Introduction
Diabetes is a syndrome presenting with chronic
hyperglycemia arising from insufficiency of insulin activity
(1). Insulin resistance is defined as
a decreased response of the peripheral tissues to insulin activity.
Individuals with insulin resistance are predisposed to developing
type 2 diabetes mellitus. Previous studies have revealed that the
plasma concentration of inflammatory mediators, such as tumor
necrosis factor-α (TNF-α), interleukin-6 (IL-6) and high
sensitivity C-reactive protein (hsCRP) is increased in the insulin
resistant states of obesity and type 2 diabetes (2–4).
Propolis is a resinous material collected by the
Apis mellifera bee from leaf buds and cracks in the bark of
various plants. Propolis contains a variety of chemical compounds,
including polyphenols, flavonoids, amino acids, vitamins (5) and caffeic acid phenethyl ester (6). Propolis presents numerous biological and
pharmacological properties, such as immunomodulatory, antitumor,
anti-inflammatory and antioxidant activity (7). In previous years, a number of studies
have identified that propolis has hypoglycemic effects in animal
models with type 2 diabetes (8–10). To the
best of our knowledge, however, there have been no studies
regarding these effects in human.
The aim of the present paper was to evaluate the
effect of the Brazilian green propolis extract on glucose
metabolism, renal function, lipid metabolism and inflammatory
cytokines in patients with type 2 diabetes.
Materials and methods
Enrolled participants
Patients with type 2 diabetes were screened and
enrolled if they were aged 35–80 years and received treatment with
diet and exercise, oral hypoglycemic agents or glucagon-like
peptide-1 receptor agonists at the Kyoto Prefectural University of
Medicine (Kyoto, Japan).
All the patients provided details of their
demographics, medical history and medication usage. Body mass index
was calculated as weight in kilograms divided by height in meters
squared. Type 2 diabetes was diagnosed according to the Report of
the Expert Committee on the Diagnosis and Classification of
Diabetes Mellitus (11). Nephropathy
was graded as follows: Normoalbuminuria, urinary albumin excretion
(UAE) <30 mg/g of creatinine (Cr); microalbuminuria, 30–300 mg/g
Cr; and macroalbumiuria, >300 mg/g Cr.
The patients treated with insulin were excluded. In
addition, the following patients were also excluded: Severe renal
dysfunction [estimated glomerular filtration rate (eGFR) <30
ml/min/1.73m2] and/or hepatic dysfunction (aspartate
aminotransferase >100 U/l or alanine aminotransferase >100
U/l), as well as pregnant females. The study was examined and
approved by the Institutional Review Board (IRB) and was
subsequently implemented in accordance with Good Clinical Practice.
The investigator or sub-investigator informed each candidate
patient of the study design using the leaflet and consent form
authorized by the IRB prior to enrolling the patient in the study,
and the patient consent was obtained in writing.
Study design and methods
The present study was a randomized, double-blind
study. For allocation of the participants, the numbered container
method was used. The placebo served as a reference drug for
comparison.
The propolis group received Brazilian green propolis
(226.8 mg, 8.4 kcal/day), whereas the placebo group received
tablets containing safflower oil, wheat germ oil and perilla oil
(8.4 kcal/day). Brazilian green propolis was provided by the Yamada
Bee Company, Inc. (Okayama, Japan). In each group, oral medication
was administered once a day for 8 weeks. The diabetic diet and
exercise regimen at baseline was continued, and was not changed
during the study.
The primary outcome was the change in homeostasis
model assessment of insulin resistance (HOMA-IR) at the end of the
study. Secondary outcomes were the changes in fasting plasma
glucose, glycated hemoglobin (HbA1c), serum insulin,
total cholesterol, high-density lipoprotein, low-density
lipoprotein, triglyceride, remnant-like particle lipoprotein
cholesterol, uric acid, eGFR, TNF-α, IL-6, hsCRP, urine pH and
UAE.
