Introduction
The prevalence of diabetes is on the increase
worldwide (1) and the global
incidence and prevalence of type 2 diabetes in adolescents varies
substantially in different countries and ethnicities (2). The estimated prevalence of diabetes
and prediabetes was 11.6 and 50.1%, respectively, in a
representative sample of Chinese adults, and these figures may
account for up to 113.9 and 493.4 million Chinese adults with
diabetes and prediabetes, respectively (3).
The fat mass and obesity-associated (FTO)
gene is situated on chromosome 16q12.2 and is composed of nine
exons (4). FTO is highly
expressed in rat hypothalamus (5),
a brain structure involved in the regulation of energy balance and
appetite (5,6). Two independent genome-wide association
studies (GWASs) indicated that a number of common variants
(including rs9939609) in the first intron of FTO have the
most significant effect on body mass index (BMI) and susceptibility
to obesity, particularly in Europeans (7,8). Since
then, the majority of follow-up genetic studies have focused on
FTO rs9939609, and the positive association between
rs9939609 and type 2 diabetes has been shown to be entirely
mediated through the effect of FTO on obesity (9).
Recently, Sällman Almén et al (10) sequenced 412 kilo base pairs of the
genome, which covered the complete FTO gene in 524 severely
obese children and 527 lean controls with massive parallel
sequencing, following adjustment for the 44 haplotype blocks in the
FTO region. Three single-nucleotide polymorphisms (SNPs)
(rs55872725, rs11642015 and rs62048402) were found to be associated
with obesity (P<0.0011). rs11642015 and rs62048402 were the
top-associated SNPs and had a stronger obesity association
(P<0.007) than the frequently studied rs9939609 (P<0.012).
The study did not detect any strongly associated variants
elsewhere, and therefore it was concluded that intron one was the
only obesity-associated region of the FTO gene (10). Additionally, it was demonstrated
that FTO rs11642015 was associated with obesity in Latvia
(10), while Kalnina et al
(11) demonstrated that the
rs11642015, rs62048402 and rs9939609 polymorphisms in the first
intron of FTO contributed to risk of type 2 diabetes, even
following the correction for BMI.
Obesity is a major predictor of the risk of type 2
diabetes (12). FTO was
initially identified as a type 2 diabetes susceptibility gene.
However, further adjustment for BMI eliminated any significant
association with type 2 diabetes (9). The association of the FTO
variants with type 2 diabetes and BMI has been mostly identified in
Europeans (7). However, Meyre
(13) examined whether FTO
was a type 2 diabetes susceptibility gene. Further studies that
focus particularly on different ethnicities are required to
investigate this hypothesis. As the association between the
FTO variants and risk of type 2 diabetes varied between
ethnicities, conducting relevant future studies in subjects with
different ethnic backgrounds is important.
As the association of FTO rs11642015 with the
risk of prediabetes, type 2 diabetes and obesity in the Chinese
population remains unclear, 490 type 2 diabetic, 471 prediabetic
and 575 healthy subjects who originated from Shenzhen (China) were
recruited. The results were also combined with those from two
previously published studies (10,14) to
establish the contribution of FTO rs11642015 to
susceptibility to obesity.
Patients and methods
Participants
The study was a population-based case-control study,
in which 1,516 individuals (490 type 2 diabetic, 471 prediabetic
and 575 healthy subjects) were consecutively recruited between
April 2010 and September 2011. All the subjects were from 16
Community Health Service Centers (Nanshan, China) under the
supervision of the Shenzhen Nanshan Center for Chronic Disease
Control (Guangdong, China). A two-stage sampling method and a
simple random procedure according to the sequence of
computer-generated random numbers were applied. The study was
approved by the Ethics Committee of Shenzhen Nanshan Center for
Chronic Disease Control and all participants provided written
informed consent. The BMI was calculated by the weight (kg)/height
(m2). The obesity (BMI≥28 kg/m2), overweight
(24≤BMI<28 kg/m2) and normal weight (BMI<24
kg/m2) classes were defined according to the definition
proposed by the Working Group on Obesity in China (15). Type 2 diabetes and prediabetes were
diagnosed according to the American Diabetes Association guidelines
of 2010 (16).
