Introduction
Acute myocardial infarction (AMI) remains the
leading cause of morbidity, mortality and heart failure worldwide.
Over the past few decades, significant efforts have been made in
the treatment of AMI based on early reperfusion of the culprit
artery, including pharmacological and mechanical therapies,
resulting in significantly reducing mortality (1). However, the lack of repair of a
substantial amount of damaged cardiac tissue may lead to congestive
heart failure. Necrosis or scarred tissue following AMI in the
heart will lead to significant ventricular remodeling characterized
by dilation of the left ventricular cavity, thinning of the
infarcted tissue and electrical remodeling, resulting in the
increase of the risk of sudden cardiac fatality. The use of stem
cells with angiogenic properties for therapy of AMI has been widely
investigated as a feasible strategy for repairing injured
myocardial muscle tissue (2,3). Mesenchymal stem cells (MSCs), as a
subpopulation of bone marrow cells, were found to differentiate
into various cell types, including functional cardiomyocytes. MSCs
are easily isolated, have a proclivity for ex vivo expansion
and a potential for allogeneic use (4). MSC therapy for AMI has been investigated
widely in animals and humans (5,6).
Positron emission tomography (PET) has been applied
to investigate the improvement of myocardial function following
stem cell therapy for AMI in humans and animals as a non-invasive
imaging tool, such as imaging of 11C-acetate,
13N-ammonia and 18F-FDG (7). These PET tracers are not always available
for numerous hospitals as their generation requires the on-site
cyclotron (except 18F-FDG) (8,9). Compared
with PET, single proton emission computed tomography (SPECT) is
much more available in practices. In particular, SPECT with dual
isotope simultaneous acquisition (DISA) allowed the assessment of
myocardial perfusion (with 99mTc-MIBI) and metabolism
(with 18F-FDG) in a single study by Slart et al
(10,11). Therefore, DISA SPECT provides an easy
procedure for stem cell study to assess myocardial function for
humans and animals. Thus, the present study was designed to
evaluate the engraftment in myocardium and the improvement of
cardiac function following intracoronary injection of
pre-differentiated MSCs in a canine model with induced AMI using
DISA SPECT, to quantify myocardial perfusion and metabolism,
respectively.
Materials and methods
Animal model of myocardial
infarction
All the surgical procedures and postoperative care
were performed in accordance with the guidelines of the Beijing
Fuwai Hospital Animal Care and Use Committee (Beijing, China). All
the study protocols were approved by the Ethics Committee of
Beijing Fuwai Hospital. A total of 12 dogs (male and female, 2–3
years old and 20–30 kg) were included in the experiment: 6 were the
control group and another 6 were the graft group. All the dogs in
the two groups were anesthetized by venous injection of ketamine
(50 mg/kg) and diazepam (0.05 mg/kg). Following the induction of
anesthesia, the animals were intubated and mechanically ventilated
through a small-animal ventilator with a minute volume of 1.5–2.5
ml. Respiratory rate and tidal volume were adjusted according to
body weight and other physiological parameters. To avoid heat loss,
the body temperature was maintained by a heating lamp and the
animals were covered with drapes, leaving only the necessary
surgical area uncovered. The electrocardiogram was monitored
throughout the surgery. Subsequent to the opening of the thoracic
cavity and pericardium, four silk suture strings were placed
underneath the vessel and the surrounding myocardium at the distal
left anterior descending (LAD) coronary artery. LAD was ligated for
5 min and the perfusion was restored for 10 min. After this,
ligation was maintained for 90 min, and LAD perfusion was restored
again. Duration ligation, a small piece of a polyethylene tube was
used to avoid damage to the vessel.
Bone marrow harvest, stem cells
isolation, expansion and labeling
Four weeks before surgery, 10–15 ml bone marrow
aspirate was collected in a syringe with 1 ml heparin from the
iliac crest from each dog after anesthesia was administrated. Bone
marrow mononuclear cells were isolated using Ficoll-Hypaque
solution (1.077 g/ml; Sigma-Aldrich, St. Louis, MI, USA) and plated
in Dulbeccos modified Eagles medium (DMEM) containing 10% fetal
bovine serum (FBS) and antibiotics (100 U/ml penicillin and 100
µg/ml streptomycin; Invitrogen, Carlsbad, CA, USA). The media were
changed and maintained in DMEM-F12 medium supplemented with 10% FBS
and antibiotics for several weeks. To induce cell differentiation,
cells were incubated in DMEM-F12 medium supplemented with 5% FBS
and antibiotics 24 h before treatment with 5-azacytidine (20
µmol/l; Sigma-Aldrich) from the third passage. Following
incubation, media were changed and maintained in DMEM-F12
supplemented with 10% FBS and antibiotics for several weeks.
