Introduction
Angiogenesis, the growth of new blood vessels, plays
an important role in tumor development and metastasis.
Antiangiogenic therapies have been demonstrated to inhibit tumor
growth and prolong progression-free survival and/or overall
survival. Vascular endothelial growth factor (VEGF) is thought to
be the major regulator of physiological and pathological
angiogenesis. A decrease of the serum VEGF level will directly
influence the downstream angiogenic process (1–3).
Chai Hu Long Gu Mu Li soup, a classic formula of
Traditional Chinese medicine (TCM), was described in a TCM
monograph (Shang Han Lun) by Ji Zhang, a Han dynasty physician
(A.D. 150–219). Feijining Decoction (FJND) was developed by
Professor Dongfeng Yin [Affiliated Hos-pital of Liaoning University
of Traditional Chinese Medicine (AHTCM), Liaoning, China] from Chai
Hu Long Gu Mu Li soup, and has been used to treat lung cancer for
decades. Our previous experimental study confir-med that FJND
inhibited the growth of A549 cells, which is possibly associated
with decreased VEGF expression (4).
According to recent research, VEGF also exerts a
systemic influence on immune cell development and function. In
cancer, VEGF is present at high levels in the tumor and the
systemic circulation. Elevated levels of circulating VEGF inhibit
T-cell immune responses (5,6). Immunotherapy is a central component of
numerous cancer treatment regimens as well.
Ginseng, a prime ingredient in FJND, is also
particularly popular as a treatment among cancer patients, since
multiple studies have associated the consumption of ginseng with
cancer prevention and treatment, and with an improved well-being
(such as cancer-related fatigue and immunity) during cancer therapy
(7,8).
Ginsenoside Rg3, an extract from ginseng, has demonstrated
anti-cancer activity in vitro and in vivo with
relatively low toxicity, particularly on vessels or angiogenesis in
tumors, and it selectively suppressed VEGF expression (9,10). In order
to explore the an-titumor effects of FJND, the present study was
performed on Lewis lung carcinoma (LLC)-bearing mice.
Materials and methods
Animals and cells
Sixty male C57BL/6 mice weighing 20±2 g were used in
the study. The mice were obtained from the Institute of Laboratory
Animal Science, Chinese Academy of Medical Sciences (Beijing,
China; Certificate of Conformity: SCXK Jing 2004-0001). The animals
were maintained in a pathogen-free facility (22±2°C, 55±5%
humidity) and a 12-h light/dark cycle with lights on from 07:00 to
19:00 h daily. Food and water were provided ad libitum. All
the procedures on treating mice were performed according to the
Animal Care Guidelines issued by the Ministry of Science and
Technology of China and the Animal Care Committee of Liaoning
University of Traditional Chinese Medicine approved the
protocols.
LLC cells were obtained from the Department of
Immunology, College of Basic Medical Science, China Medical
University (Wuhan, China) and maintained in Dulbecco's modified
Eagle's medium supplemented with 100 ml/l fetal bovine serum,
penicillin (1×105U/l) and streptomycin (100 mg/l) with
5% CO2 at 37°C in a humidified environment.
Chemicals and reagents
Cisplatinum (DDP; lot no. 6120251DB) was obtained
from Qilu Pharmaceutical Co., Ltd., Jinan, Shandong, China. FJND
consisted of 13 herbal materials, including Radix Bupleuri
(10 g), Scutellaria baicalensis Georgi (15 g), Rhizoma
Pinelliae (10 g), Ginseng (10 g), Fossilia Ossis
Mastodi (25 g), Concha Ostreae (25 g), Psuedobulbus
Cremastrae (15 g), Zedoary Rhizoma (15 g),
Fritillariae Thunbergii (15 g), Radix Platycodi (15
g), Hedyotis Diffusa Willd (20 g), Indian buead (15 g) and
Radix Glycyrrhizae (10 g). All were purchased from the
Hospital Pharmacy of AHTCM. FJND was extracted by a routine method
which is used in the Key Laboratory of Department of Integrated
Traditional Chinese and Western Medicine, Peking University School
of Oncology, as reported previously (11–13).