Genotyping
Human genomic DNA was separated from peripheral
blood samples using the nucleic acid extraction automatic analyzer
(Lab-Aid 820; Zeesan Biotech, Xiamen, China) and all the DNA
samples were stored in Tris-EDTA buffer. The DNA concentration was
determined using the PicoGreen® double-strand DNA
Quantification kit (Molecular Probes Inc., Eugene, OR, USA). All
subjects were genotyped for FTO rs11642015 using the
MassARRAY compact analyzer based on the chip-based matrix-assisted
laser desorption ionization time-of-flight mass spectrometry
platform (Sequenom Inc., San Diego, CA, USA). Polymerase chain
reaction cycles were initiated with an initial denaturation stage
at 94°C for 15 min, followed by 45 cycles at 94°C for 20 sec for
denaturation, 56°C for 30 sec for annealing, 72°C for 1 min for
primer extension and 72°C for 3 min for a final extension. DNA
amplification for the FTO rs11642015 genotyping was
performed on 5% of the total samples and were randomly selected for
a second genotype. The results remained consistent.
Statistical analysis
Consistency of the genotype frequencies were
assessed with Hardy-Weinberg equilibrium (HWE) and percentages were
analyzed by the χ2 test. A one-way analysis of variance
was used to compare the continuous variables among the three groups
(Table I). Allele and genotype
frequencies were compared between cases and controls with the
χ2 test. Binary logistic regression analysis was used to
estimate the association of FTO rs11642015 with the risk of
prediabetes, type 2 diabetes and obesity, subsequent to adjustment
for the corresponding confounders. To reduce type I error induced
by multiple tests, Bonferroni’s adjustment was applied to determine
the significance thresholds. This employed the following formula to
adjust the significance level and maintain an error rate of 0.05:
1-(1-∂)1/n. P<0.008 was adopted as the significant
threshold (Tables II and III). Statistical analysis was performed
with the SPSS package, version 17.0 (SPSS, Inc., Chicago, IL, USA).
All tests were two-tailed and P<0.05 was considered to indicate
a statistically significant difference. Power analysis was
performed using the Power and Sample Size Calculation software
(version 3.0.43) designed by William D. Dupont and Walton D.
Plummer Jr (17).
 | Table ICharacteristics of the subjects. |
Table I
Characteristics of the subjects.
| Characteristic | Controls | Prediabetes | Type 2
diabetes | P1 | P2 | P3 |
|---|
| Subjects, n | 575 | 471 | 490 | | | |
| Female/male, n | 289/286 | 241/230 | 248/242 | 0.81 | 0.91 | 0.95 |
| Age, years | 57.94±10.81 | 61.39±11.43 | 62.76±11.14 | 0.001 | 0.055 | 0.001 |
| BMI,
kg/m2 | 23.52±3.17 | 25.28±3.82 | 24.95±3.46 | 0.001 | 0.143 | 0.001 |
| Female |
| Age, years | 58.50±10.00 | 61.66±10.43 | 63.99±10.28 | 0.001 | 0.01 | 0.001 |
| BMI,
kg/m2 | 23.18±3.09 | 25.18±4.04 | 24.69±3.58 | 0.001 | 0.12 | 0.001 |
| Male |
| Age, years | 57.38±11.56 | 61.10±12.42 | 61.51±11.85 | 0.001 | 0.70 | 0.001 |
| BMI,
kg/m2 | 23.87±3.22 | 25.37±3.59 | 25.20±3.32 | 0.001 | 0.59 | 0.001 |
 | Table IIComparison of genotype and allele
frequencies between cases and controls in the whole sample,
stratified by gender. |
Table II
Comparison of genotype and allele
frequencies between cases and controls in the whole sample,
stratified by gender.