Cell differentiation was evaluated morphologically
and immunohistochemically. Briefly, after 4 weeks of culture,
differentiated cells were evaluated by phase contrast microscopy
and electron microscopy, respectively. Furthermore, cultured cells
were immunostained by monoclonal antibodies to sarcomeric α-actine,
cardiac myosin heavy chain and troponin I (Sigma-Aldrich).
Cellular transplant
For the graft group, at 2 or 3 h after perfusion was
restored following 90 min ligation of LAD, 2–3 ml (107
cells) of in vitro culture-expanded MSCs were implanted to
the AMI area by intracoronary injection using a thin syringe. To
identify the grafted cells in the host myocardium, one-third of the
implanted MSCs were labeled with bromodeoxyuridine (BrdU) prior to
implantation.
For dogs in the control group, 0.9% NaCl was
injected in the same way. Electrocardiography (ECG) was performed
during surgical procedure and continued to 24–48 h.
Myocardial perfusion and metabolism
assessment by SPECT
DISA SPECT with 18F-FDG and
99mTc-MIBI was employed to assess myocardial perfusion
and metabolism simultaneously, using a double-head γ-camera
equipped with ultra-high-energy collimators (VariCam; GE
Healthcare, Cleveland, OH, USA). The imaging protocol was as
previously described [Slart et al (11) and De Boer et al (12)]. Briefly, at week 10 after implantation,
99mTc-MIBI (925 MBq) and 18F-FDG (222 MBq)
were injected into the ear vein of a dog after administration of
anesthesia, and scanning started at 60–90 min after injection. The
acquisition was set to continuous mode with a 64×64 word matrix.
Dual isotope acquisition was obtained with two separate energy
windows: 140 keV with 20% window and 511 keV with 20% window.
Following this, a standard gated SPECT myocardial perfusion scan
was performed for LVEF measurement.
Image analysis
Myocardial perfusion and metabolic imaging data were
analyzed semi-quantitatively by two experienced nuclear
cardiologists independently, with a five-point scoring system: 0,
normal uptake; 1, mild hypoperfusion; 2, moderate hypoperfusion; 3,
severe hypoperfusion; and 4, no uptake. Two cardiologists were
blinded to the experimental groups.
Histology, pathology and
immunohistochemistry
All the dogs were sacrificed following SPECT
imaging. The heart was arrested by infusion of cadmium chloride
(0.1 M) into the left atrium. The heart and circulation were
perfused with phosphate-buffered saline [PBS (pH 7.4) 50 nM sodium
nitroprusside] and 5% formalin in PBS at a physiological pressure
for 10 min. Subsequently, the hearts were dissected and
immersion-fixed in 10% formalin in PBS for 24 h, processed and
embedded in paraffin for further morphological, histological and
immunohistochemical analyses. The heart was cut longitudinally,
perpendicular to the infarcted area, resulting in an anterior and
posterior section. From the two sections, 10 µm slices were
cut.
