release of Cor and EPI from the adrenal gland (44,45).
Melin et al showed that when rats were exposed to heat
stress followed by exhaustive exercise, the levels of EPI and
norepinephrine were increased (46). In the present study, it was
demonstrated that high temperature exposure increased the plasma
level of EPI, and that there was no significant change in the HTL
group compared with the HT group. This suggests that LBP had no
obvious effect on alleviating the increased plasma EPI level that
was caused by heat stress.
NPY is a 36-amino acid peptide, which is widely
distributed in the central and peripheral nervous system of
mammals. NPY plays a decisive role in maintaining the homeostasis
of the internal environment (47).
Whether in humans or animals, stressors, including cold,
electroshock and exercise, can increase the plasma NPY levels has
also been investigated (48). The
NPY gene expression was elevated by a acute stress, whereas the NPY
gene expression was different in a reduplicated stress (49). NPY is a primary neurotransmitter
activated by stress that has been widely studied in the field of
anxiety and stress. In the present study, in the rats exposed to
high ambient temperature, a significant downregulation in the mRNA
expression of NPY was observed. This may indicate that prolonged
heat stress may cause injury to the body. The downregulation was
entirely reversed by the intervention of LBPs in the HTL group.
The present study has shown that LBP enhanced the
expression of HSP70 in plasma. LBP treatment was also shown to
reverse the downregulation of NPY mRNA expression. This may
indicate that LBP alleviates the harm caused by prolonged heat
stress. However, the mechanism of the increased NPY mRNA expression
in LBP-treated rats has not examined thoroughly in the study, and
further investigation is required to investigate this
mechanism.
In conclusion, results of the present study have
shown a significant increase in the plasma concentration of CRH,
Cor, HSP70 and EPI in rats exposed to high ambient temperature. By
contrast, LBP significantly increased the plasma level of HSP70 and
the mRNA expression of NPY, to protect the body from the damage
caused by heat stress.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81060230) and the
Ningxia Natural Science Foundation Key Project (grant no.
NZ13055).
References
|
1
|
Yun SH, Moon YS, Sohn SH and Jang IS:
Effects of cyclic heat stress or vitamin C supplementation during
cyclic heat stress on HSP70, inflammatory cytokines, and the
antioxidant defense system in Sprague Dawley rats. Exp Anim.
61:543–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sohail MU, Ijaz A, Yousaf MS, et al:
Alleviation of cyclic heat stress in broilers by dietary
supplementation of mannan-oligosaccharide and Lactobacillus-based
probiotic: dynamics of cortisol, thyroid hormones, cholesterol,
C-reactive protein, and humoral immunity. Poult Sci. 89:1934–1938.
2010. View Article : Google Scholar
|
|
3
|
Lamb JR, Bal V, Mendez-Samperio P, et al:
Stress proteins may provide a link between the immune response to
infection and autoimmunity. Int Immunol. 1:191–196. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Power ML and Schulkin J: Functions of
corticotropin-releasing hormone in anthropoid primates: from brain
to placenta. Am J Hum Biol. 18:431–447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li SY, Yang D, Yeung CM, et al: Lycium
barbarum polysaccharides reduce neuronal damage, blood-retinal
barrier disruption and oxidative stress in retinal
ischemia/reperfusion injury. PLoS One. 6:e163802011. View Article : Google Scholar
|
|
6
|
Chang RC and So KF: Use of anti-aging
herbal medicine, Lycium barbarum, against aging-associated
diseases. What do we know so far? Cell Mol Neurobiol. 28:643–652.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng D and Kong H: The effect of Lycium
barbarum polysaccharide on alcohol-induced oxidative stress in
rats. Molecules. 16:2542–2550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li XM, Ma YL and Liu XJ: Effect of the
Lycium barbarum polysaccharides on age-related oxidative stress in
aged mice. J Ethnopharmacol. 111:504–511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu MS, Lai CS, Ho YS, et al:
Characterization of the effects of anti-aging medicine Fructus
lycii on beta-amyloid peptide neurotoxicity. Int J Mol Med.
20:261–268. 2007.PubMed/NCBI
|
|
10
|
Du G, Liu L and Fang J: Experimental study
on the enhancement of murine splenic lymphocyte proliferation by
Lycium barbarum glycopeptide. J Huazhong Univ Sci Technolog Med
Sci. 24:518–520. 5272004.PubMed/NCBI
|
|
11
|
Gan L, Zhang SH, Liu Q and Xu HB: A
polysaccharide-protein complex from Lycium barbarum upregulates
cytokine expression in human peripheral blood mononuclear cells.