| FTO
(rs11642015) | CC, n | CT, n | TT, n | χ2 | P-value | C, n | T, n | χ2 | OR (95% CI) | OR (95% CI) | HWE |
|---|
|
|
|---|
| P-value | Unadjusted | P-value | Adjusteda |
|---|
| Whole
sampleb |
| Controls | 435 | 111 | 7 | | | 981 | 131 | | | | | | |
| Prediabetes | 330 | 121 | 18 | 12.86 | 0.002c | 781 | 157 | 9.94 | 0.002c | 1.50
(1.17–1.93) | 0.001c | 1.55
(1.19–2.01) | 0.97 |
| Type 2
diabetes | 345 | 133 | 9 | 8.46 | 0.01 | 823 | 151 | 5.84 | 0.01 | 1.37
(1.06–1.76) | 0.50 | 0.91
(0.70–1.18) | |
| Female |
| Controls | 218 | 57 | 5 | | | 493 | 67 | | | | | | |
| Prediabetes | 162 | 66 | 11 | 7.97 | 0.01 | 390 | 88 | 7.93 | 0.005c | 1.66
(1.17–2.34) | 0.006c | 1.64
(1.14–2.34) | 0.57 |
| Type 2
diabetes | 174 | 66 | 5 | 3.27 | 0.19 | 414 | 76 | 2.50 | 0.11 | 1.35
(0.94–1.92) | 0.55 | 1.11
(0.77–1.62) | |
| Male |
| Controls | 217 | 54 | 2 | | | 488 | 58 | | | | | | |
| Prediabetes | 168 | 55 | 7 | 5.38 | 0.06 | 391 | 69 | 3.94 | 0.04 | 1.48
(1.02–2.15) | 0.06 | 1.44
(0.98–2.14) | 0.49 |
| Type 2
diabetes | 171 | 67 | 4 | 5.67 | 0.059 | 409 | 75 | 4.99 | 0.02 | 1.54
(1.06–2.22) | 0.02 | 1.57
(1.05–2.33) | |
 | Table IIIAssociation between FTO
rs11642015 with obesity and Hardy-Weinberg equilibrium (HWE). |
Table III
Association between FTO
rs11642015 with obesity and Hardy-Weinberg equilibrium (HWE).
| FTO
(rs11642015) | CC, n | CT, n | TT, n | χ2 | P-value | C, n | T, n | χ2 | OR (95% CI) | OR (95% CI) | HWE |
|---|
|
|
|---|
| P-value | Unadjusted | P-value | Adjusted |
|---|
| Whole sample |
| Normal weight | 500 | 189 | 18 | | | 1189 | 225 | | | | | | 0.97 |
| Overweight | 483 | 137 | 14 | 5.13 | 0.07 | 1103 | 165 | 4.52 | 0.03 | 0.79
(0.63–0.98) | 0.03 | 1.55
(1.19–2.01) | |
| Obese | 127 | 39 | 2 | 2.18 | 0.33 | 293 | 43 | 1.79 | 0.18 | 0.77
(0.54–1.10) | 0.20 | 0.91
(0.70–1.18)a | |
| Female |
| Normal weight | 274 | 106 | 7 | | | 654 | 120 | | | | | | 0.37 |
| Overweight | 218 | 63 | 9 | 3.74 | 0.15 | 499 | 81 | 0.50 | 0.47 | 0.88
(0.65–1.20) | 0.47 | 1.64
(1.14–2.34) | |
| Obese | 60 | 23 | 2 | 0.11 | 0.94 | 143 | 27 | 0.01 | 0.99 | 1.02
(0.65–1.62) | 0.80 | 1.11
(0.77–1.62)a | |
| Male |
| Normal weight | 226 | 83 | 11 | | | 535 | 105 | | | | | | 0.33 |
| Overweight | 265 | 74 | 5 | 5.00 | 0.08 | 604 | 84 | 4.44 | 0.03 | 0.70
(0.52–0.96) | 0.03 | 1.44
(0.98–2.14) | |
| Obese | 67 | 16 | 0 | 4.96 | 0.08 | 150 | 16 | 4.21 | 0.04 | 0.54
(0.31–0.94) | 0.04 | 1.57
(1.05–2.33)a | |
The meta-analysis was performed by Stata version
11.0 (Stata Corporation, College Station, TX, USA). The Z-test was
used to calculate the P-value of the overall effect for the
meta-analysis. The combined odds ratios (ORs) together with their
95% confidence intervals (CIs) were assessed with the
random-effects method. The between-study heterogeneity was
estimated by the χ2-based Q test (significance level,
P<0.10) and I2 statistics (I2 values of
<25, 25–75 and >75% were defined as low, moderate and high
heterogeneity, respectively), which can be interpreted as the
percentage of total variation across several studies due to
heterogeneity. Publication bias was evaluated by Egger’s regression
test, Begg’s adjusted rank correlation test and a funnel plot. A
two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
Characteristics of subjects
The characteristics of individuals included in the
study are shown in Table I. A total
of 490 subjects with type 2 diabetes, 471 with prediabetes and 575
non-diabetic subjects were involved in the present study. The
genotype frequencies were assessed by HWE (Table I).