LLC-bearing mice
Solid-type LLC was prepared by subcutaneous
transplantation of 1×107 cells (0.2 ml) into the armpits
of 60 C57BL/6 mice. The tumor volume was determined every two days
by direct measurement with calipers and calculated using the
formula: [Width2 (mm2) × length (mm)]/2. The
treatment was initiated while all the tumor volumes were >100
mm3 in ≥40 mice. Forty mice were randomly assigned to
four groups (10 animals/group); control (CG), DDP (DG), FJND (FG)
and FJND + DDP groups (FDG). A 0.9% NaCl solution (0.4 ml/20 g) was
administered intragastrically once daily for two weeks in CG and DG
mice, and a 0.9% NaCl solution (0.1 ml/20 g) was administered
intraperitoneally once daily for 1, 3 and 5 days in CG and FG mice.
DG and FDG mice were provided DDP (0.1 ml, 0.1 mg/20 g)
intraperitoneally once daily for 1, 3 and 5 days. FG and FDG mice
were administered FJND (0.4 ml, 0.62 g/20 g) intragastrically on
the same schedule. The mice were treated daily for two weeks and
sacrificed on day 15.
Spontaneous activity, tumor weight,
thoracic gland and spleen index
Spontaneous activity in 5 min was observed by the
photoelectric counting method and all the mice were weighed every
other day. Tumor, thoracic gland and spleen tissues were cut to
calculate the inhibitory rate (IR), thoracic gland index and spleen
index. The IR was calculated as: [(Average tumor weight in the CG
group - average tumor weight in the treatment group) / average
tumor weight in the control group] × 100%. The thoracic (spleen)
index was calculated as: (Thoracic (spleen) weight / body weight) ×
100%.
Flow cytometry for CD4+ and
CD8+
On day 15, the mice were sacrificed by cervical
dislocation, and the spleen and thymus were quickly removed and
weighed. The spleen tissues were gently teased to release cells by
means of dissecting forceps in phosphate-buffered saline (PBS; pH
7.4). The lymphocytes were isolated using density gradient
centrifugation (lymphocyte separation medium). The number of
lymphocytes was measured using a Coulter counter. The cell
concentration was adjusted to 1×1010 cells/l and 10 µl
fluorescein isothiocyanate-labeled anti-mouse CD4 and CD8 (cat.
.no. 11-0041-82 and cat.no. 12-0081-82, respectively; both from
eBioscience, San Diego, CA, USA) was added to 100 µl of the cell
suspension. After incubation for 30 min at 4°C, the lymphocytes
were rinsed three times with 1 ml PBS and centrifuged at 500 × g
for 3 min. Subsequently, CD4+ and CD8+ were
analyzed by flow cytometry (BD Biosciences, San Jose, CA, USA).
Pathological section
A portion of tumor tissue was fixed by immersion in
10% buffered formalin (pH 7.4) for 24 h. The fixed tissue was
dehydrated in graded ethanol, paraffin-embedded and sectioned at a
thickness of 4 µm. The tumor histology of each group was observed
under a light microscope.
Western blot analysis
VEGF protein expression was determined for each
group by western blot analysis of the protein extracts obtained
from the tumor tissue. The protein concentrations were determined
by a bicinchoninic acid protein assay (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Proteins (30 µg) were separated by 12%
SDS-PAGE, transferred to polyvinylidene fluoride and blocked with
skimmed milk for 2 h at room temperature and incubated with the
primary antibody (1:1,000; rabbit polyclonal antibody; BA0407;
Wuhan Boster Biological Technology, Ltd., Wuhan, China) at 4°C
overnight. The next day the membranes were incubated with the
secondary antibody for 1 h (1:5,000; peroxidase-conjugated
AffiniPure goat anti-rabbit IgG (H+L); cat. no. ZB-2301; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China).
The targeted proteins were visualized by autoradiography. The
grayscale was measured using the software BandScan 4.0 (Glyko,
Inc., Novato, CA, USA). The relative densities of VEGF protein
verses β-actin were calculated.
Statistical analysis
The values are presented as the mean ± standard
deviation relative to the control values. Statistically significant
differences from the control group were identified by one-way
analysis of variance for the data. P<0.05 was considered to
indicate a statistically significant difference.
