Introduction
Inflammation is a complex process mediated by the
activation of various immune cells. Macrophages play a central role
in mediating a number of different immunopathological phenomena
during inflammation by the overproduction of inflammatory
mediators, known as prostaglandins (PGs) (1). Cyclooxygenase (COX) catalyzes the
synthesis of PGs from arachidonic acid (AA). There are two isoforms
of COX. COX-1 is a reference gene that is expressed
constitutively in the majority of tissues. COX-2 is an
immediate, early-response gene that is highly inducible by
inflammatory stimuli, including endotoxin lipopolysaccharides (LPS)
(2). This indicates that targeted
inhibition of COX-2 is a promising approach in preventing
inflammation and inflammation-associated cancer.
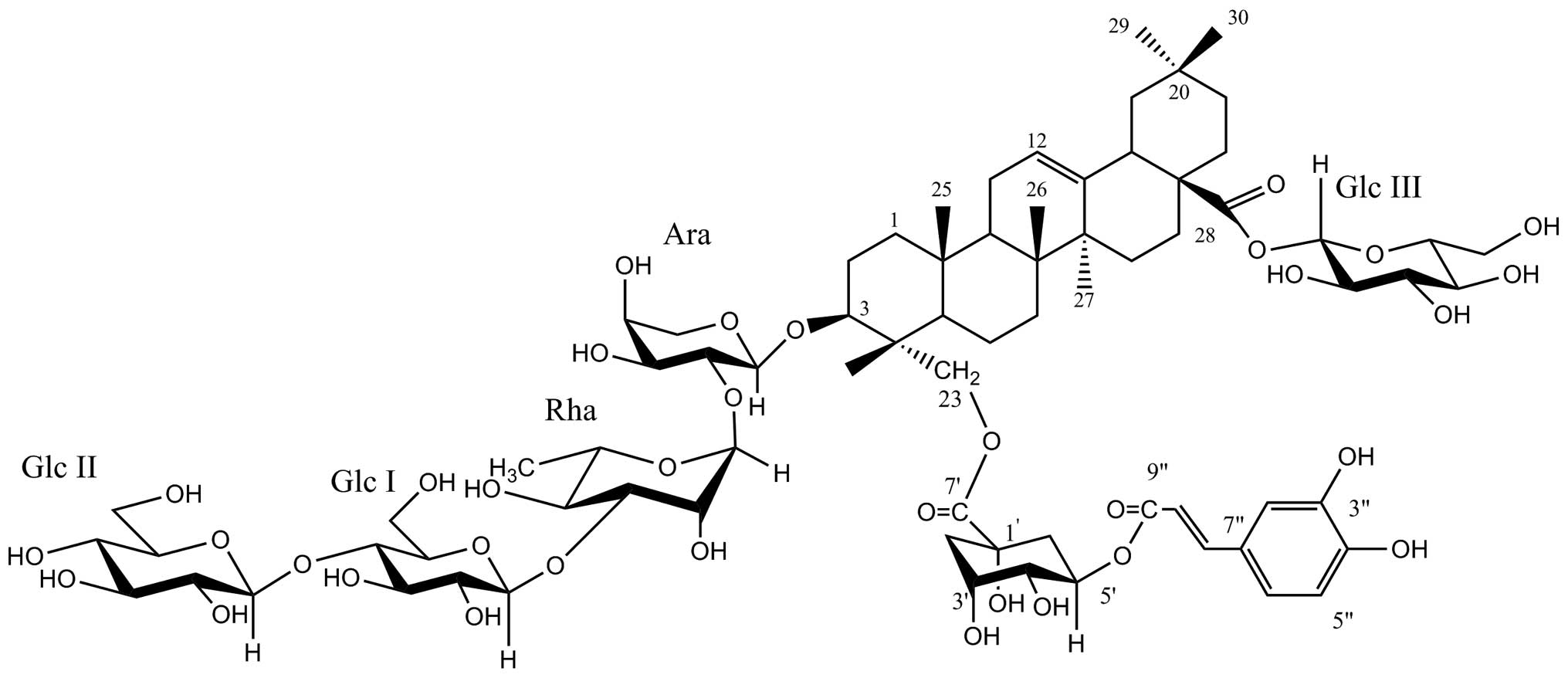
Lonimacranthoide VI (Fig. 1), which was first isolated from the
flower buds of Lonicera macranthoides (Caprifoliaceae) in
our present study, is a rare chlorogenic acid ester acylated at
C-23 of hederagenin (3). Shan et
al (4) reported that
chlorogenic acid significantly decreased LPS-induced upregulation
of COX-2 at protein and mRNA levels in RAW264.7 cells and
consequently inhibited PGE2 release from LPS-treated
RAW264.7 cells, indicating that chlorogenic acid exerted
anti-inflammatory effects. Previously, several studies have
reported that triterpene saponins can also inhibit COX-2 expression
(5,6). However, thus far, the effects of
chlorogenic acid ester saponin on the cyclooxygenase isoforms
(COX-1 and COX-2) have not been analyzed. In the present study, the
effects of lonimacranthoide VI were observed on PGE2
synthesis, in vitro activity of COX-1 and COX-2 and the gene
expression of COX-1 and COX-2. These data provide a mechanistic
basis for the chemopreventive and anti-inflammatory properties of
lonimacranthoide VI.
