Introduction
Osteoporosis is a systemic skeletal disease
characterized by low bone mass and a deterioration of trabecular
microarchitecture, resulting in an increase in bone fragility and a
tendency to fractures (1).
Osteoporosis is standardly diagnosed via the measurement of bone
mineral density (BMD) by dual-energy X-ray absorptiometry (DXA).
The operational World Health Organization definition of
osteoporosis is based on a T-score of ≤-2.5 (2). Bone turnover markers (BTM) do not
establish the diagnosis of osteoporosis. While DXA reflects changes
in BMD 1–2 years after anti-osteoporotic treatment, BTM levels were
altered after 3–6 months of therapy so they may provide earlier
information on the response to treatment (3–7). In
the present study, the clinical potential of cathepsin K (CatK)
serum levels for monitoring the treatment of osteoporosis with
zoledronic acid is studied.
The prevalence of osteoporosis in Germany among
persons aged ≥50, as revealed by diagnoses of osteoporosis or
osteoporotic fractures, or by the prescription of medication for
osteoporosis, was found to be 14% in the year 2009. The
gender-specific prevalence was 24% in women and 6% in men (8).
CatK is mainly expressed in mature osteoclasts,
cleaves both helical and telopeptide regions of collagen I and is
therefore essential for bone resorption (9). This cysteine protease was first
described by Inaoka et al (10). Deficiency of CatK results in
pyknodysostosis (Toulouse Lautrec syndrome), an autosomal recessive
osteosclerotic skeletal dysplasia with impaired bone resorption,
which is characterized by decreased bone turnover and an
accumulation of undigested collagen fibrils (11). Saftig et al (12) showed that in CatK-deficient mice
impaired osteoclastic bone resorption leads to osteopetrosis. Due
to the fact that CatK is expressed and secreted by osteoclasts
during active bone resorption, it may be a useful and specific
biochemical marker of osteoclastic activity. Henriksen et al
(13) postulated that circulating
levels of CatK are proportional to the number of osteoclasts, and
thus can be used as a surrogate marker of osteoclast number.
Materials and methods
Study subjects
In total, nine postmenopausal females aged 62–75
years were included. Of these, seven females could be monitored
completely with DXA before and 1 year after anti-osteoporotic
therapy with intravenous infusion of zoledronic acid, and
furthermore, the levels of serum CatK before and 3, 6 and 12 months
after therapy could be assessed. Two individuals had missing
observations after 1 year. The indication for anti-osteoporotic
treatment was according to the guidelines of the Dachverband
Osteologie (DVO) by the lowest T-score in DXA and associated risk
factors (14). Surgery or fracture
≤12 months, malignant tumor, ovariectomy and the intake of drugs
prior to the beginning of treatment, including cortisone,
strontium, fluorides, bisphosphonates, selective estrogen receptor
modulators, estrogens and steroids, were exclusion criteria.
Ethical approval
The study was conducted according to the ethical
guidelines of the Declaration of Helsinki as revised in 1989 and
approved by the Ethical Committee of the Otto von Guericke
University (91/08) and the German Office of Radiation Protection in
Salzgitter (no. Z5-22462/2-2009-022).
BMD measurements
All the female patients underwent bone density
measurements before and 1 year after treatment. Radiographs of the
spine were not performed, as there were no references for vertebral
fractures, such as back pain and loss of height, in the medical
history or examination. The measurement of BMD was performed at the
lumbar spine (L1-L4), femoral neck and total hip with DXA as
described previously (15).
Laboratory
Serum parameters, according to the DVO guidelines,
were examined to exclude secondary osteoporosis, and furthermore,
25-(OH)-vitamin D. The serum samples were aliquoted and stored at
−80°C until analysis. The measurement of CatK was performed before
and 3, 6 and 12 months after the beginning of treatment [ELISA;
Biomedica, Wien, Austria; intra-assay coefficient of variance (CV)
4–6%, inter-assay CV 6–8%, detection level 1.1 pmol/l] as described
previously (15).