Eur J Pharmacol. 471:217–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X: Experimental research on the role
of Lycium barbarum polysaccharide in anti-peroxidation. China J
Chin Mater Med. 18:110–112. 1993.(In Chinese).
|
|
13
|
Luo Q, Yan J and Zhang S: Isolation and
purification of Lycium barbarum polysaccharides and its antifatigue
effect. J Hyg Res. 29:115–117. 2000.(In Chinese).
|
|
14
|
Yao LQ and Li FL: Lycium barbarum
polysaccharides ameliorates physical fatigue. Afr J Agric Res.
5:2153–2157. 2010.
|
|
15
|
Xiao J, Liong EC and Ching YP: Lycium
barbarum polysaccharides protect rat liver from non-alcoholic
steatohepatitis-induced injury. Nutr Diabetes. 3:e812013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ha KT, Yoon SJ, Choi DY, Kim DW, Kim JK
and Kim CH: Protective effect of Lycium chinense fruit on carbon
tetrachloride-induced hepatotoxicity. J Ethnopharmacol. 96:529–535.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo Q, Cai Y, Yan J, Sun M and Corke H:
Hypoglycemic and hypolipidemic effects and antioxidant activity of
fruit extracts from Lycium barbarum. Life Sci. 76:137–149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miao Y, Xiao B, Jiang Z, et al: Growth
inhibition and cell-cycle arrest of human gastric cancer cells by
Lycium barbarum polysaccharide. Med Oncol. 27:785–790. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong H, Shen P, Jin L, Xing C and Tang F:
Therapeutic effects of Lycium barbarum polysaccharide (LBP) on
irradiation or chemotherapy-induced myelosuppressive mice. Cancer
Biother Radiopharm. 20:155–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hai-Yang G, Ping S, Li JI, Chang-Hong X
and Fu T: Therapeutic effects of Lycium barbarum polysaccharide
(LBP) on mitomycin C (MMC)-induced myelosuppressive mice. J Exp
Ther Oncol. 4:181–187. 2004.PubMed/NCBI
|
|
21
|
Zhao Z, Luo Y, Li G, Zhu L, Wang Y and
Zhang X: Thoracic aorta vasoreactivity in rats under exhaustive
exercise: effects of Lycium barbarum polysaccharides
supplementation. J Int Soc Sports Nutr. 10:472013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li G, Li H, Zhang Q, Liu H, Zhou X and
Osamu S: Effects of heat exposure on weight of stress organ and
intervention of LBP in rats. Liaoning J Trad Chin Med. 34:45–47.
2012.
|
|
23
|
Mahmoud KZ, Edens FW, Eisen EJ and
Havenstein GB: Effect of ascorbic acid and acute heat exposure on
heat shock protein 70 expression by young white Leghorn chickens.
Comp Biochem Physiol C Toxicol Pharmacol. 136:329–335. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Collip D, Nicolson NA, Lardinois M, et al;
G.R.O.U.P. Daily cortisol, stress reactivity and psychotic
experiences in individuals at above average genetic risk for
psychosis. Psychol Med. 41:2305–2315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rivier CL and Plotsky PM: Mediation by
corticotropin releasing factor (CRF) of adenohypophysial hormone
secretion. Annu Rev Physiol. 48:475–494. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thorsell A: Brain neuropeptide Y and
corticotropin-releasing hormone in mediating stress and anxiety.
Exp Biol Med (Maywood). 235:1163–1167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang SS, Yan XB, Hofman MA, Swaab DF and
Zhou JN: Increased expression level of corticotropin-releasing
hormone in the amygdala and in the hypothalamus in rats exposed to
chronic unpredictable mild stress. Neurosci Bull. 26:297–303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ayensu WK, Pucilowski O, Mason GA,
Overstreet DH, Rezvani AH and Janowsky DS: Effects of chronic mild
stress on serum complement activity, saccharin preference, and
corticosterone levels in Flinders lines of rats. Physiol Behav.