Results
Changes in body weight
The body weights of the DG mice were significantly
reduced to 14.5±1.01 from 20±0.54 g compared to 16.8±1.24 from
20±0.87 g for the FDG mice. The DG mice became significantly less
active in their cages, assessed by rearing and ambulating, compared
to the active FDG mice, and the FG mice were more active than the
CG mice (Table I).
 | Table I.Effects of Feijining Decoction (FJDN)
on the body weight and spontaneous activity in Lewis lung
carcinoma-bearing mice. |
Table I.
Effects of Feijining Decoction (FJDN)
on the body weight and spontaneous activity in Lewis lung
carcinoma-bearing mice.
|
|
| Body weights, g | Rearing and
ambulating, counts/min |
|---|
|
|
|
|
|
|---|
| Groups | n | Pre | Post |
|---|
| CG | 10 | 20±0.56 | 19.6±0.94 | 19±2.52 |
| FG | 10 | 20±0.47 | 22.5±0.83 | 25±2.13a |
| DG | 9 | 20±0.54 | 14.5±1.01 | 2±1.98 |
| FDG | 8 | 20±0.87 | 16.8±1.24 | 12±0.87b |
Changes in tumor, spleen and thoracic
index
Tumor growth and final tumor weight were
significantly inhibited in the FDG mice. FJND significantly reduced
the tumor compared to the CG mice. FJND may have enhanced the
antitumor effects of cisplatin. FJND had a synergistic effect with
cisplatin in the treatment of LLC-bearing mice (Table II). The spleen weights of FG mice were
greater than those of the DG mice. The number of lymphocytes in the
spleens of FG mice significantly increased compared to the CG mice.
In DG, the number of lymphocytes in the spleens was significantly
reduced compared to the FDG mice. However, FJND had no effect on