Comparison of genotype and allele
frequencies
A multivariate analysis with covariates of age and
BMI revealed that FTO rs11642015 was significantly
associated with the risk of prediabetes in the whole sample (T vs.
C: OR, 1.55; 95% CI, 1.19–2.01; P=0.001; CC vs. CT + TT: OR, 1.55;
95% CI, 1.15–2.09; P=0.004; Table
II). A further breakdown analysis by gender showed that
FTO rs11642015 was associated with prediabetes, OR, 1.66 (T
vs. C: 95% CI, 1.17–2.34; P=0.005), in females. The significant
results remained following adjustment of age and BMI (OR, 1.64; 95%
CI: 1.14–2.34; P=0.006, Table
II).
Statistically significant differences were observed
in the genotype frequencies of rs11642015 between subjects with
type 2 diabetes and controls in the whole sample, particularly
under the dominant model following adjustment for age and BMI (OR,
1.58; 95% CI, 1.17–2.12; P=0.002). Further gender-stratified
analysis did not reveal any significant results (Table II). There was no association of
rs11642015 with type 2 diabetes for the comparison of the T and C
allele and the additional adjustment for age and BMI did not change
the results (Table II). According
to power calculations, the sample size provided a 67.2% power
(∂=0.05) to detect a relative risk for rs11642015.
Breakdown analysis by the BMI categories (14) was then conducted. However, no
significant associations were found under the stringent
Bonferroni’s correction (Table
III).
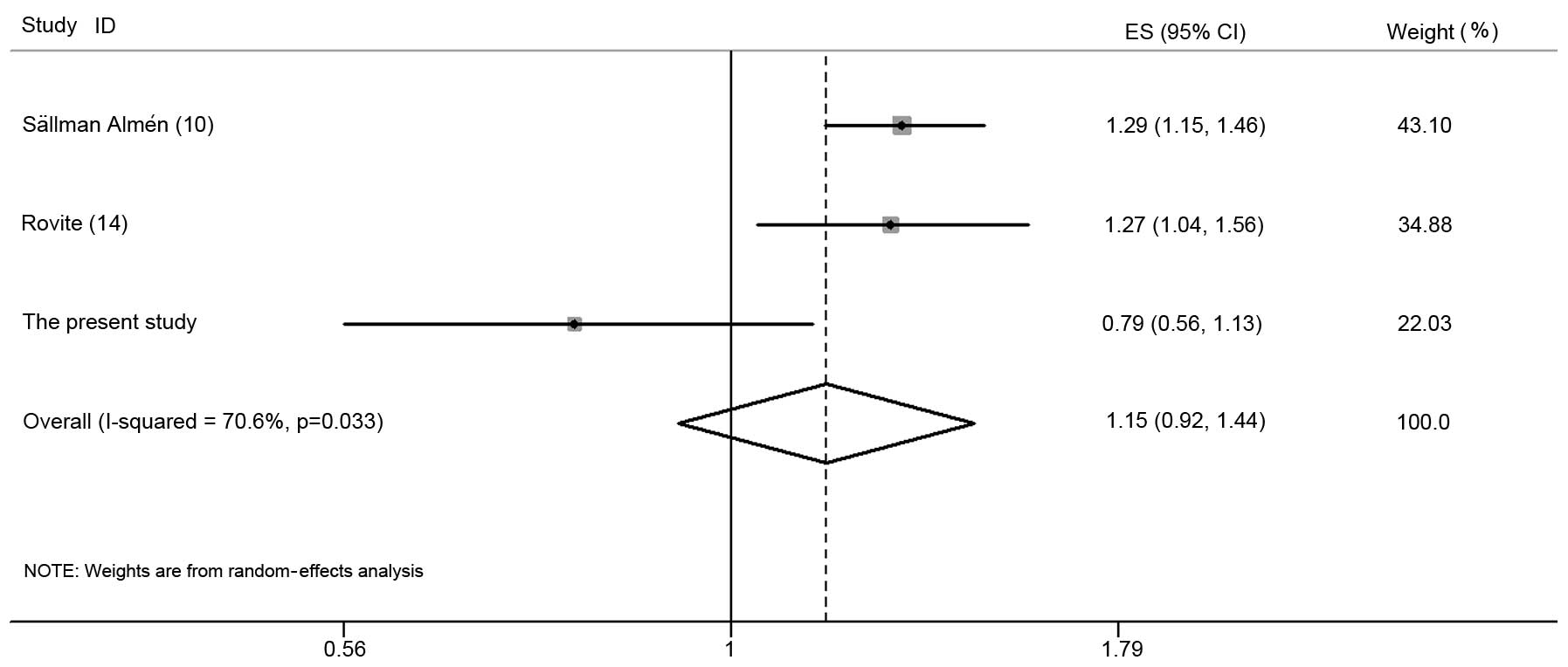
To assess the contribution of FTO rs11642015
to obesity, a meta-analysis of the data was performed together with
data from two previous publications (10,14). A
meta-analysis suggested that the association between FTO
rs11642015 and obesity under the additive model was not significant
with high heterogeneity (OR, 1.15; 95% CI, 0.92–1.44; P=0.21;
Pheterogeneity=0.03; I2=70.6%; Fig. 1). There was no evidence of any
publication bias observed (Begg’s test = 0.29 and Egger’s test =
0.32).
Discussion
The association between the FTO rs11642015
polymorphism with prediabetes, type 2 diabetes and obesity was
examined in the present study, and to the best of our knowledge,
the association has not been reported previously in the Chinese
population. The association of the FTO locus with type 2
diabetes may vary in different populations.
The findings demonstrated that the FTO
rs11642015 T allele was associated with the risk of prediabetes,
particularly in females, and the association retained its
significance following correction for age and BMI. Subjects