Materials and methods
Reagents and chemicals
Lonimacranthoide VI was isolated from the flowers of
Lonicera macranthoides Hand.-Mazz. and the structure is
shown in Fig. 1. Lonimacranthoide
VI was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St.
Louis, MO, USA) as a stock solution stored at −20°C and was
subsequently diluted with medium prior to each experiment. The
final DMSO concentration did not exceed 0.1% DMSO throughout the
study (all the control groups comprised 0.1% DMSO).
Cell culture and cell viability
assay
RAW264.7 murine macrophages were obtained from the
Cell Bank of Shanghai Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China) and cultured in
Dulbecco’s modified Eagle’s medium containing 100 U/ml penicillin
and 100 μg/ml streptomycin at 37°C in 5% CO2. The effect
of lonimacranthoide VI on cell viability was assessed by the
2-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, which is based on the reduction of MTT by the mitochondrial
dehydrogenase of intact cells to a purple formazan product.
Briefly, the RAW264.7 cells were seeded at 5×104
cells/well in a 96-well plate and treated with various
concentrations of lonimacranthoide VI or vehicles. Each treated or
control group contained six parallel wells. After incubation for 24
h at 37°C in a humidified incubator, cell viability was determined.
MTT (5 mg/ml in phosphate-buffered saline) was added to each well
and incubated for 4 h; subsequently, 100 μl of the solubilization
solution (10% sodium dodecyl sulphate in 0.012 M HCl) was added
into each well and the plate remained in the incubator overnight.
Absorbance was recorded on a microplate reader (Tecan Austria GmbH,
Salzburg, Austria) at a wavelength of 570 nm (reference wavelength,
690 nm). The percentage of cell proliferation was calculated as a
ratio of the optical density (OD) value of the sample to the OD
value of the control. All the experiments were performed under the
same conditions at least three times. The cell inhibitory ratio was
calculated by the following formula: Inhibitory ratio (%) =
(1−average absorbance of treated group/average absorbance of
control group) ×100%.
Measurement of PGE2
RAW264.7 cells were plated at a density of
2.5×105/ml cells in a 24-well plate with 1 ml of culture
medium per well and cultured overnight. The cells were
pre-incubated for 2 h with various doses of lonimacranthoide VI and
stimulated for 24 h with 100 ng/ml LPS. The cell culture
supernatants were collected immediately following treatment and
centrifuged at 1,000 × g for 15 min to remove the particulate
matter. PGE2 was determined using an enzyme immunoassay
(EIA) kit (catalog no. ADI-900-001, Enzo Life Sciences,
Switzerland). The medium and PGE2 EIA conjugate was
added to a 96-well plate pre-coated with goat anti-mouse IgG and
left to react for 2 h, followed by a final wash to remove any
unbound antibody-enzyme reagent. A substrate solution was added and
the intensity of the color produced was measured at 405 nm
(correction wavelength set at 570–590 nm).
In vitro COX inhibition assay
The ability of lonimacranthoide VI to inhibit ovine
COX-1 and COX-2 was determined using an enzyme immunoassay (EIA)
kit (catalog no. 560101; Cayman Chemical Co., Ann Arbor, MI, USA).