thymus weight.
 | Table II.Effects of Feijining Decoction (FJDN)
on tumor, spleen and thoracic index in Lewis lung carcinoma-bearing
mice. |
Table II.
Effects of Feijining Decoction (FJDN)
on tumor, spleen and thoracic index in Lewis lung carcinoma-bearing
mice.
| Groups | n | Tumor inhibitory
rate | Spleen index, % | Thoracic gland index,
% |
|---|
| CG | 10 | – | 11.24 | 2.31 |
| FG | 10 | 26.1a | 18.21a | 2.79 |
| DG | 9 | 51.2 | 6.23 | 1.69 |
| FDG | 8 | 62.7b | 14.34b | 1.90 |
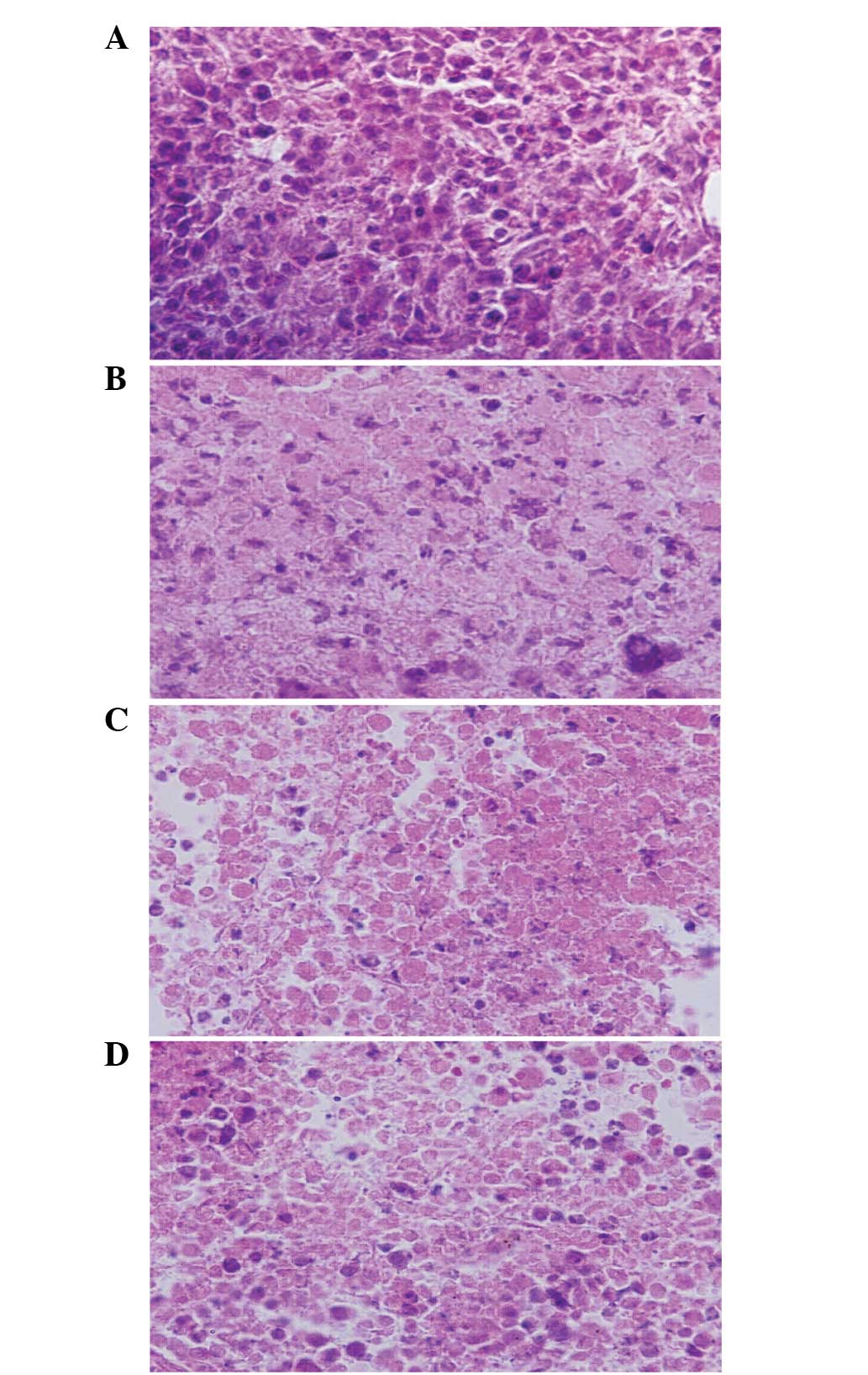
Morphological changes of tumor
tissues
Following hematoxylin-eosin staining, the change of
tumor structure was observed under a light microscope (Fig. 1). Tumor cells grew more productively,
proliferated markedly atypical and had a deeper nuclear stain in
the CG mice, as compared to the FG mice. In the tumor tissue of the
FDG mice, there was a large necrosis area, and a scattered and
abnormal small necrosis area. The plasmocytes and lymphocytes
infiltrated with scattering or gathering around the necrosis area.
The number of plasmocytes and lymphocytes in the DG mice were less
in comparison to the FDG mice.
CD+ cells
In the FG mice, the splenic CD4+ cells
and CD4+/CD8+ were significantly increased
compared to CG mice, and in the FDG mice were significantly
increased compared to DG mice (Table
III).
 | Table III.T cell subsets of the groups were
measured and compared following therapy. |
Table III.
T cell subsets of the groups were
measured and compared following therapy.