Biochemical analysis
Laboratory tests (hematology, biochemistry and
urinalysis) were carried out before and 8 weeks after the start of
taking tablets. Fasting blood and urine samples were obtained in
the morning. The data of the laboratory tests were measured at a
central laboratory institute at the Kyoto Prefectural University of
Medicine. Fasting plasma glucose and insulin levels were used to
calculate HOMA-IR, as previously reported (12). HbA1c was assayed using
high-performance liquid chromatography and was expressed as a
National Glycohemoglobin Standardization Program unit. eGFR was
calculated using the Japanese Society of Nephrology equation: eGFR
= 194 × Cr−1.094 × age−0.287
(ml/min/1.73m2) for males, and for females the eGFR was
multiplied by a correction factor of 0.739 (13). Urinary albumin and Cr concentrations
were determined using early morning spot urine. UAE was measured
with an immunoturbidimetric assay.
Statistical analysis
With a study sample of 82 patients (41 in each
group), the study was estimated to have 80% power to detect a
clinically important difference in the absolute difference in
HOMA-IR of ~1 between the two groups, assuming a standard deviation
(SD) of 1.4 and a two-sided type one error rate of 5%.
The mean values are expressed as the mean ± SD. All
the statistical tests were two-tailed and P<0.05 was considered
to indicate a statistically significant difference. As
triglycerides, hsCRP and UAE showed skewed distributions, the data
were normalized by logarithmic transformation for further
statistical analysis. The differences in categorical variables
between the two groups were evaluated using Fisher's exact test.
Continuous variables were compared using a Student's t-test and a
Mann-Whitney U test if appropriate for non-normally distributed
data. All the statistical analyses were carried out using the JMP
software, version 10.0 (SAS Institute Inc., Cary, NC, USA).
Results
Demographic information of
patients
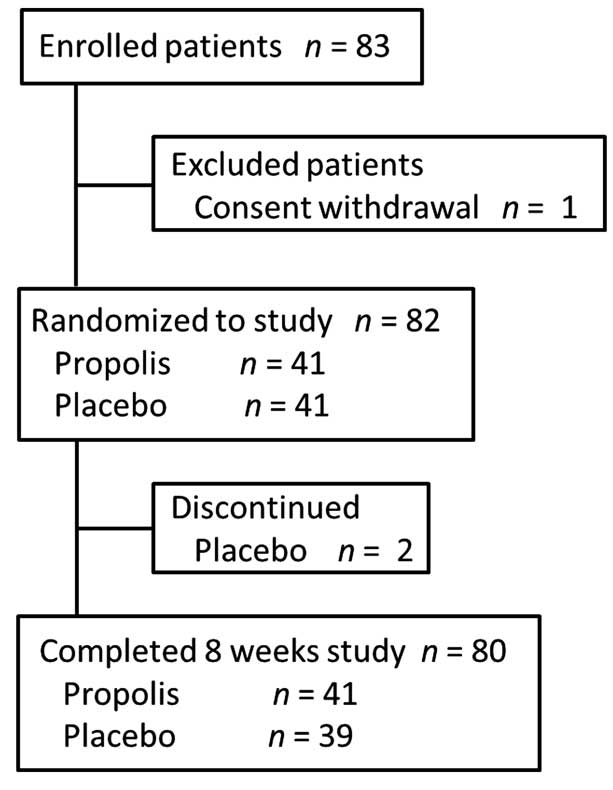
Between June and August 2012, a total of 82 patients
were randomized to administration with propolis (n=41) or placebo
(n=41) and 80 patients were finally included in the full analysis
set: 41 for the propolis group and 39 for the placebo group
(Fig. 1). Two patients in the placebo
group discontinued due to the onset of acute myocardial infarction
and cellulitis, respectively. Demographic and baseline
characteristics of the 80 randomized patients are summarized in
Table I. The mean age, proportion of
males and proportion of diabetic microangiopathic complications
were similar between the propolis and the placebo groups. There
were no significant differences in the use of sulfonylureas,
dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor
agonists, pioglitazones and biguanides at study entry between the
two groups. Furthermore, the mean systolic/diastolic blood pressure
and all the biochemical data in the baseline were similar between
the two groups (Table I). The two
groups differed significantly in the duration of diabetes only
(P<0.05).
 | Table I.Clinical and laboratory
characteristics of patients. |
Table I.