COX catalyzes the first step in the biosynthesis of AA to
PGH2. PGF2α, produced from PGH2 by
reduction with stannous chloride, was measured by EIA (ACE™
competitive EIA, Cayman Chemical, Ann Arbor, MI, USA). Briefly, to
a series of supplied reaction buffer solutions [960 μl 0.1 M
Tris-HCl (pH 8.0) containing 5 mM EDTA and 2 mM phenol] with either
COX-1 or COX-2 (10 μl) enzyme in the presence of heme (10 μl), 10
μl of various concentrations of test drug solutions (1, 10, or 100
μM in a final volume of 1 μl) were added. These solutions were
incubated for 5 min at 37°C and subsequently 10 μl AA solution (100
μM) was added. The COX reaction was stopped by the addition of 50
μl 1 M HCl after 2 min. Then 100 μl of stannus chloride was added
to produce PGF2α, which was measured by EIA. This assay
is based on the competition between PGs and a
PG-acetylcholinesterase conjugate (PG tracer) for a limited amount
of PG antiserum. The amount of PG tracer that is able to bind to
the PG antiserum is inversely proportional to the concentration of
PGs in the wells since the concentration of the PG tracer is held
at a constant while the concentration of PGs varies. The specific
antiserum-PG complex bound to a mouse anti-rabbit IgG that had been
previously attached to the well. The plate was washed to remove any
unbound reagents and 200 μl Ellman’s reagent, which contains the
substrate to acetylcholine esterase, was added to the well. The
product of this enzymatic reaction generates a distinct yellow
color that absorbs at 406 nm. The intensity of this color,
determined by spectrophotometry, is proportional to the amount of
PG tracer bound to the well, which is inversely proportional to the
amount of PGs present in the well during the incubation. Percent
inhibition was calculated by the comparison of the compounds
treated to the various control incubations.
Quantification of mRNA by reverse
transcription-polymerase chain reaction (PCR)
Total cellular RNA was extracted using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and the
concentration of RNA was determined at 260 nm. cDNA was synthesized
by extension of oligo (dT) primers with 10 units of avian
myeloblastosis virus reverse transcriptase in a mixture containing
1 μg total RNA. The cDNA amplification was performed using the PCR
kit (Takara Bio, Inc., Shiga, Japan), denaturation at 95°C for 30
sec, annealing at 55°C for 30 sec and extension at 72°C for 1 min.
The primer sequences used were as follows: COX-2 sense,
5′-GGGAAGCCTTCTCCAACC-3′ and antisense, 5′-GAACCCAGGTCCTCGCTT-3′;
and GADPH sense, 5′-AACGACCCCTTCATTGACC-3′ and antisense,
5′-TCAGATGCCTGCTTCACC-3′, which was used as an internal control.
The PCR products (10 μl) were separated on 2% agarose gel and
visualized by ethidium bromide staining. The gel image was captured
and analyzed using Quantity One software (Tanon Science and
Technology Co., Shanghai, China).
Statistical analysis
All the experiments were repeated at least three
times. Data are reported as means ± standard deviation. The
statistically significant differences of the test compounds
compared to the untreated control were calculated using the
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of lonimacranthoide VI on the cell
viability
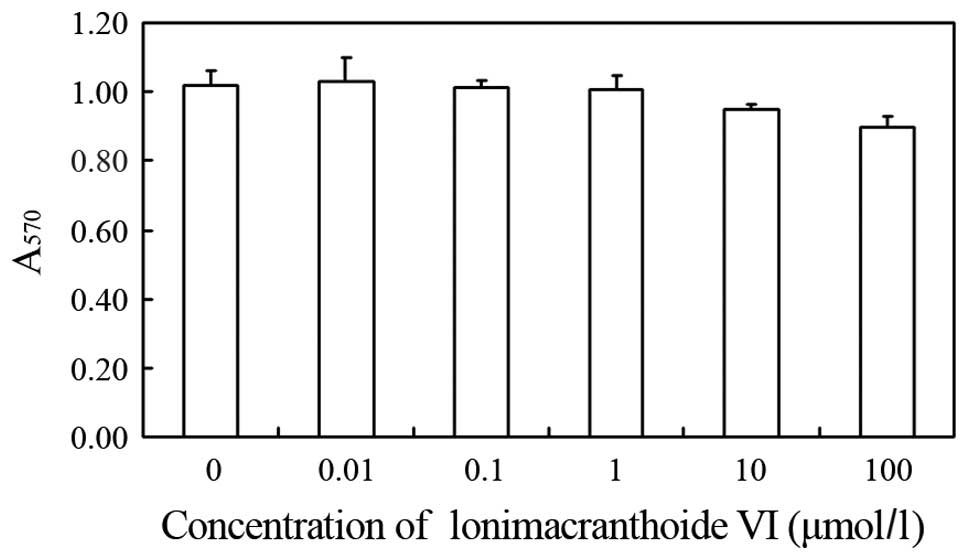
To evaluate the anti-inflammatory effects of
lonimacranthoide VI, a murine RAW264.7 macrophages in vitro
model was used. RAW264.7 cells were treated with various
concentrations of lonimacranthoide VI and cell viability was
measured using the MTT assay. As shown in Fig. 2, the resulting survival curve shows
that lonimacranthoide VI does not have cytotoxic effects on the
proliferation of cells. As lonimacranthoide VI showed no
cytotoxicity at concentrations ≤100 μM in RAW264.7 macrophages,
lonimacranthoide VI was used at a concentration of 0–100 μM for the
remaining experiments.