| Groups | n | CD4, % | CD8, % |
CD4+/CD8+ |
|---|
| CG | 10 | 12.04±3.01 | 16.57±3.49 | 0.73±0.34 |
| FG | 10 |
25.21±4.67a | 17.59±2.15 |
1.43±0.54a |
| DG | 9 | 9.01±1.78 | 16.58±3.72 | 0.54±0.31 |
| FDG | 8 |
15.37±2.34b | 15.89±2.97 |
0.97±0.47b |
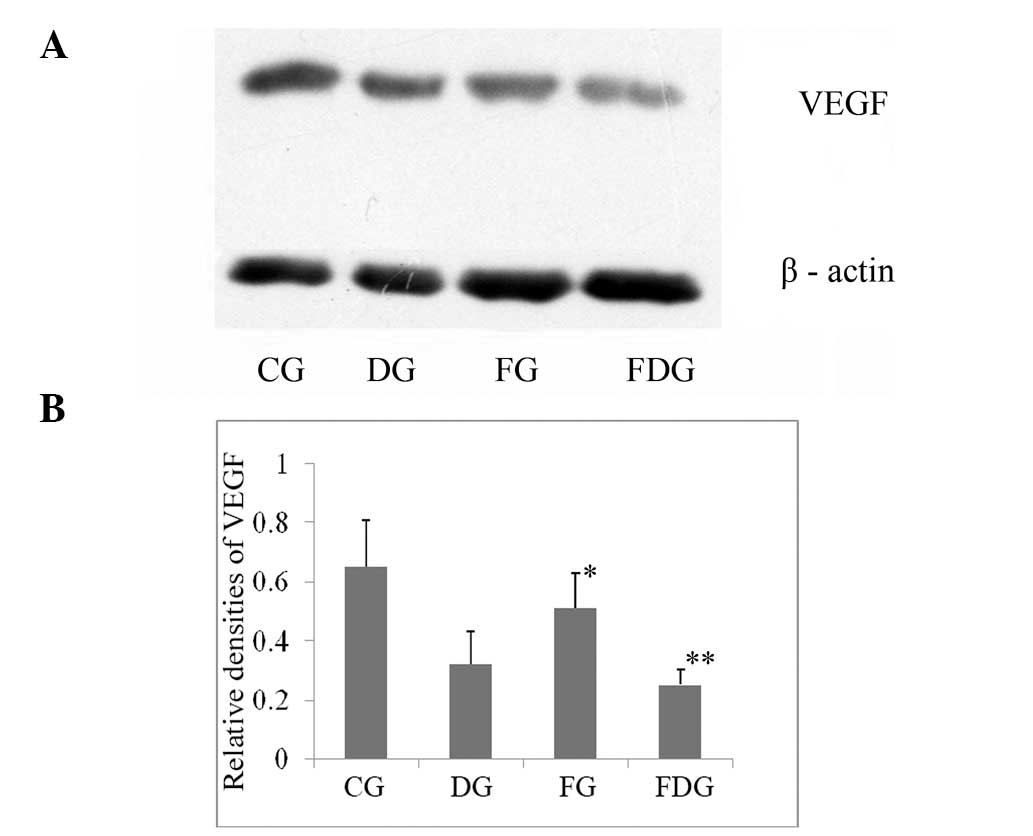
VEGF expression
The protein expression of VEGF significantly
downregulated in carcinoma of the FNJD mice compared to the CG
mice. The expression of VEGF was significantly reduced in the FDG
mice than DG mice (Fig. 2).
Discussion
Angiogenesis in tumors was described by Folkman
(14), and it was proposed that tumor
growth and metastasis are angiogenesis-dependent. It is now widely
accepted that tumor-angiogenesis plays a crucial role in tumor
growth and metastasis formation. Among several angiogenic
activators, VEGF and its receptors represent one of the major
inducers of tumor angiogenesis. Thus, this system has become the
focus of therapeutic interventions.
TCM has been confirmed to effectively reduce toxic
side effects and enhance curative effects of chemotherapy, palliate
clinical syndrome, prevent recurrence and metastasis, and improve
quality of life and immune function (15,16). The
study guided by TCM theory believes that disharmony of Qi function
(according to the fundamental theory of TCM, Qi is often translated
as vital energy) is crucial to lung cancer. The herbs in the
compound formula, FJND, for regulating Qi flow in the lungs, as
well as for anticancer, played the roles of assistant ingredients.
Ginsenosides, belonging to dammaranes, beneficially target the
inhibition of tumor angiogenesis by suppressing its inducer in the
endothelial cells of blood vessels and prevent adhering, invasion
and metastasis of tumor cells (17).
Polysaccharides from the mycelia of Indian buead also showed
antitumor effects (18). An ethanol
extract of Scutellaria baicalensis inhibited the growth of
tumor cells, arrested the cell cycle, induced cell apoptosis and
increased the content of tumor necrosis factor-α in serum (19). Hedyotis Diffusa Willd extract
induces apoptosis via activation of the mitochondrion-dependent
pathway in human colon carcinoma cells (20–22).