Clinical and laboratory
characteristics of patients.
| Variables | Propolis (n=41) | Placebo (n=39) | P-value |
|---|
| Age, years | 63.7±9.3 | 62.9±7.8 | 0.69 |
| Gender,
male/female | 27/14 | 19/20 | 0.12 |
| Body weight, kg | 62.8±15.0 | 65.1±9.9 | 0.43 |
| BMI,
kg/m2 | 25.0±4.8 | 25.0±3.5 | 0.98 |
| Duration of diabetes,
years | 13.7±10.3 | 9.8±6.2 | <0.05 |
| Retinopathy,
NDR/SDR/PDR | 31/5/5 | 30/3/6 | 0.76 |
| Nephropathy,
normo/micro/macroalbuminuria | 29/9/3 | 29/8/2 | 0.95 |
| Neuropathy, –/+ | 26/15 | 25/14 | 0.95 |
| Hypertension,
–/+ | 18/23 | 11/28 | 0.14 |
| Hyperlipidemia,
–/+ | 22/19 | 18/21 | 0.50 |
| Sulfonylurea,
–/+ | 17/24 | 20/19 | 0.38 |
| Dipeptidyl
peptidase-4 inhibitor, –/+ | 21/20 | 20/19 | 1.00 |
| Glucagon like
peptide-1 receptor agonist, –/+ | 37/4 | 35/4 | 0.94 |
| Pioglitazone,
–/+ | 37/4 | 35/4 | 0.94 |
| Biguanide, –/+ | 19/22 | 20/19 | 0.75 |
| Systolic blood
pressure, mmHg | 124.8±15.5 | 125.4±14.6 | 0.88 |
| Diastolic blood
pressure, mmHg | 71.0±12.9 | 71.1±10.9 | 0.97 |
| Fasting plasma
glucose, mg/dl | 133.8±25.3 | 138.8±33.6 | 0.46 |
| Hemoglobin
A1c, % | 7.09±0.79 | 7.21±0.85 | 0.52 |
| Insulin,
μIU/ml | 6.94±3.75 | 7.74±3.42 | 0.32 |
| HOMA-IR | 2.34±1.36 | 2.64±1.39 | 0.33 |
| AST, IU/l | 24.4±10.3 | 24.1±7.5 | 0.88 |
| ALT, IU/l | 23.8±17.1 | 26.7±12.0 | 0.39 |
| Uric acid,
mg/dl | 5.51±1.23 | 5.23±1.30 | 0.33 |
| Serum Cr,
mg/dl | 0.73±0.26 | 0.75±0.22 | 0.69 |
| eGFR, ml/min/1.73
m2 | 75.5±21.7 | 74.9±17.3 | 0.90 |
| Total cholesterol,
mg/dl | 180.4±29.4 | 181.6±34.9 | 0.87 |
| HDL cholesterol,
mg/dl | 59.7±15.2 | 54.5±16.4 | 0.14 |
| LDL cholesterol,
mg/dl | 108.5±24.2 | 114.6±29.1 | 0.30 |
| Log Triglycerides,
mg/dl | 4.70±0.53 | 4.78±0.36 | 0.44 |
| RLP cholesterol,
mg/dl | 4.79±2.90 | 4.77±2.49 | 0.97 |
| TNF-α, ng/ml | 1.4 (1.2–1.9) | 1.4 (1.2–1.7) | 0.32 |
| IL-6, pg/ml | 2.0 (1.3–2.8) | 1.4 (1.0–2.3) | 0.69 |
| Log hsCRP,
mg/l | 6.38±1.28 | 6.52±1.12 | 0.60 |
| Urine, pH | 6.00±0.63 | 5.95±0.57 | 0.69 |
| Log UAE, mg/g
Cr | 3.07±1.54 | 2.67±1.43 | 0.24 |
Efficacy
In the control group, HOMA-IR changed from 2.64±1.39
at baseline to 2.73±1.52 at 8 weeks after administration of the
placebo. In the propolis group, HOMA-IR changed from 2.34±1.36 at
baseline to 2.33±1.56 at 8 weeks after administration. The mean
difference of HOMA-IR, which was a primary outcome between the
baseline data and the data at 8 weeks after administration, was not
significant between the two groups (P=0.62) (Table II). Furthermore, each group showed no
significant change in HOMA-IR 8 weeks after the intake of propolis
or placebo compared to the baseline.
 | Table II.Changes in the parameters during 8
weeks of placebo or propolis administration. |
Table II.