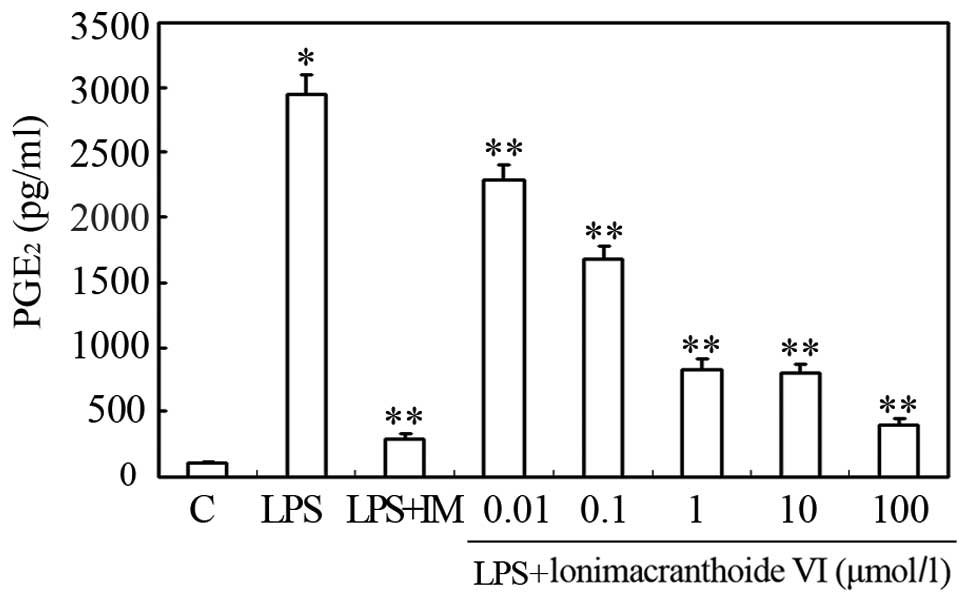
Effect of lonimacranthoide VI on serum
PGE2 concentration
Since PGE2 is one of the most important
inflammatory mediators, the effects of lonimacranthoide VI on the
LPS-induced release of PGE2 from RAW264.7 cells were
observed. The cells were pretreated with lonimacranthoide VI for 2
h followed by incubation with 100 ng/ml LPS. One day after LPS
treatment, the PGE2 contents in the culture medium were
detected. The LPS-induced PGE2 secretion level was
inhibited by treatment with the compound at all the doses examined
(IC50=0.25 μM) and the maximum inhibition was observed
at a dose of 100 μM (Fig. 3).
Indomethacin (IM) was used as a positive control.