Anti-osteoporotic therapy
A total of 100 ml (5 mg) zoledronic acid
(Aclasta®; Novartis, Basel, Switzerland) was infused
intravenously within 15 min. In all females, calcium-supplementing
was completed with 500 mg daily. Individually, a daily
supplementation of 400–2,000 U of vitamin D3 was carried out.
Statistical analysis
The statistical analyses were performed with SPSS
(version 21; SPSS, Inc., Chicago, IL, USA) using non-parametric
tests, as graphical inspection indicated deviations from the normal
distribution. Data for the BMD were evaluated with the Wilcoxon
signed rank test due to the small random sample. The serum
parameters with four time measurements were analysed by Friedman
test. The individual time points were compared to the baseline
value with the Wilcoxon matched-pairs signed rank test. A two-sided
P-value of 0.05 was set to be the level of significance and was
considered to indicate a statistically significant difference.
Bonferroni adjustment was performed to control for family-wise
error rate. The results are illustrated in median, minimum and
maximum.
Results
CatK serum levels
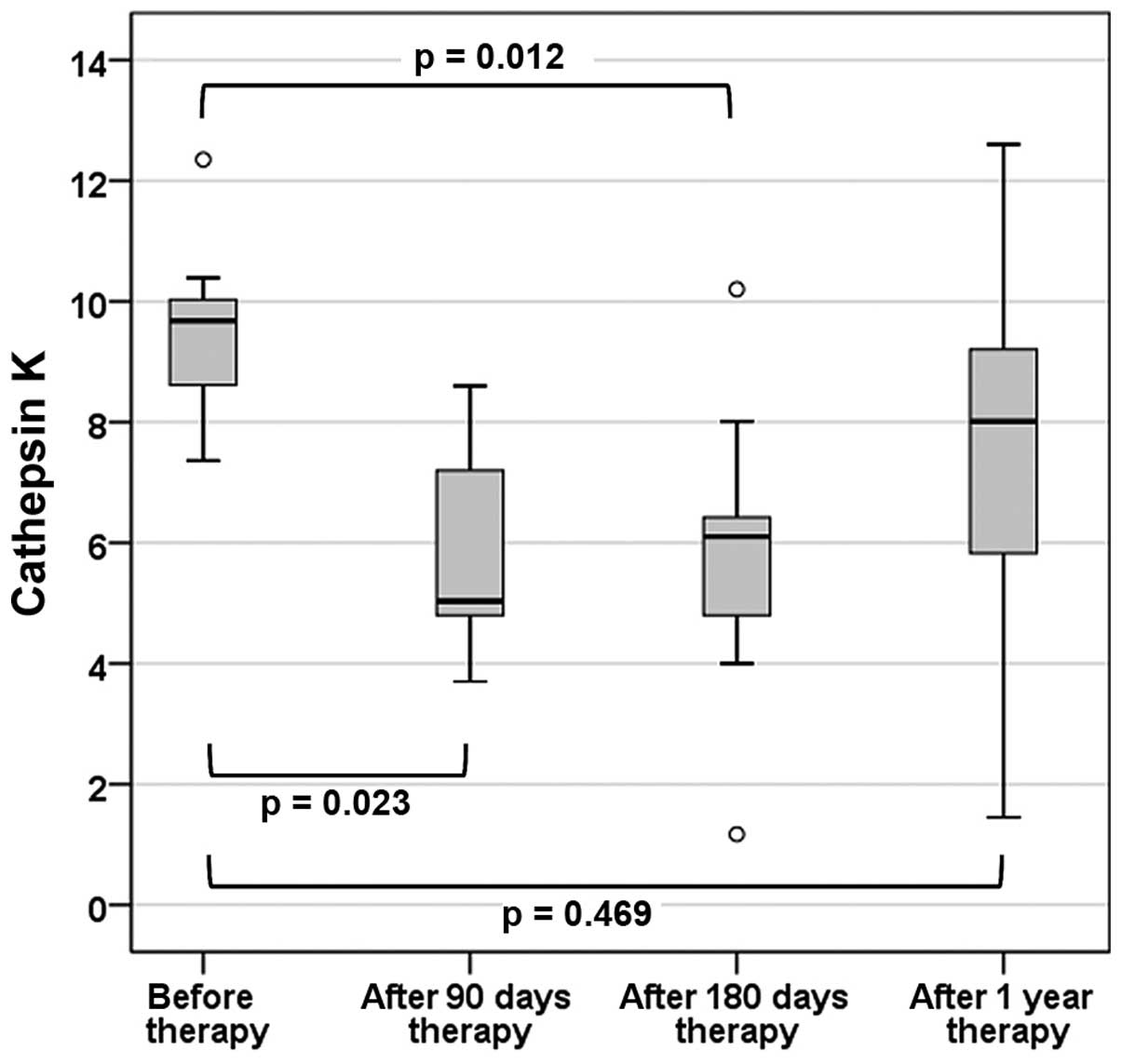
Overall, no significant changes in the lowest
T-Score were observed 1 year after anti-osteoporotic treatment
(P=0.523). A significant improvement was only observed in the
lumbar spine (P=0.008). Serum CatK presented a significant change
over the four visits (P=0.003). In particular, there was a
significant decrease in the first 3 (P=0.023) and 6 months