Changes in the parameters during 8
weeks of placebo or propolis administration.
|
| Placebo (n=39) | Propolis
(n=41) |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | Baseline | After 8 weeks | Baseline | After 8 weeks | Difference (95%
CI) |
P-valuea |
|---|
| Primary
outcome |
|
|
|
|
|
|
|
HOMA-IR | 2.64±1.39 | 2.73±1.52 | 2.34±1.36 | 2.33±1.56 | −0.10 (−0.51 to
0.31) | 0.62 |
| Secondary
outcome |
|
|
|
|
|
|
| Fasting
plasma glucose, mg/dl | 138.8±33.6 | 136.8±27.8 | 133.8±25.3 | 130.2±28.1 | −1.63 (−11.81 to
5.11) | 0.75 |
|
Hemoglobin A1c,
% | 7.21±0.85 | 7.23±0.89 | 7.09±0.79 | 7.08±0.79 | −0.03 (−0.13 to
0.19) | 0.69 |
|
Insulin, μIU/ml | 7.74±3.42 | 8.15±4.50 | 6.94±3.75 | 6.92±3.82 | −0.43 (−1.55 to
0.69) | 0.45 |
| Uric
acid, mg/dl | 5.23±1.30 |
5.44±1.44b | 5.51±1.23 | 5.48±1.38 | −0.23 (−0.52 to
0.06) | 0.11 |
| eGFR,
ml/min/1.73 m2 | 74.9±17.3 |
72.2±18.4b | 75.5±21.7 | 74.5±19.7 | 1.74
(−1.99 to 5.48) | 0.36 |
| Total
cholesterol, mg/dl | 181.6±34.9 | 180.7±32.7 | 180.4±29.4 | 177.7±32.5 | −1.91 (−8.31 to
4.49) | 0.55 |
| HDL
cholesterol, mg/dl | 54.5±16.4 | 54.4±16.5 | 59.7±15.2 | 59.3±15.8 | −0.39 (−2.49 to
1.71) | 0.71 |
| LDL
cholesterol, mg/dl | 114.6±29.1 | 115.0±27.0 | 108.5±24.2 | 107.6±26.7 | −1.16 (−6.72 to
4.39) | 0.68 |
| Log
triglycerides, mg/dl | 4.78±0.36 | 4.75±0.38 | 4.70±0.53 | 4.63±0.48 | −0.04 (−0.16 to
0.08) | 0.51 |
| RLP
cholesterol, mg/dl | 4.77±2.49 | 4.09±2.10 | 4.79±2.90 | 4.83±2.68 | 0.76
(−0.28 to 1.80) | 0.15 |
| TNF-α,
ng/ml | 1.4 (1.2–1.7) | 1.5 (1.4–1.9) | 1.4 (1.2–1.9) | 1.4 (1.2–1.8) | −0.15 (−0.66 to
0.36) | 0.56 |
| IL-6,
pg/ml | 1.4 (1.0–2.3) | 1.6 (1.1–2.2) | 2.0 (1.3–2.8) | 1.6 (1.2–2.6) | −0.51 (−1.61 to
0.59) | 0.36 |
| Log
hsCRP, mg/l | 6.38±1.28 | 6.26±1.07 | 6.52±1.12 | 6.45±1.22 | 0.05
(−0.36 to 0.47) | 0.81 |
| Urine,
pH | 5.95±0.57 | 5.95±0.74 | 6.00±0.63 | 5.93±0.76 | −0.07 (−0.32 to
0.17) | 0.56 |
| Log
UAE, mg/g Cr | 2.67±1.43 | 2.65±1.04 | 3.07±1.54 | 2.97±1.50 | −0.07 (−0.35 to
0.21) | 0.62 |
All the parameters of glucose metabolism, renal
function, lipid metabolism and inflammatory cytokines, which were
secondary outcomes between baseline data and data at 8 weeks after
administration, between the two groups did not have significant
differences (Table II). However, it
is notable that the concentration of uric acid in the blood of
patients taking the placebo significantly increased 8 weeks later
compared to the baseline (P<0.05; Table II), while in patients taking propolis
it was maintained at a level similar to the baseline but did not
significantly increase 8 weeks later (P=0.80; Table II). Furthermore, eGFR was decreased in
patients taking placebo 8 weeks later compared to the baseline
(P<0.01; Table II), while eGFR in
patients taking propolis was maintained at a similar level to the
baseline without any decrease 8 weeks later (P=0.52; Table II). Although TNF-α did not
significantly increase in either group, patients taking the placebo
showed a tendency to increase TNF-α (P=0.08; Table II), the value of which in patients
taking propolis was stable (P=0.98; Table
II). No reports on side effects, including allergy, were noted
in patients who finished the protocol.