In vitro COX inhibition
COX-1 and COX-2 catalyze the biosynthesis of
PGH2 from the AA substrate. The inhibition of COX-1
results in certain undesirable side-effects, whereas COX-2
inhibition provides therapeutic effects in pain, inflammation,
cancer, glaucoma, Alzheimer’s and Parkinson disease (7). Therefore, the present study aimed to
examine the COX-1 and COX-2 inhibitory activity of lonimacranthoide
VI on purified enzymes as a mechanism of topical anti-inflammatory
action. The compound showed inhibitory effects on COX-1 and COX-2
(Tables I and II). Furthermore, 10 μM lonimacranthoide
VI inhibited COX-2, whereas the dose had no effect on COX-1.
 | Table IInhibitory effects of lonimacranthoide
VI on in vitro COX-1 enzyme activity. |
Table I
Inhibitory effects of lonimacranthoide
VI on in vitro COX-1 enzyme activity.
| Group | PGF2α,
pg/ml | Inhibitory rate,
% |
|---|
| COX-1 inhibitor
tubes | 3.5±0.3 | - |
| COX-1 100% initial
activity tubes | 151.4±21.5 | - |
| IM, μmol/l | | |
| 10 | 3.5±0.5 | 97.68 |
| Lonimacranthoide VI,
μmol/l | | |
| 100 | 15.0±0.8 | 90.08 |
| 10 | 143.1±45.5 | 5.45 |
| 1 | 205.5±3.0 | −35.80 |
 | Table IIInhibitory effects of lonimacranthoide
VI on in vitro COX-2 enzyme activity. |
Table II
Inhibitory effects of lonimacranthoide
VI on in vitro COX-2 enzyme activity.
| Group | PGF2α,
pg/ml | Inhibitory rate,
% |
|---|
| COX-2 inhibitor
tubes | 0.9±0.1 | - |
| COX-2 100% initial
activity tubes | 144.5±29.3 | - |
| NS-398, μmol/l | | |
| 10 | 37.3±4.1 | 74.19 |
| Lonimacranthoide VI,
μmol/l | | |
| 100 | 40.5±7.0 | 71.99 |
| 10 | 116.5±5.6 | 19.39 |
| 1 | 143.5±6.5 | 0.66 |
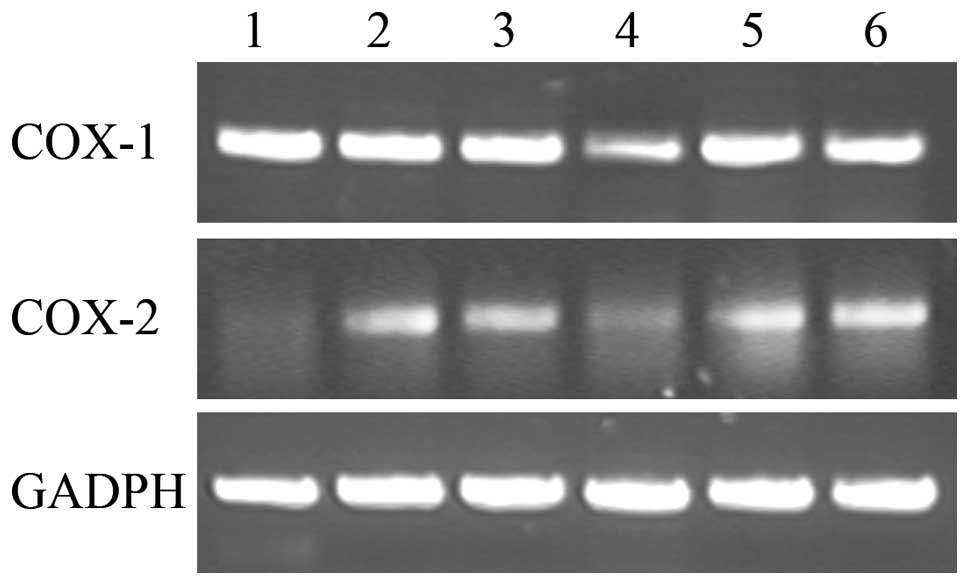
Effects of lonimacranthoide VI on mRNA
expression of COX-1 and COX-2
To investigate the effects of lonimacranthoide VI on
mRNA expression of COX-1 and COX-2, the RAW264.7
macrophage cells were pre-treated with the compound at various
concentrations ranging 1–100 μM and were stimulated with 100 ng/ml
LPS for 24 h. The COX-2 non-selective inhibitor, IM, was used as a
standard drug for comparing the ability of lonimacranthoide VI in
modulating the pro-inflammatory genes, COX-1 and
COX-2. As shown in Fig. 4,
100 ng/ml LPS can stimulate COX-2 mRNA expression, whereas
it had no significant effects on the COX-1 mRNA expression.