Statistical analysis
Differences among groups were compared using one-way
analysis of variance with Students t-test using SPSS v.12 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Mesenchymal stem cell culture and
differentiation
Differentiation of mesenchymal stem cells to
myocytes induced by 5-azacytidine was evaluated by microscopic and
electron microscopic observations, as well as immunohistochemical
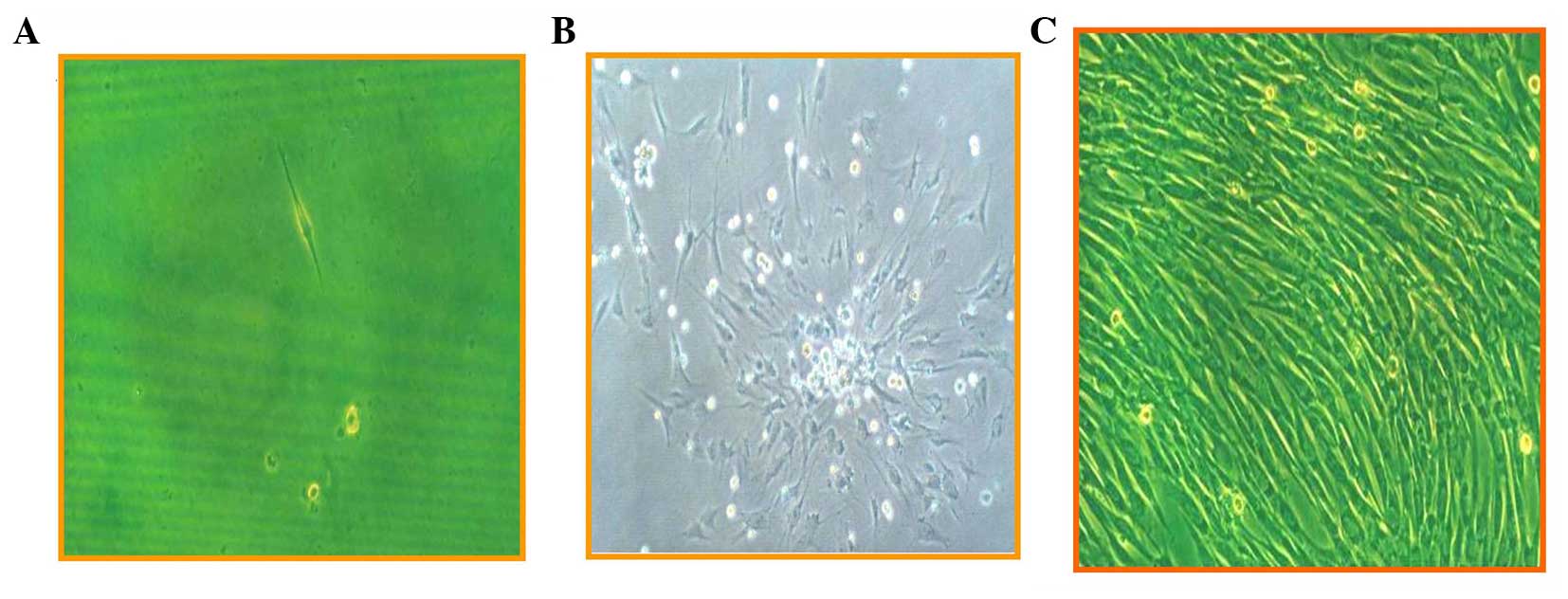
staining. Fig. 1 shows the
morphological changes following induction, from rod-like (Fig. 1A) to colony formation (Fig. 1B) and spindle-like cells (Fig. 1C). Using an electron microscope,
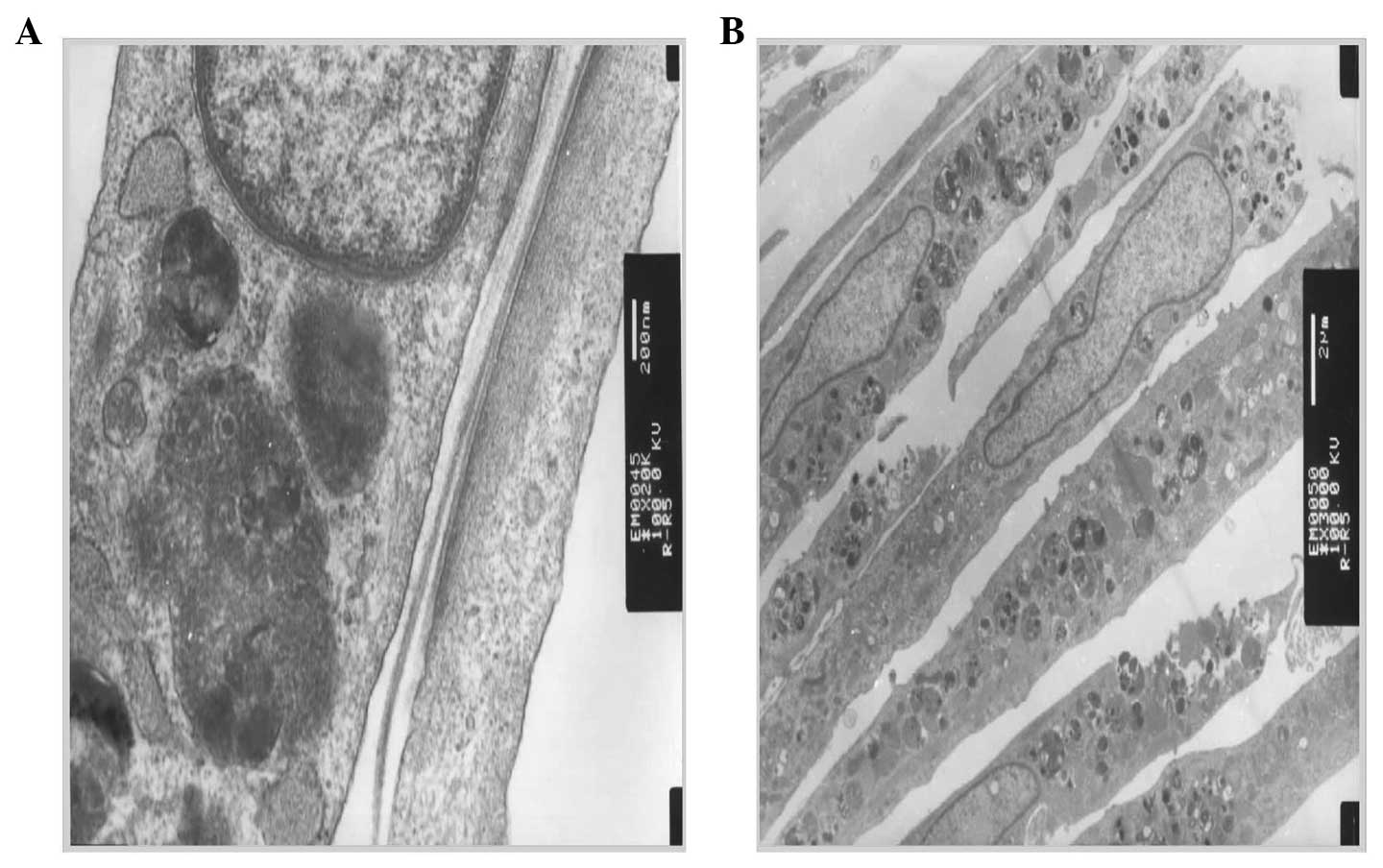
microfilaments and atrial granules around the nucleus were
identified (Fig. 2). Furthermore, by
immunohistochemical staining, sarcomeric α-actinin, cardiac myosin
heavy head and troponin I were positive (Fig. 3). These observations confirmed that
bone marrow mesenchymal stem cells have differentiated into
cardiomyocytes.
Serum enzyme increase following