Discussion
The present study examined the efficacy of Brazilian
green propolis compared to the placebo for 8 weeks in Japanese
patients with type 2 diabetes. To the best of our knowledge, this
is the first double-blind randomized placebo-controlled study to
investigate the effectiveness of propolis in patients with type 2
diabetes. In the study, there were no evident differences between
the propolis and the placebo groups in the changes of HOMA-IR,
HbA1c, fasting blood glucose or serum insulin level, so
therefore, intake of 226.8 mg propolis/day for 8 weeks did not
improve glucose metabolism in the patients with type 2 diabetes.
However, as shown in Table II, the
concentration of uric acid in the blood of patients taking propolis
for 8 weeks was maintained at a level identical to the baseline
without any significant increase (P=0.80; Table II), while for the patients taking the
placebo, the concentration significantly elevated 8 weeks later
(P<0.05; Table II). Furthermore,
intake of 226.8 mg propolis/day did not improve eGFR, but
maintained eGFR at the baseline without any decrease for 8 weeks
(P=0.52; Table II). By contrast, eGFR
of diabetic patients taking the placebo decreased 8 weeks later
compared to the baseline (P<0.01; Table II). Furthermore, daily intake of
propolis maintained TNF-α at a stable value for 8 weeks without any
significant elevation (P=0.98; Table
II), while diabetic patients taking the placebo showed a
tendency to increase TNF-α (P=0.08; Table
II). These observations suggest that daily intake of 226.8 mg
propolis for 8 weeks prevents diabetic patients from developing a
worse renal glomerular filtrating function and elevation of blood
uric acid, and may have an anti-inflammatory action.
The biological action of propolis originates from
its active constituents, which differ in type and amount in the
various types of propolis (14).
Brazilian propolis represents 10–15% of the worldwide production
and Brazil is the third world producer behind Russia and China
(15). Among the types produced in
Brazil, green propolis prevails and gains preference in the world
propolis market. In the present study, the high quality Brazilian
green propolis was used. Although toxic data for propolis are
limited, various allergens have been isolated from propolis; such
as 3-methyl-2-butenyl caffeate, phenylethyl caffeate, benzyl
caffeate, geranyl caffeate, benzyl alcohol benzyl cinnammate,
methyl cinnammate, ferulic acid and tectochrysin (16). Therefore, allergic reactions were of
concern in the present study; however, no patient in the propolis
group suffered from allergic reactions.
Multifold pathways, including increased polyol
pathway flux, overactivity of the hexosamine pathway, increased
formation of advanced glycation end-products and activation of
protein kinase C isoforms, are involved in diabetic complications
(17). Increased reactive oxygen
species inducing hyperglycemia-activated electron-transport chain
in mitochondria are believed to be a main underlying mechanism
linking all of these factors, and oxidative stress is possibly
involved in the progression of pancreatic β-cell dysfunction
(18). Propolis has a strong
antioxidative activity and is confirmed to inhibit the increase of
the malonaldehyde (MDA) level and improve antioxidase activity in
the animal model and patients (19,20).
Previous studies have reported that propolis can prevent oxidative
stress-induced tissue damage by decreasing the overproduction of
MDA and superoxide anion, and by restoring the respiratory control
ration in mitochondrial tissue (21,22).
Therefore, propolis may improve glucose metabolism by an
attenuation of mitochondrial oxidative stress.