Rhizoma Curcumae is a popular type of TCM whose essential
oils are widely used in the treatment of cancer in China. Elemene,
one of the chemical compositions of Rhizoma Curcumae, has
already been approved by China's State Food and Drug Administration
as an anticancer adjuvant drug and has been prescribed as a part of
certain cancer treatment regimens in China. Another ingredient,
furanodiene, significantly inhibits the proliferation of human
umbilical vascular endothelial cells and inhibits VEGF-induced
proliferation (23,24).
Chai Hu Long Gu Mu Li soup is a commonly used
formula in the treatment of diseases of the neuropsychological
system (such as chest congestion and anxiety). Therefore, FJND from
Chai Hu Long Gu Mu Li soup, the herbs of which have the function of
regulating Qi flow of the lungs, played the role of the sovereign
ingredient. The effect of mood and depression on immunity has been
widely discussed. Cancer patients frequently suffer from
psychological comorbidities, such as depression and elevated
perceived stress (25–28).
The results show that FJND can improve spontaneous
activity of carcinoma-bearing mice. The present study analysed
CD4+ and CD8+ in carcinoma-bearing mice
receiving FJND therapy during chemotherapy. Compared to the DG
mice, the level of CD4+ and
CD4+/CD8+ in FDG mice increased
significantly. T cells are also key members of adaptive immunity
against tumorigenesis. VEGF solid tumor secreted through autocrine
and paracrine prevents the expression and secretion of cytokines
(interleukins) and suppresses immune cell proliferation. Thus,
tumor cells escape from the immune surveillance of the
tumor-bearing host (29).
Therefore, these findings suggest that the antitumor
mechanism of FJND may be due to the inhibition expression of the
VEGF protein and the recovery of the immune balance.
References
|
1
|
Ebos JM and Kerbel RS: Antiangiogenic
therapy: impact on invasion, disease progression, and metastasis.
Nat Rev Clin Oncol. 8:210–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramlau R, Gorbunova V, Ciuleanu TE, et al:
Aflibercept and Docetaxel versus Docetaxel alone after platinum
failure in patients with advanced or metastatic non-small-cell lung
cancer: a randomized, controlled phase III trial. J Clin Oncol.
30:3640–3647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cook KM and Figg WD: Angiogenesis
inhibitors: current strategies and future prospects. CA Cancer J
Clin. 60:222–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang W and Yin DF: Effects of Feijining
Decoction on apoptosis down-regulation of expression of VEGF in
human lung adenocarcinoma cell line A549. Xian Dai Zhong Liu Xue Za
Zhi. 21:330–332. 2007.[(In Chinese)].
|
|
5
|
Terme M1, Pernot S, Marcheteau E, et al:
VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory
T-cell proliferation in colorectal cancer. Cancer Res. 73:539–549.
2007. View Article : Google Scholar
|
|
6
|
Hamzah J, Jugold M, Kiessling F, et al:
Vascular normalization in Rgs5-deficient tumours promotes immune
destruction. Nature. 453:410–414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barton DL, Soori GS, Bauer BA, et al:
Pilot study of Panax quinquefolius (American ginseng) to improve
cancer-related fatigue: a randomized, double-blind, dose-finding
evaluation: NCCTG trial N03CA. Support Care Cancer. 18:179–187.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suh SO, Kroh M, Kim NR, Joh YG and Cho MY:
Effects of red ginseng upon postoperative immunity and survival in
patients with stage III gastric cancer. Am J Chin Med. 30:483–494.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen QJ, Zhang MZ and Wang LX: Gensenoside
Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells.
Cell Physiol Biochem. 26:849–858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lü JM1, Yao Q and Chen C: Ginseng
compounds: an update on their molecular mechanisms and medical
applications. Curr Vasc Pharmacol. 7:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y and Li PP: Evaluation of
estrogenic potential of Shu-Gan-Liang-Xue Decoction by
dual-luciferase reporter based bioluminescent measurements in
vitro. J Ethnopharmacol. 126:345–349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y1 and Li PP: Shu-Gan-Liang-Xue
Decoction, a Chinese herbal formula, down-regulates the expression
of steroid sulfatase genes in human breast carcinoma MCF-7 cells. J
Ethnopharmacol. 127:620–624. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W and Yin DF: Effects of Feijining
Decoction on apoptosis down-regulation of expression of VEGF in
human lung adenocarcinoma cell line A549. Xian Dai Zhong Liu Xue Za
Zhi. 21:330–332. 2007.[(In Chinese)].
|
|
14
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Jiang P and Zhang W: Molecular
networks for the study of TCM pharmacology. Brief Bioinform.