ligation of LAD
The levels of serum enzymes after 9–12 h following
LAD ligation, including creatine kinase (CK), isoenzymes of
creatine kinase (CK-MB), glutamic oxaloacetic transaminase (GOT),
glutamate-pyruvate transaminase (GPT) and troponine T (TNT), were
significantly increased, as shown in Table
I, in the two groups. High levels of cardiomyocytes enzyme in
the serum indicated that myocardial ischemia or AMI occurred in the
dogs of the two groups.
 | Table I.Levels of serum enzymes at 9–12 h
after LAD ligation. |
Table I.
Levels of serum enzymes at 9–12 h
after LAD ligation.
| Group | CK, IU/l | CK-MB, IU/l | GOT, IU/l | GPT, IU/l | TNT |
|---|
| Control | 4,998.6±198.4 | 423.7±67.9 | 494.8±36.7 | 231.3±45.6 | +++ |
| Graft | 4,987.5±158.3 | 428.3±58.2 | 487.6±40.4 | 235.4±41.8 | +++ |
Myocardial function changes following
MSCs implantation
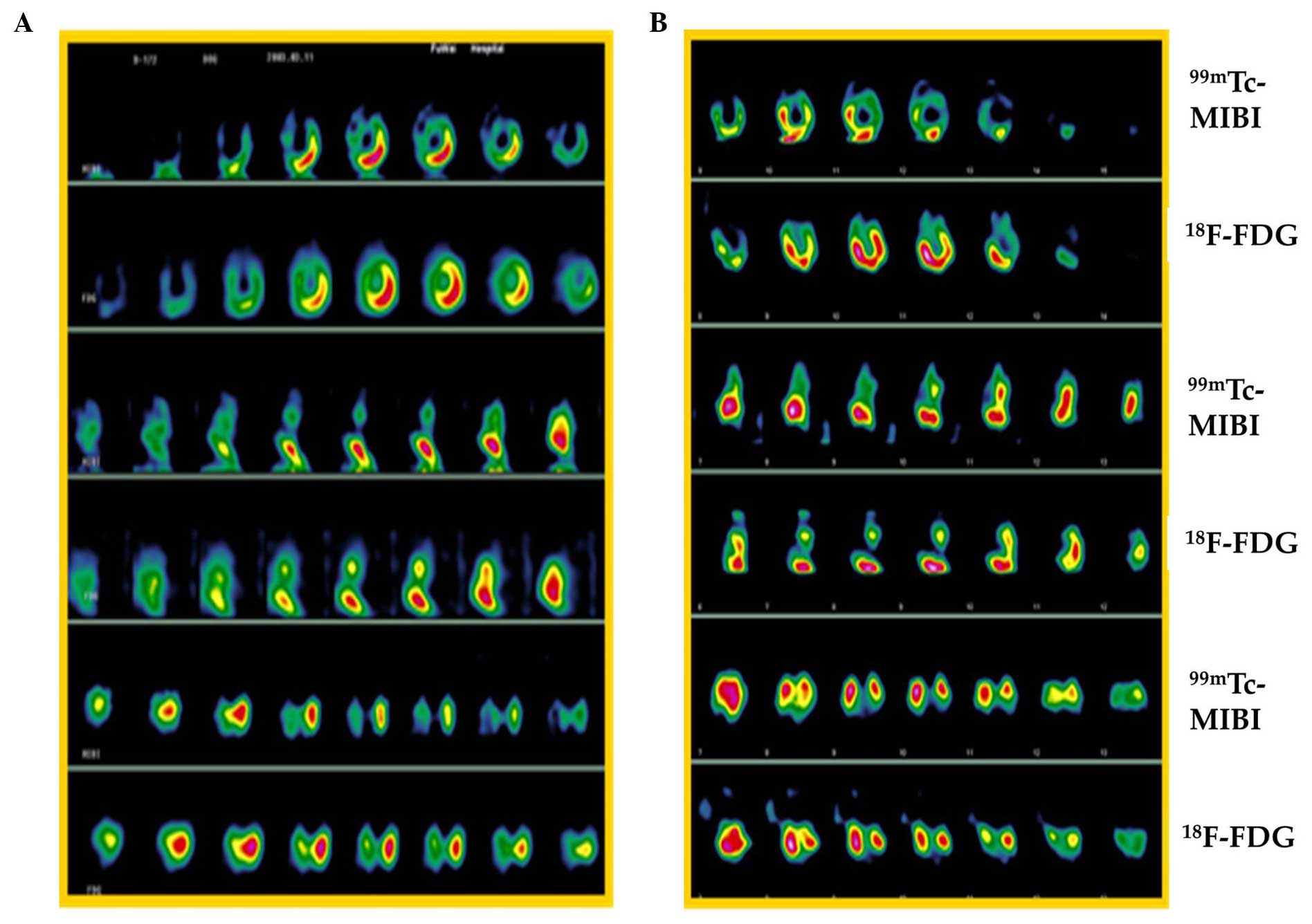
One myocardial viability case is shown in Fig. 4 at different time points [1 (Fig. 4A) and 4 weeks (Fig. 4B)] after ligation of LAD. The change in
the number of viable/infarcted segments in myocardium is presented
as a summary in Table II. The
infarcted number of myocardial segments was decreased significantly
in the graft group, from 25 (1 week) to 15 (10 weeks), following
MSCs implantation, indicating that myocardial regeneration occurred
in the infarction area. Cardiac function was also improved
significantly in the graft group, as LVEF increased from 53.80% (1
week) to 70.00% (10 weeks), but in the control group, LVEF had a
small increase (from 50.5 to 56.5%) at the same time-points, as
shown in Table III.
 | Table II.Infarcted segment number change at
different time points after mesenchymal stem cell implantation. |
Table II.
Infarcted segment number change at
different time points after mesenchymal stem cell implantation.
| Group | 1 week | 10 weeks | Change |
|---|
| Control | 20 | 18 | 2 |
| Graft | 25 | 15 | 10 |
 | Table III.Change of LVEF (%) following
mesenchymal stem cell implantation. |
Table III.
Change of LVEF (%) following
mesenchymal stem cell implantation.