carrying the CT + TT genotype were predisposed to prediabetes and
type 2 diabetes following correction for age and BMI. This was
consistent with the study by Kalnina et al (11), in which FTO rs11642015 was
linked with a higher type 2 diabetes prevalence and the
significance was retained subsequent to correction for BMI.
Notably, a significant association was not observed
between the FTO rs11642015 polymorphism and obesity in the
whole sample, females and males under the stringent Bonferroni’s
correction. Under the stringent Bonferroni’s correction, the
correction may be extremely conservative and may increase the
likelihood of type II error. In contrast to the present study,
Sällman Almén et al (10)
observed a stronger association of FTO rs11642015 with
obesity compared to rs9939609 by massive parallel sequencing in
Latvia. The minor allele frequency (MAF) observed in the present
study was 0.18 for rs11642015, whereas the corresponding MAF in the
study by Sällman Almén et al (10) was 0.48. The differences among the
studies may be due to the differences in the risk allele
frequencies and linkage disequilibrium structure across different
ethnicities. Other reasons include the varying study designs,
different sample sizes and ethnic differences in genetic background
and environmental factors (18).
Whether there was an association of the FTO rs11642015 with
obesity was not clear, and therefore a meta-analysis was also
conducted with available studies to provide more definitive
evidence. The meta-analysis, including a total of 2,711 subjects,
provided evidence that FTO rs11642015 is not associated with
obesity.
Obesity is one of the most important risk factors
for the development of type 2 diabetes (19). The most common genetic variant of
the FTO gene is rs9939609, and the first GWAS detected the
contribution of rs9939609 to type 2 diabetes. However, the
significant association was not apparent following adjustment for
BMI (9), indicating that the
association between the FTO locus and type 2 diabetes was
mediated by BMI and that FTO is a susceptibility locus for
obesity instead of type 2 diabetes. Several studies have reported
that the association between the FTO locus and risk of type
2 diabetes remained significant following adjustment for BMI
(20–22). However, a recent study showed a
significant association between the FTO rs9939609
polymorphism and type 2 diabetes in a Vietnamese population,
independent of obesity-related measurements, socio-economic status
and lifestyle factors (23).