(P=0.012) after the start of therapy. One year after the initiation
of treatment, the CatK levels almost reached the base level
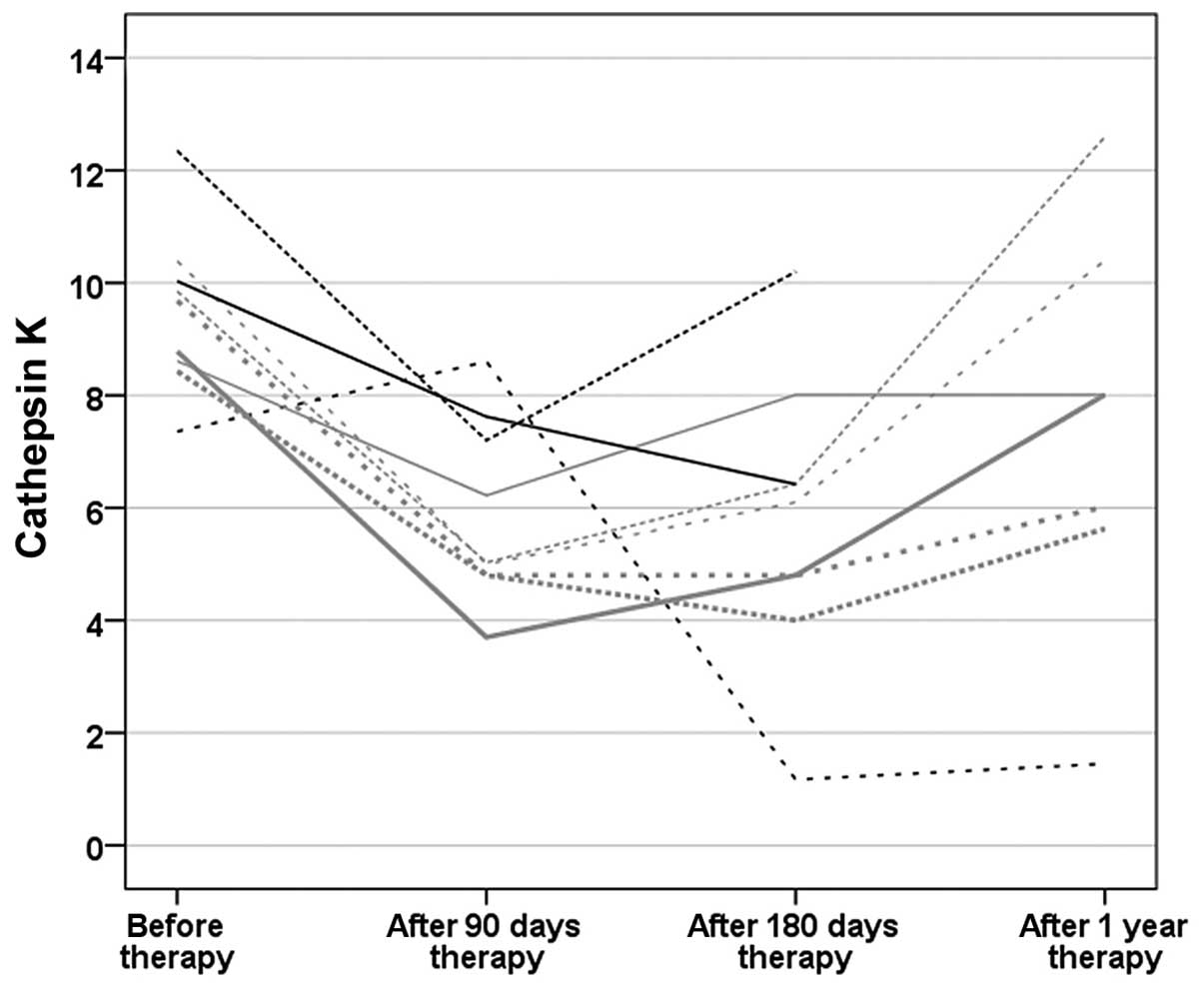
(P=0.469) (Fig. 1). Individual
serum CatK levels at 3, 6 and 12 months after antiresorptive
therapy are shown in Fig. 2. The
strongest decrease in the majority of cases was observed after 3
months. In one case, the decrease set on 6 months after therapy was
started, with low residual values. In the other females the CatK
serum levels increased after 1 year following intravenous
zoledronic acid.
Discussion
The study by Holzer et al (16) monitored serum CatK concentrations of
18 patients for 14.25±11.57 months (range, 1–48 months) after the
start of osteoporosis treatment (oral bisphosphonate plus a
combination of calcium and vitamin D). Serum CatK concentrations
clearly decreased (mean at initial examination, 7.10±3.8 pmol/l;
after treatment, 5.9±3.4 pmol/l), but did not demonstrate
significance (t=1.362, df=17, P=0.191). Meier et al
(17) examined longitudinally 21
females with postmenopausal osteoporosis and 10 patients with
Paget’s disease of bone. All the patients started on oral
(alendronate or risedronate) or intravenous bisphosphonate
(pamidronate or zoledronate) treatment and were followed
prospectively over 6 months. In postmenopausal osteoporotic
females, oral and intravenous bisphosphonate treatment resulted in
a significant reduction in serum CatK levels (P=0.03) with the
majority of the effect occurring after 1 month (mean % change,