In addition, extracellular pH plays an important
role in glucose metabolism. In a rat skeletal muscle-derived cell
line, L6 cell, the phosphorylation level of the insulin receptor
and Akt is significantly diminished in low pH media, and binding of
insulin to its receptor on the plasma membrane is reduced by
lowering the extracellular pH, while the expression of insulin
receptors on the plasma membrane is not affected. As a result,
insulin-stimulated 2-deoxyglucose uptake in L6 cells is diminished
in low pH media (23). An ex
vivo study by Yamauchi et al (24) reported that insulin-mediated
2-deoxy-D-glucose uptake by rat soleus muscle is inhibited by a
reduction in the pH of the medium from 7.4 to 6.8. Furthermore, Aoi
et al (25) have reported that
a propolis-contained diet improved, by increasing, the pH of
ascites and metabolic tissues compared to the normal diet and
improved glucose metabolism in Otsuka Long-Evans Tokushima Fatty
rats, whose pH in various tissues was lower compared to normal rats
(25–27). Therefore, propolis may improve insulin
sensitivity by an increase of extracellular pH.
In the present study, at least two limitations
should be noted. First, the trial may have been too short to
observe the change of glucose metabolism. Propolis may require a
longer duration to improve glucose metabolism in humans, so
administration of propolis for 8 weeks appears to be unlikely to
elicit significant improvements in HbA1c. Second, the
dose of propolis may not have been sufficient. Numerous studies
report the efficacy of propolis on diabetes; however, the majority
are animal experiments and administered doses of propolis were
extremely high (such as 50–300 mg/kg of the body weight) (8–10,28–30).
Therefore, if a higher dose of propolis was administered to
patients, the effect of propolis on glucose metabolism may take
effect.
In conclusion, 226.8 mg/day of Brazilian green
propolis for 8 weeks prevented the actions of hyperuricemia and
dysfunction of renal glomerular filtrating function that commonly
develop in patients suffering from diabetes mellitus. Therefore,
further clinical studies should be continued to verify whether much
higher doses and/or longer administration of Brazilian green
propolis are useful in the prevention and care of diabetes
mellitus.
Acknowledgements
The present study was supported by Grants-in-Aid,
mainly from the Yamada Research Grant.
References
|
1
|
Defronzo RA: Banting Lecture. From the
triumvirate to the ominous octet: A new paradigm for the treatment
of type 2 diabetes mellitus. Diabetes. 58:773–795. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hotamisligil GS, Shargill NS and
Spiegelman BM: Adipose expression of tumor necrosis factor-alpha:
Direct role in obesity-linked insulin resistance. Science.
259:87–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pickup JC, Mattock MB, Chusney GD and Burt
D: NIDDM as a disease of the innate immune system: Association of
acute-phase reactants and interleukin-6 with metabolic syndrome X.
Diabetologia. 40:1286–1292. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang ES, Kim HJ, Ahn CW, Park CW, Cha BS,
Lim SK, Kim KR and Lee HC: Relationship of serum high sensitivity
C-reactive protein to metabolic syndrome and microvascular
complications in type 2 diabetes. Diabetes Res Clin Pract.
69:151–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khalil ML: Biological activity of bee
propolis in health and disease. Asian Pac J Cancer Prev. 7:22–31.
2006.PubMed/NCBI
|
|
6
|
Grunberger D, Banerjee R, Eisinger K, Oltz
EM, Efros L, Caldwell M, Estevez V and Nakanishi K: Preferential
cytotoxicity on tumor cells by caffeic acid phenethyl ester
isolated from propolis. Experientia. 44:230–232. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banskota AH, Tezuka Y and Kadota S: Recent
progress in pharmacological research of propolis. Phytother Res.
15:561–571. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Hariri MT: Propolis and its direct and
indirect hypoglycemic effect. J Fam Community Med. 18:152–154.
2011. View Article : Google Scholar
|
|
9
|
Matsui T, Ebuchi S, Fujise T, Abesundara
KJ, Doi S, Yamada H and Matsumoto K: Strong antihyperglycemic
effects of water-soluble fraction of Brazilian propolis and its
bioactive constituent, 3,4,5-tri-O-caffeoylquinic acid. Biol Pharm
Bull. 27:1797–1803. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu W, Chen M, Shou Q, Li Y and Hu F:
Biological activities of chinese propolis and brazilian propolis on
streptozotocin-induced type 1 diabetes mellitus in rats. Evid Based
Complement Alternat Med. 2011:4685292011.PubMed/NCBI
|
|
11
|
Expert Committee on the Diagnosis and
Classification of Diabetes Mellitus: Report of the expert committee
on the diagnosis and classification of diabetes mellitus. Diabetes
Care. 26 (Suppl 1):S5–S20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuo S, Imai E, Horio M, Yasuda Y,
Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H and Hishida A:
Collaborators developing the Japanese equation for estimated GFR:
Revised equations for estimated GFR from serum creatinine in Japan.