Findings of that study showed that the association between the
FTO locus and type 2 diabetes was not completely moderated
through BMI, as the accurate estimates may not be revealed in
particular populations.
There have been fewer studies reported in South
Asians. Of three studies that have been reported, there were two
that confirmed the association between the FTO locus and
obesity susceptibility (24,25)
and one that did not (26). The
association of the FTO variants with type 2 diabetes and BMI
has been independently identified in a number of Caucasian European
populations. However, Asians and Europeans have differences in body
composition, and therefore, FTO may have a smaller effect on
obesity in Asians compared to Europeans. A population-based
homogeneous population with 100% Han Chinese was determined, which
eliminated the ethnical heterogeneity. A group of prediabetes
subjects were also included. The majority of the previous studies
only focused on two groups (type 2 diabetes and non-diabetic
subjects). Including the group of prediabetes subjects is useful
for identifying high-risk individuals at early stages and providing
improved early prevention. Identification of the FTO
variations in multi-ethnic groups is useful for understanding the
diverse genetic backgrounds in different ethnicities. Additionally,
the P-values are also corrected for multiple comparisons to avoid
false-positive associations.
However, there are certain limitations that require
highlighting. Firstly, due to the cross-sectional design, the
selection bias cannot be neglected. In addition, the sample size
was relatively small and the power at 67.2% to detect the
association. Thirdly, there is a possibility that the interaction
between gene-gene or gene-environment factors may conceal the true
effect of specific genetic variants.
In conclusion, a significant association of the
FTO rs11642015 T allele was observed with prediabetes
following correction for age and BMI, particularly in females.
Subjects carrying the CT + TT genotype are predisposed to a
prediabetes and type 2 diabetes risk. Future investigation is
required to elucidate the molecular function of FTO,
downstream pathways and interactions, as well as the biological
pathways that are fundamental for the independent association
between the FTO variation with obesity and type 2 diabetes
(27).
Acknowledgements
The authors are grateful to the doctors and nurses
for their help in the data and sample collection at the Community
Health Centers. The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31100919
and 81371469), the Natural Science Foundation of Zhejiang Province
(grant no. LR13H020003), the K.C. Wong Magna Fund in Ningbo
University and Ningbo Social Development Research Projects (grant
no. 2012C50032), the Ningbo University Talent Project (grant no.
ZX2012000046) and the Natural Science Foundation of Zhejiang
Province (LQ13H260002), Zhejiang Province Scientific Research
Projects of Education (no. Y201326971).
References
|
1
|
Chen L, Magliano DJ and Zimmet PZ: The
worldwide epidemiology of type 2 diabetes mellitus - present and
future perspectives. Nat Rev Endocrinol. 8:228–236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fazeli Farsani S, van der Aa MP, van der
Vorst MM, et al: Global trends in the incidence and prevalence of
type 2 diabetes in children and adolescents: a systematic review
and evaluation of methodological approaches. Diabetologia.
56:1471–1488. 2013.PubMed/NCBI
|
|
3
|
Xu Y, Wang L, He J, et al; 2010 China
Noncommunicable Disease Surveillance Group. Prevalence and control
of diabetes in Chinese adults. JAMA. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fredriksson R, Hägglund M, Olszewski PK,
et al: The obesity gene, FTO, is of ancient origin, up-regulated
during food deprivation and expressed in neurons of feeding-related
nuclei of the brain. Endocrinology. 149:2062–2071. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cecil J, Dalton M, Finlayson G, et al:
Obesity and eating behaviour in children and adolescents:
contribution of common gene polymorphisms. Int Rev Psychiatry.