| Group | 1 week | 10 weeks | Diffference |
|---|
| Control | 50.50±8.02 | 56.50±7.24 | 5.50±2.69 |
| Graft | 53.80±9.58 |
70.00±7.52a |
16.20±2.93a |
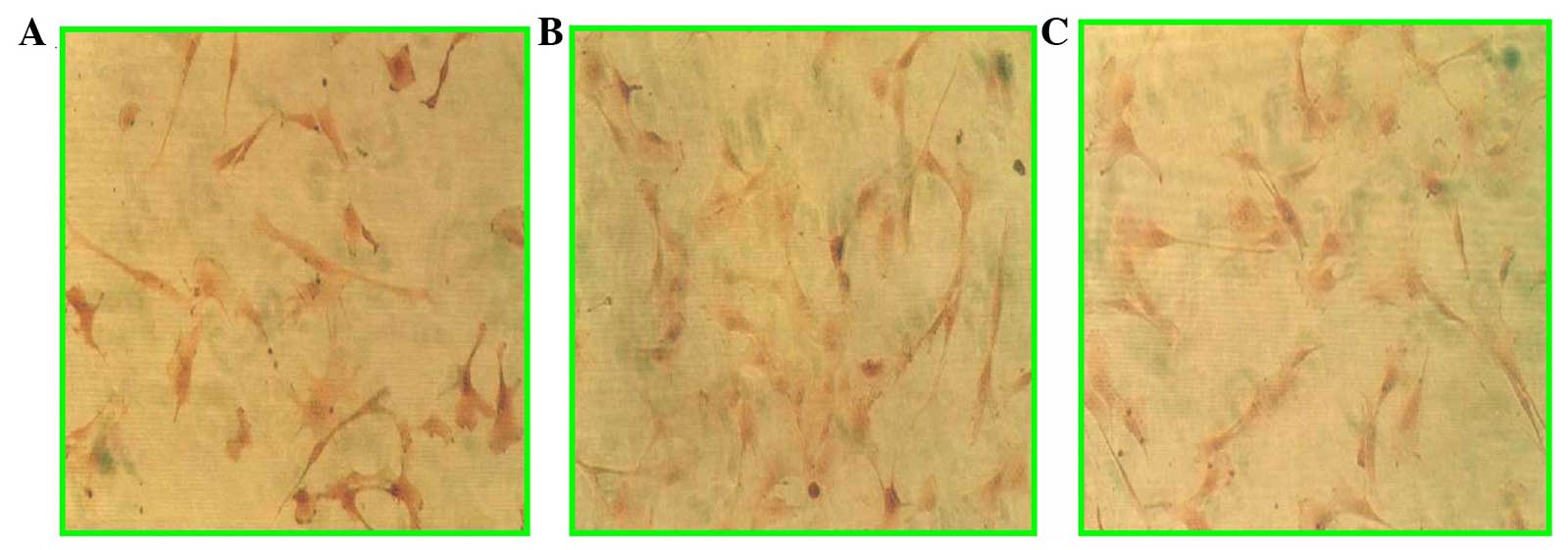
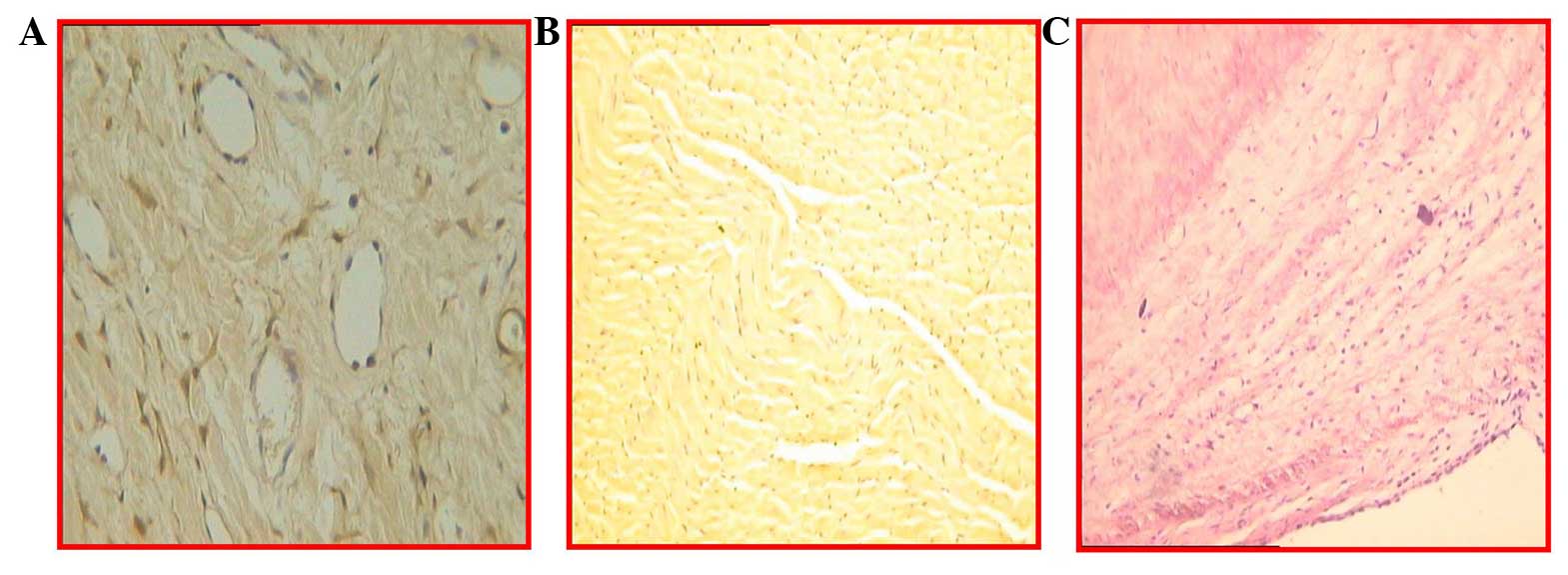
Immunohistochemical staining
Evidence from immunohistochemical staining
demonstrated the engraftment of MSCs in the infarcted myocardial
area (Fig. 5). In the antibody
staining of BrdU (Fig. 5A and B),
angiogenesis and troponin I were found, and similarly, lymphocytic
infiltration was present in the infarcted myocardium (Fig. 5C).