57:165–169. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ostrander MM, Ulrich-Lai YM, Choi DC,
Richtand NM and Herman JP: Hypoactivity of the
hypothalamo-pituitary-adrenocortical axis during recovery from
chronic variable stress. Endocrinology. 147:2008–2017. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Elias AN, Wilson AF, Pandian MR, et al:
Corticotropin releasing hormone and gonadotropin secretion in
physically active males after acute exercise. Eur J Appl Physiol
Occup Physiol. 62:171–174. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dai X, Thavundayil J and Gianoulakis C:
Response of the hypothalamic-pituitary-adrenal axis to stress in
the absence and presence of ethanol in subjects at high and low
risk of alcoholism. Neuropsychopharmacology. 27:442–452. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lucassen EA and Cizza G: The
hypothalamic-pituitary-adrenal axis, obesity, and chronic stress
exposure: Sleep and the HPA axis in obesity. Curr Obes Rep.
1:208–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jones T and Moller MD: Implications of
hypothalamic-pituitary-adrenal axis functioning in posttraumatic
stress disorder. J Am Psychiatr Nurses Assoc. 17:393–403.
2011.PubMed/NCBI
|
|
34
|
Galina ZH, Sutherland CJ and Amit Z:
Effects of heat-stress on behavior and the pituitary adrenal axis
in rats. Pharmacol Biochem Behav. 19:251–256. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Megahed GA, Anwar MM, Wasfy SI and
Hammadeh ME: Influence of heat stress on the cortisol and
oxidant-antioxidants balance during oestrous phase in buffalo-cows
(Bubalus bubalis): thermo-protective role of antioxidant treatment.
Reprod Domest Anim. 43:672–677. 2008. View Article : Google Scholar
|
|
36
|
Sørensen JG, Kristensen TN and Loeschcke
V: The evolutionary and ecological role of heat shock proteins.
Ecol Lett. 6:1025–1037. 2003.
|
|
37
|
Gupta SC, Sharma A, Mishra M, Mishra RK
and Chowdhuri DK: Heat shock proteins in toxicology: how close and
how far? Life Sci. 86:377–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Georgopoulos C and Welch WJ: Role of the
major heat shock proteins as molecular chaperones. Annu Rev Cell
Biol. 9:601–634. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Piano A, Valbonesi P and Fabbri E:
Expression of cytoprotective proteins, heat shock protein 70 and
metallothionins, in tissues of Ostrea edulis exposed to heat and
heavy metals. Cell Stress Chaperones. 9:134–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rhee JS, Raisuddin S, Lee KW, et al: Heat
shock protein (Hsp) gene responses of the intertidal copepod
Tigriopus japonicus to environmental toxicants. Comp Biochem
Physiol C Toxicol Pharmacol. 149:104–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Horowitz M: From molecular and cellular to
integrative heat defense during exposure to chronic heat. Comp
Biochem Physiol A Mol Integr Physiol. 131:475–483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu J and Bao E: Effect of acute heat
stress on heat shock protein 70 and its corresponding mRNA
expression in the heart, liver, and kidney of broilers. Asian-Aust
J Anim Sci. 21:1116–1126. 2008. View Article : Google Scholar
|
|
43
|
Hiremagalur B, Kvetnansky R, Nankova B, et
al: Stress elicits trans-synaptic activation of adrenal
neuropeptide Y gene expression. Brain Res Mol Brain Res.
27:138–144. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McMorris T, Swain J, Smith M, et al: Heat
stress, plasma concentrations of adrenaline, noradrenaline,
5-hydroxytryptamine and cortisol, mood state and cognitive
performance. Int J Psychophysiol. 61:204–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tai TC, Claycomb R, Siddall BJ, Bell RA,
Kvetnansky R and Wong DL: Stress-induced changes in epinephrine
expression in the adrenal medulla in vivo. J Neurochem.
101:1108–1118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Melin B, Curé M, Jimenez C, Koulmann N,
Savourey G and Bittel J: Effect of ingestion pattern on rehydrating
and exercise performance subsequent to passive dehydration. Eur J
Appl Physiol Occup Physiol. 68:281–284. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tatemoto K, Carlquist M and Mutt V:
Neuropeptide Y - a novel brain peptide with structural similarities
to peptide YY and pancreatic polypeptide. Nature. 296:659–660.
1982. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kaijser L, Pernow J, Berglund B and
Lundberg JM: Neuropeptide Y is released together with noradrenaline
from the human heart during exercise and hypoxia. Clin Physiol.
10:179–188. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fuller RW: Stress. Neurochemical and
humoral mechanisms. Neurochem Int. 18:1431991.
|