11:417–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Jiedu Xiaozheng Yin, a
Chinese herbal formula, inhibits tumor angiogenesis via
downregulation of VEGF-A and VEGFR-2 expression in vivo and in
vitro. Oncol Rep. 29:1080–1086. 2013.PubMed/NCBI
|
|
17
|
Wang W, Zhao Y, Rayburn ER, Hill DL, Wang
H and Zhang R: In vitro anti-cancer activity and structure-activity
relationships of natural products isolated from fruits of
Panaxginseng. Cancer Chemother Pharmacol. 59:589–601. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanayama H, Adachi N and Togami M: A new
antitumor polysaccharide from the mycelia of Poria cocos wolf. Chem
Pharm Bull (Tokyo). 31:1115–1118. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng Y, Sun M, Di Y, et al: Anti-tumor
effect of the ethanol extract of Scutellaria baicalensis on the
mice bearing U14 cervical cancer. Afr J Biotechnol. 11:6542–6549.
2012.
|
|
20
|
Lin J, Wei L, Xu W, Hong Z, Liu X and Peng
J: Effect of Hedyotis Diffusa Willd extract on tumor angiogenesis.
Mol Med Rep. 4:1283–1288. 2011.PubMed/NCBI
|
|
21
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis Diffusa Willd extract induces
apoptosis via activation of the mitochondrion-dependent pathway in
human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.PubMed/NCBI
|
|
22
|
Chen XZ, Cao ZY, Chen TS, Zhang YQ, Liu
ZZ, Su YT, Liao LM and Du J: Water extract of Hedyotis Diffusa
Willd suppresses proliferation of human HepG2 cells and potentiates
the anticancer efficacy of low-dose 5-fluorouracil by inhibiting
the CDK2-E2F1 pathway. Oncol Rep. 28:742–748. 2012.PubMed/NCBI
|
|
23
|
Zhong ZF, Hoi PM, Wu GS, Xu ZT, Tan W,
Chen XP, Cui L, Wu T and Wang YT: Anti-angiogenic effect of
furanodiene on HUVECs in vitro and on zebrafish in vivo. J
Ethnopharmacol. 141:721–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen W, Lu Y, Wu J, Gao M, Wang A and Xu
B: Beta-elemene inhibits melanoma growth and metastasis via
suppressing vascular endothelial growth factor-mediated
angiogenesis. Cancer Chemother Pharmacol. 67:799–808. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mehnert A and Koch U: Prevalence of acute
and post-traumatic stress disorder and comorbid mental disorders in
breast cancer patients during primary cancer care: A prospective
study. Psychooncology. 16:181–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maneeton B, Maneeton N, Reungyos J,
Intaprasert S, Leelarphat S and Thongprasert S: Prevalence and
relationship between major depressive disorder and lung cancer: A
cross-sectional study. Onco Targets Ther. 7:815–821. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arrieta O, Angulo LP, Nunez-Valencia C,
Dorantes-Gallareta Y, Macedo EO, Martinez-Lopez D, Alvarado S,
Corona-Cruz JF and Onate-Ocaña LF: Association of depression and
anxiety on quality of life, treatment adherence, and prognosis in
patients with advanced non-small cell lung cancer. Ann Surg Oncol.
20:1941–1948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Piet J, Würtzen H and Zachariae R: The
effect of mindfulness-based therapy on symptoms of anxiety and
depression in adult cancer patients and survivors: A systematic
review and meta-analysis. J Consult Clin Psychol. 80:1007–1020.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Honggang Z, Bingkui P, Yongming Z, et al:
Effect of Fei Liu Ping Ointment and its modified formula on
dendritic cell. Beijing Zhong Yi Yao Da Xue Xue Bao. 30:525–527.
2007.[(In Chinese)].
|