Discussion
Using the dog AMI model in the present study,
intracoronary injection of bone marrow-derived MSCs into injured
myocardium significantly improved cardiac functions, including
reduction of the infarcted area and increase of left ventricle
(LVEF). Notably, DISA and gated SPECT can be used for assessment of
viability of newly-formed myocardium following MSCs
transplantation, and this is of importance in practice, as SPECT is
significantly less expensive with longer-lived more easily-obtained
radioisotopes, that that of PET (11,13).
Previously, stem cell-based treatments of AMI have
been a focus of investigation. Autologous bone marrow-derived MSCs
have been widely utilized as a result of plasticity, availability
and lack of immunological rejection or ethical issues (14,15).
However, poor survival of transplanted MSCs in the injured
myocardial area is one barrier to the success of stem cell therapy
for AMI (16). Previous studies have
shown that improvement of the periinfarct milieu with
pharmacological treatment, such as simvastatin and atorvastatin, is
beneficial for the survival of implanted stem cells (17–19). In the
present study, pharmacological treatment was not used for
improvement of the microenvironment for implanted stem cells, but a
suitable injection time was selected; MSCs were injected after 2 h
of myocardial reperfusion with 90 min of LAD ligation. Immediate
injection of MSCs may be better that that of implantation within a
week after AMI.
DISA SPECT offers the advantage of obtaining
information on myocardial perfusion using 99mTc-MIBI and
metabolism using 18F-FDG in a single study, with
shortened duration of the procedure and an identical geometric
registration of different isotope images (9). Direct comparison of the perfusion tracer
99mTc-MIBI with metabolic tracer 18F-FDG can
determine the scarce and viable myocardium [Slarts et al
(10)]. Comparing with PET perfusion
tracers, such as 15O-water, 13N-ammonia
(which requires on-site cyclotron for radionuclide production) and
82Rb-rubidium (requires on-site generation for
radionuclide production), DISA SPECT with
99mTc-MIBI/18F-FDG is much more widely used
in hospital routine practice. Therefore, in the future, with rapid
development of stem cell implantation-based therapy for treatment
of AMI, DISP SPECT and gated SPECT will play more roles in
assessment of cardiomyogenesis, angiogenesis, cardiac function and
viable myocardial change following stem cell implantation for the
injured heart.
In conclusion, direct intracoronary injection of
bone marrow MSCs into injured myocardium in the experimental dog
AMI model can significantly improve cardiac function with new
vessel formation and myocyte-specific biomarker expression. In
particular, the study further shows that DISA SPECT can be used for
assessment of stem cells transplantation in the heart.
Acknowledgements
The present study was funded by a grant from the
Fujian Provincial Youth Personnel of Scientific and Technology
innovation item (no. 2005J070) and from the Affiliated Hospital of
Inner Mongolia Medical College (no. nmgfy 200802). The authors
thank Professors Xiejie Liu, Rongfang Shi and Zuoxiang He at
Beijing Fuwai Hospital.
References
|
1
|
Keeley EC and Hillis LD: Primary PCI for
myocardial infarction with ST-segment elevation. N Engl J Med.
356:47–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin-Rendon E, Brunskill SJ, Hyde CJ,
Stanworth SJ, Mathur A and Watt SM: Autologous bone marrow stem
cells to treat acute myocardial infarction: A systematic review.