In addition, 100 μM lonimacranthoide VI can significantly suppress
the mRNA expression of COX-1 and COX-2 in the
LPS-stimulated RAW264.7 macrophage cells as compared to LPS-treated
cells alone. However, the presence of IM only produced a
significant reduction of COX-2 mRNA expression in
LPS-treated cells.
Discussion
Natural products play a significant role in drug
discovery and development. The search for natural products with
anti-inflammatory activity has increased in recent years (8). The dried flower buds of Lonicera
macranthoides Hand.-Mazz., a plant of Lonicera in
Caprifoliaceae, are commonly used in traditional Chinese medicine
in the Southwest of China (9).
Lonicera plants have antipyretic and detoxification
properties and have been widely used to treat carbuncles and boils,
toxins in blood, fever and colds (9). The study by Liu et al (10) reported that the total saponins of
Lonicera fulvotnetosa Hsu et S. C. Cheng. Ms could inhibit
the mouse ear edema provoked by croton oil and also the
carrageenan-induced hind paw edema in rats. Following this,
loniceroside A and loniceroside C, isolated from Lonicera
japonica, were shown to possess anti-inflammatory activity in a
croton oil-induced ear edema model in vivo (11,12).
In the present study, lonimacranthoide VI was demonstrated to
inhibit the LPS-induced production of PGE2, indicating
anti-inflammatory effects. Therefore, lonimacranthoide VI is an
important anti-inflammatory constituent of Lonicera
macranthoides.
Inflammation is the response towards the presence of
pathogens, chemicals or mechanical injury. The inflammatory
response is induced by inflammatory mediators generated via a
series of inducible genes that have critical functions in the host
immune defence, signal transduction pathways and vascular
regulation. The cyclooxygenase isoforms (COX-1 and COX-2) are among
the most thoroughly studied mammalian oxygenases involved in the
inflammation response pathway (13). The results of Fig. 4 and Table II indicated that lonimacranthoide
VI appeared to produce a significant dose-dependent reduction on
the mRNA expression and in vitro activity of COX-2.
By contrast, lonimacranthoide VI (concentration range, 1–10 μM) did
not suppress the mRNA expression and in vitro activity of
COX-1 (Fig. 4, Table I) in the LPS-stimulated RAW264.7
macrophage cells as compared to LPS-treated cells alone. However,
the COX-1 gene expression and in vitro activity of 100
μmol/l lonimacranthoide VI was significantly lower compared to
LPS-treated cells alone (Fig. 4,
Table I). This indicates that
higher concentrations of lonimacranthoide VI may induce inhibition
of COX-1 expression and activity. The decrease in PGE2
production following lonimacranthoide VI treatment corresponded
with the decrease in COX (COX-1 and COX-2)
mRNA expression and in vitro activity, particularly for
COX-2.
A number of studies have demonstrated that the
expression of COX-2 is largely regulated by transcriptional
activation (14,15). Lipopolysaccharide and other
pro-inflammatory cytokines activate NF-κB, which is a mammalian
transcription factor that regulates several genes important in
immunity and inflammation. NF-κB binding sites have been identified
on the murine COX-2 promoter, which plays a role in LPS-mediated
induction of COX-2 in macrophages. In addition, binding of
CCAAT-enhancer-binding proteins (C/EBPs), c-AMP response element
binding proteins (CREBs) and c-Jun to the COX-2 promoter enhances
its transcriptional activation (16). The present study is limited to
understanding the gene expression and in vitro activity of
COX-1 and COX-2 and the production of PGE2, therefore,
it may be noteworthy to understand the effect of lonimacranthoide
VI at the transcriptional activation level involving the NF-κB,
C/EBP, CREB and c-Jun proteins.
In conclusion, lonimacranthoide VI was found to
inhibit mRNA expression and in vitro activity of
COX-2 and PGE2 production in a dose-dependent
manner. Although lower concentrations of lonimacranthoide VI did
not significantly reduce the mRNA expression and in vitro
activity of COX-1 in the LPS-stimulated RAW264.7 macrophage
cells, a higher concentration may possibly reduce COX-1
expression and in vitro activity further. To the best of our
knowledge, this is the first study explaining the anti-inflammatory
pathway of lonimacranthoide VI, which provides support to the
traditional utilization of this plant in pain and inflammation. The
study clearly indicates that lonimacranthoide VI inhibits the
production of PGE2 via the inhibition of COX-2
expression and activity; however, caution is recommended as LMS4-1
may inhibit the COX-1 expression at higher doses.