24:200–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmid PM, Heid I, Buechler C, et al:
Expression of fourteen novel obesity-related genes in Zucker
diabetic fatty rats. Cardiovasc Diabetol. 11:482012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dina C, Meyre D, Gallina S, et al:
Variation in FTO contributes to childhood obesity and severe adult
obesity. Nat Genet. 39:724–726. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scott LJ, Mohlke KL, Bonnycastle LL, et
al: A genome-wide association study of type 2 diabetes in Finns
detects multiple susceptibility variants. Science. 316:1341–1345.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frayling TM, Timpson NJ, Weedon MN, et al:
A common variant in the FTO gene is associated with body mass index
and predisposes to childhood and adult obesity. Science.
316:889–894. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sällman Almén M, Rask-Andersen M,
Jacobsson JA, et al: Determination of the obesity-associated gene
variants within the entire FTO gene by ultra-deep targeted
sequencing in obese and lean children. Int J Obes (Lond).
37:424–431. 2013.PubMed/NCBI
|
|
11
|
Kalnina I, Zaharenko L, Vaivade I, et al:
Polymorphisms in FTO and near TMEM18 associate with type 2 diabetes
and predispose to younger age at diagnosis of diabetes. Gene.
527:462–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abdullah A, Peeters A, de Courten M and
Stoelwinder J: The magnitude of association between overweight and
obesity and the risk of diabetes: a meta-analysis of prospective
cohort studies. Diabetes Res Clin Pract. 89:309–319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meyre D: Is FTO a type 2 diabetes
susceptibility gene? Diabetologia. 55:873–876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rovite V, Petrovska R, Vaivade I, et al:
The role of common and rare MC4R variants and FTO polymorphisms in
extreme form of obesity. Mol Biol Rep. 41:1491–1500. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bei-Fan Z; Cooperative Meta-Analysis Group
of Working Group on Obesity in China. Predictive values of body
mass index and waist circumference for risk factors of certain
related diseases in Chinese adults: study on optimal cut-off points
of body mass index and waist circumference in Chinese adults. Asia
Pac J Clin Nutr. 11(Suppl 8): S685–S693. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
American Diabetes Association. Diagnosis
and classification of diabetes mellitus. Diabetes Care. 33(Suppl
1): S62–S69. 2010. View Article : Google Scholar
|
|
17
|
Dupont WD and Plummer WD Jr: Power and
sample size calculations. A review and computer program. Control
Clin Trials. 11:116–128. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adeyemo A and Rotimi C: Genetic variants
associated with complex human diseases show wide variation across
multiple populations. Public Health Genomics. 13:72–79. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weber MB, Oza-Frank R, Staimez LR, et al:
Type 2 diabetes in Asians: prevalence, risk factors and
effectiveness of behavioral intervention at individual and
population levels. Annu Rev Nutr. 32:417–439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hertel JK, Johansson S, Sonestedt E, et
al: FTO, type 2 diabetes and weight gain throughout adult life: a
meta-analysis of 41,504 subjects from the Scandinavian HUNT, MDC
and MPP studies. Diabetes. 60:1637–1644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Liu Z, Song Y, et al: Meta-analysis
added power to identify variants in FTO associated with type 2
diabetes and obesity in the Asian population. Obesity (Silver
Spring). 18:1619–1624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takeuchi F, Yamamoto K, Katsuya T, et al:
Association of genetic variants for susceptibility to obesity with
type 2 diabetes in Japanese individuals. Diabetologia.
54:1350–1359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Binh TQ, Phuong PT, Nhung BT, et al:
Association of the common FTO-rs9939609 polymorphism with type 2
diabetes, independent of obesity-related traits in a Vietnamese
population. Gene. 513:31–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramya K, Radha V, Ghosh S, et al: Genetic
variations in the FTO gene are associated with type 2 diabetes and
obesity in south Indians (CURES-79). Diabetes Technol Ther.
13:33–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dorajoo R, Blakemore AI, Sim X, et al:
Replication of 13 obesity loci among Singaporean Chinese, Malay and
Asian-Indian populations. Int J Obes (Lond). 36:159–163. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yajnik CS, Janipalli CS, Bhaskar S, et al:
FTO gene variants are strongly associated with type 2 diabetes in
South Asian Indians. Diabetologia. 52:247–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hess ME and Brüning JC: The fat mass and
obesity-associated (FTO) gene: Obesity and beyond? Biochim Biophys
Acta. Feb 8–2014.(Epub ahead of print). View Article : Google Scholar
|