Eur Heart J. 29:1807–1818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Penn MS: Stem Cells and Myocardial
Regeneration. 1st. Humana Press; Totowa: pp. 2772007
|
|
4
|
Silva GV, Litovsky S, Assad JA, Sousa AL,
Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, et al:
Mesenchymal stem cells differentiate into an endothelial phenotype,
enhance vascular density, and improve heart function in a canine
chronic ischemia model. Circulation. 111:150–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodrigo SF, van Ramshorst J, Hoogslag GE,
Boden H, Velders MA, Cannegieter SC, Roelofs H, Al Younis I,
Dibbets-Schneider P, Fibbe WE, et al: Intramyocardial injection of
autologous bone marrow-derived ex vivo expanded mesenchymal stem
cells in acute myocardial infarction patients is feasible and safe
up to 5 years of follow-up. J Cardiovasc Transl Res. 6:816–825.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fukuda K and Fujita J: Mesenchymal, but
not hematopoietic, stem cells can be mobilized and differentiate
into cardiomyocytes after myocardial infarction in mice. Kidney
Int. 68:1940–1943. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castellani M, Colombo A, Giordano R,
Pusineri E, Canzi C, Longari V, Piccaluga E, Palatresi S,
Dellavedova L, Soligo D, et al: The role of PET with
13N-ammonia and 18F-FDG in the assessment of
myocardial perfusion and metabolism in patients with recent AMI and
intracoronary stem cell injection. J Nucl Med. 51:1908–1916. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saraste A, Kajander S, Han C, Nesterov SV
and Knuuti J: PET: Is myocardial flow quantification a clinical
reality? J Nucl Cardiol. 19:1044–1059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rahmim A and Zaidi H: PET versus SPECT:
Strengths, limitations and challenges. Nucl Med Commun. 29:193–207.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Slart RH, Bax JJ, van Veldhuisen DJ, van
der Wall EE, Dierckx RA and Jager PL: Imaging techniques in nuclear
cardiology for the assessment of myocardial viability. Int J
Cardiovasc Imaging. 22:63–80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slart RH, Bax JJ, van Veldhuisen DJ, van
der Wall EE, Irwan R, Sluiter WJ, Dierckx RA, de Boer J and Jager
PL: Prediction of functional recovery after revascularization in
patients with chronic ischaemic left ventricular dysfunction:
Head-to-head comparison between
99mTc-sestamibi/18F-FDG DISA SPECT and
13N-ammonia/18F-FDG PET. Eur J Nucl Med Mol
Imaging. 33:716–723. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Boer J, Slart RH, Blanksma PK,
Willemsen AT, Jager PL, Paans AM, Vaalburg W and Piers DA:
Comparison of
99mTc-sestamibi-18F-fluorodeoxyglucose dual
isotope simultaneous acquisition and rest-stress
99mTc-sestamibi single photon emission computed
tomography for the assessment of myocardial viability. Nucl Med
Commun. 24:251–257. 2003. View Article : Google Scholar
|
|
13
|
Hutton BF: The origins of SPECT and
SPECT/CT. Eur J Nucl Med Mol Imaging. 41 (Suppl 1):S3–S16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Psaltis PJ, Zannettino AC, Worthley SG and
Gronthos S: Concise review: Mesenchymal stromal cells: Potential
for cardiovascular repair. Stem Cells. 26:2201–2210. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Circ Res.
95:9–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robey TE, Saiget MK, Reinecke H and Murry
CE: Systems approaches to preventing transplanted cell death in
cardiac repair. J Mol Cell Cardiol. 45:567–581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song L, Yang YJ, Dong QT, Qian HY, Gao RL,
Qiao SB, Shen R, He ZX, Lu MJ, Zhao SH, et al: Atorvastatin enhance
efficacy of mesenchymal stem cells treatment for swine myocardial
infarction via activation of nitric oxide synthase. PLoS One.
8:e657022013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang YJ, Qian HY, Huang J, Li JJ, Gao RL,
Dou KF, Yang GS, Willerson JT and Geng YJ: Combined therapy with
simvastatin and bone marrow-derived mesenchymal stem cells
increases benefits in infarcted swine hearts. Arterioscler Thromb
Vasc Biol. 29:2076–2082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soboleva EL, Gabbasov ZA, Agapov AA,
Akchurin RS, Saburova OS, Romanov YA and Smirnov VN: Circulating
bone marrow stem/progenitor cells in vascular atherogenesis and in
the noninvasive diagnosis of coronary stenosis. Exp Clin Cardiol.
10:184–188. 2005.PubMed/NCBI
|