Acknowledgements
The present study was financially supported by the
Open Fund of Jiangsu Key Laboratory for Bioresources of Saline
Soils (grant no. JKLBS2013003), the Institute of Botany, Jiangsu
Province and the Chinese Academy of Sciences (grant no. 201201),
the Jiangsu Provincial Natural Science Foundation of China (grant
no. BK20131338) and the National Key Technologies R&D Program
of China during the 11th Five-Year Plan Period (grant no.
2009ZX09103-397).
References
|
1
|
Ricciotti E and FitzGerald GA:
Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol.
31:986–1000. 2011. View Article : Google Scholar
|
|
2
|
Williams CS, Mann M and DuBois RN: The
role of cyclooxygenases in inflammation, cancer, and development.
Oncogene. 18:7908–7916. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shan Y, Feng X, Guan FQ, et al: The
preparation and pharmaceutical applications of a new triterpenoid
saponin from Lonicera macranthoides. Patent 201110284742.8.
Filed September 23, 2011; issued April 25, 2012.
|
|
4
|
Shan J, Fu J, Zhao Z, Kong X, Huang H, Luo
L and Yin Z: Chlorogenic acid inhibits lipopolysaccharide-induced
cyclooxygenase-2 expression in RAW264.7 cells through suppressing
NF-kappaB and JNK/AP-1 activation. Int Immunopharmacol.
9:1042–1048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi S, Jiang D, Dong C and Tu P:
Triterpene saponins from Clematis mandshurica. J Nat Prod.
69:1591–1595. 2006. View Article : Google Scholar
|
|
6
|
Kim DH, Shin EK, Kim YH, Lee BW, Jun JG,
Park JH and Kim JK: Suppression of inflammatory responses by
celastrol, a quinone methide triterpenoid isolated from
Celastrus regelii. Eur J Clin Invest. 39:819–827. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blobaum AL and Marnett LJ: Structural and
functional basis of cyclooxygenase inhibition. J Med Chem.
50:1425–1441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen S: Natural products triggering
biological targets - a review of the anti-inflammatory
phytochemicals targeting the arachidonic acid pathway in allergy
asthma and rheumatoid arthritis. Curr Drug Targets. 12:288–301.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu XF, Li HJ, Li P, Feng X and Yuan CQ:
Chemical constituents in bud of Lonicera macranthoides. Chin
J Nat Med. 1:45–48. 2006.
|
|
10
|
Liu J, Xia L and Chen XF: The
antiinflammatory of total saponins from Lonicera fulvotnetosa
Hsu et S. C. Cheng Ms. Chin Pharmacol Bull. 9:3951988.
|
|
11
|
Lee SJ, Shin EJ, Son KH, Chang HW, Kang SS
and Kim HP: Anti-inflammatory activity of the major constituents of
Lonicera japonica. Arch Pharm Res. 18:133–135. 1995.
View Article : Google Scholar
|
|
12
|
Kwak WJ, Han CK, Chang HW, Kim HP, Kang SS
and Son KH: Loniceroside C, an antiinflammatory saponin from
Lonicera japonica. Chem Pharm Bull (Tokyo). 51:333–335.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clària J: Cyclooxygenase-2 biology. Curr
Pharm Des. 9:2177–2190. 2003.
|
|
14
|
Mestre JR, Rivadeneira DE, Mackrell PJ, et
al: Overlapping CRE and E-box promoter elements can independently
regulate COX-2 gene transcription in macrophages. FEBS Lett.
496:147–151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang YJ, Wingerd BA, Arakawa T and Smith
WL: Cyclooxygenase-2 gene transcription in a macrophage model of
inflammation. J Immunol. 177:8111–8122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Srivastava JK, Pandey M and Gupta S:
Chamomile, a novel and selective COX-2 inhibitor with
anti-inflammatory activity. Life Sci. 85:663–669. 2009. View Article : Google Scholar : PubMed/NCBI
|