−33%). In patients with mild Paget’s disease, serum CatK levels
decreased during bisphosphonate treatment. The study by
Munoz-Torres et al (18)
included 46 postmenopausal females with osteoporosis, which were
treated with oral bisphosphonate (alendronat) plus calcium and
vitamin D. The serum CatK levels gradually decreased after
alendronate treatment (17, 22 and 41% at 3, 6 and 12 months,
respectively; P<0.01). According to the current literature and
the data of the present explorative analysis, serum CatK appears to
be a putative marker for monitoring and controlling
anti-osteoporotic treatment. The fast decrease of serum levels in
the majority of the study subjects, already after 3 months, may
provide early information on the response to treatment. Future
studies are required to explore whether this information can be
used for early allocation of patients to different treatments.
Acknowledgements
The present study was supported by the ‘LOM program’
of the Otto von Guericke University.
References
|
1
|
No authors listed. Consensus Development
Conference on Osteoporosis. Hong Kong, April 1–2, 1993. Am J Med.
95:S1–S78. 1993.PubMed/NCBI
|
|
2
|
No authors listed. Assessment of fracture
risk and its application to screening for postmenopausal
osteoporosis. Report of a WHO Study Group. World Health Organ Tech
Rep Ser. 843:1–129. 1994.PubMed/NCBI
|
|
3
|
Delmas PD: Markers of bone turnover for
monitoring treatment of osteoporosis with antiresorptive drugs.