Am J Kidney Dis. 53:982–992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bankova V: Chemical diversity of propolis
and the problem of standardization. J Ethnopharmacol. 100:114–117.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paviani LC, Dariva C, Marcucci MC and
Cabral FA: Super critical carbon dioxide selectivity to fractionate
phenolic compounds from the dry ethanolic extract of propolis. J
Food Process Eng. 33:15–27. 2010. View Article : Google Scholar
|
|
16
|
Gardana C and Simonetti P: Evaluation of
allergens in propolis by ultra-performance liquid
chromatography/tandem mass spectrometry. Rapid Commun Mass
Spectrom. 25:1675–1682. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kajimoto Y and Kaneto H: Role of oxidative
stress in pancreatic beta-cell dysfunction. Ann N Y Acad Sci.
1011:168–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jasprica I, Mornar A, Debeljak Z, et al:
In vivo study of propolis supplementation effects on antioxidative
status and red blood cells. J Ethnopharmacol. 110:548–554. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanbur M, Eraslan G and Silici S:
Antioxidant effect of propolis against exposure to propetamphos in
rats. Ecotoxicol Environ Saf. 72:909–915. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Majiene D, Trumbeckaite S, Savickas A and
Toleikis A: Influence of propolis water solution on heart
mitochondrial function. J Pharm Pharmacol. 58:709–713. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alyane M, Kebsa LBW, Boussenane H, Rouibah
H and Lahouel M: Cardioprotective effects and mechanism of action
of polyphenols extracted from propolis against doxorubicin
toxicity. Pak J Pharm Sci. 21:201–209. 2008.PubMed/NCBI
|
|
23
|
Hayata H, Miyazaki H, Niisato N, Yokoyama
N and Marunaka Y: Lowered extracellular pH is involved in the
pathogenesis of skeletal muscle insulin resistance. Biochem Biophys
Res Commun. 445:170–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamauchi T, Sekiya K, Okuda H and Kimura
S: Role of Na+/H+ exchanger in insulin-stimulated glucose uptake
into skeletal muscle. Agressologie. 32:115–120. 1991.PubMed/NCBI
|
|
25
|
Aoi W, Hosogi S, Niisato N, et al:
Improvement of insulin resistance, blood pressure and interstitial
pH in early developmental stage of insulin resistance in OLETF rats
by intake of propolis extracts. Biochem Biophys Res Commun.
432:650–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marunaka Y, Yoshimoto K, Aoi W, Hosogi S
and Ikegaya H: Low pH of interstitial fluid around hippocampus of
the brain in diabetic OLETF rats. Mol Cell Ther. 2:62014.
View Article : Google Scholar
|
|
27
|
Aoi W and Marunaka Y: Importance of pH
homeostasis in metabolic health and diseases: Crucial role of
membrane proton transport. Biomed Res Int. 2014:5989862014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El-Sayed SM, Abo-Salem OM, Aly HA and
Mansour AM: Potential antidiabetic and hypolipidemic effects of
propolis extract in streptozotocin-induced diabetic rats. Pak J
Pharm Sci. 22:168–174. 2009.PubMed/NCBI
|
|
29
|
Kang L-J, Lee HB, Bae H-J and Lee SG:
Antidiabetic effect of propolis: Reduction of expression of
glucose-6-phosphatase through inhibition of Y279 and Y216
autophosphorylation of GSK-3α/β in HepG2 cells. Phytother Res.
24:1554–1561. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Chen M, Xuan H and Hu F: Effects of
encapsulated propolis on blood glycemic control, lipid metabolism,
and insulin resistance in type 2 diabetes mellitus rats. Evid Based
Complement Alternat Med. 2012:9818962012. View Article : Google Scholar : PubMed/NCBI
|