Osteoporos Int. 11(Suppl 6): S66–S76. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delmas PD, Eastell R, Garnero P, Seibel MJ
and Stepan J; Committee of Scientific Advisors of the International
Osteoporosis Foundation. The use of biochemical markers of bone
turnover in osteoporosis. Committee of Scientific Advisors of the
International Osteoporosis Foundation. Osteoporos Int. 11(Suppl 6):
S2–S17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Delmas PD, Hardy P, Garnero P and Dain M:
Monitoring individual response to hormone replacement therapy with
bone markers. Bone. 26:553–560. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seibel MJ: Biochemical markers of bone
remodeling. Endocrinol Metab Clin North Am. 32:83–113. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szulc P: The role of bone turnover markers
in monitoring treatment in postmenopausal osteoporosis. Clin
Biochem. 45:907–919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hadji P, Klein S, Gothe H, Häussler B,
Kless T, Schmidt T, Steinle T, Verheyen F and Linder R: The
epidemiology of osteoporosis - Bone Evaluation Study (BEST): an
analysis of routine health insurance data. Dtsch Arztebl Int.
110:52–57. 2013.PubMed/NCBI
|
|
9
|
Troen BR: The role of cathepsin K in
normal bone resorption. Drug News Perspect. 17:19–28. 2004.
View Article : Google Scholar
|
|
10
|
Inaoka T, Bilbe G, Ishibashi O, Tezuka K,
Kumegawa M and Kokubo T: Molecular cloning of human cDNA for
cathepsin K: novel cystein protease predominantly expressed in
bone. Biochem Biophys Res Commun. 206:89–96. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gelb BD, Shi G, Chapman HA and Desnick RJ:
Pycnodysostosis, a lysosomal disease caused by cathepsin K
deficiency. Science. 273:1236–1238. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saftig P, Hunziker E, Wehmeyer O, Jones S,
Boyde A, Rommerskirch W, Moritz JD, Schu P and von Figura K:
Impaired osteoclastic bone resorption leads to osteopetrosis in
cathepsin-K-deficient mice. Proc Natl Acad Sci USA. 95:13453–13458.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henriksen K, Tanko LB, Qvist P, Delmas PD,
Christiansen C and Karsdal MA: Assessment of osteoclast number and
function: application in the development of new and impoved
treatment modalities for bone diseases. Osteoporos Int. 18:681–685.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dachverband Osteologie e.v. DVO-Leitlinie
2009 zur Prophylaxe, Diagnostik und Therapie der Osteoporose bei
Erwachsenen. Osteologie. 18:304–328. 2009.
|
|
15
|
Adolf D, Wex T, Jahn O, Riebau C, Halangk
W, Klose S, Westphal S, Amthauer H, Winckler S and Piatek S: Serum
cathepsin K levels are not suitable to differentiate women with
chronic bone disorders such as osteopenia and osteoporosis from
healthy pre- and postmenopausal women. Maturitas. 71:169–172. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holzer G, Noske H, Lang T, Holzer L and
Willinger U: Soluble cathepsin K: a novel marker for the prediction
of nontraumatic fractures? J Lab Clin Med. 146:13–17. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meier C, Meinhard U, Greenfield JR, De
Winter J, Nguyen TV, Dunstan CR and Seibel MJ: Serum cathepsin K
concentrations reflect osteoclastic activity in women with
postmenopausal osteoporosis and patients with Paget’s disease. Clin
Lab. 52:1–10. 2006.PubMed/NCBI
|
|
18
|
Munoz-Torres M, Reyes-Garcia R,
Mezquita-Raya P, Fernandes-Garcia D, Alonso G, de Luna JD,
Ruiz-Requena ME and Escobar-Jimenez F: Serum cathepsin K as a
marker of bone metabolism in postmenopausal women treated with
alendronate. Maturitas. 64:188–192. 2009. View Article : Google Scholar : PubMed/NCBI
|