Effects of risedronate on the morphology and viability of gingiva-derived mesenchymal stem cells
- Authors:
- Published online on: September 28, 2015 https://doi.org/10.3892/br.2015.520
- Pages: 845-848
Abstract
Introduction
Bisphosphonates are pyrophosphate analogues with a high affinity to hydroxyapatite crystals. These compounds can be classified into nitrogen-containing bisphosphonates (including alendronate, ibandronate, risedronate and zoledronate) and non-nitrogen-containing bisphosphonates (including etidronate and clodronate) (1). Risedronate has been approved for the prevention and treatment of postmenopausal and corticosteroid-induced osteoporosis (2). The drug is reported to reduce bone turnover and decrease resorption, chiefly through the effects on osteoclasts, with no undesirable effect on cortical porosity, thickness or on cancellous bone volume (3).
Previous studies have reported that risedronate reduces the risk for vertebral and non-vertebral fractures in postmenopausal women with osteoporosis (2). A 24-month treatment with risedronate (5 mg/day) was associated with the prevention of bone loss at the spine and hip, with reduced bone resorption and with significantly reduced concentrations of urinary type I collagen cross-linked N-terminal telopeptide and serum bone-specific alkaline phosphatase (4). Risedronate reduced the number, formation, vitality and activity of circulating osteoclast precursors in cultures and cytokines, including the receptor activator of the nuclear factor κB ligand; risedronate also reduced tumor necrosis factor-α production (5).
Mesenchymal stem cells are characterized by the osteogenic, adipogenic and chondrogenic differentiation capabilities (6). Risedronate has been shown to exert a variety of actions on mesenchymal stem cells. A previous study reported that risedronate positively affected the osteogenic differentiation of human mesenchymal stromal cells (1). However, another study showed that risedronate suppressed the osteoblast differentiation of mesenchymal stem cells (7).
The aim of the present study was to evaluate the effects of risedronate on the morphology and viability of human stem cells derived from the gingiva. Mesenchymal stem cells from the gingiva can be isolated from a minimally-invasive procedure. To the best of our knowledge, this investigation is the first to elucidate the effect of risedronate on stem cells derived from the gingiva.
Materials and methods
Materials
Minimum essential medium-α (MEM-α), fetal bovine serum (FBS) and trypsin/EDTA solution were Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Unless otherwise stated, all the other chemicals and reagents were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA).
Isolation and culture of the stem cells derived from gingiva
Gingival tissues removed during clinical crown-lengthening procedures were collected from healthy patients. The study was reviewed and approved by the Institutional Review Board of Seoul St. Mary's Hospital College of Medicine (Catholic University of Korea, Seoul, Republic of Korea), and informed consent was obtained from the participants.
The tissues were de-epithelialized, minced into fragments, and digested with dispase (1 mg/ml) and collagenase IV (2 mg/ml). The cells were incubated at 37°C in a humidified incubator with 5% CO2 and 95% O2.
Evaluation of stem cell morphology
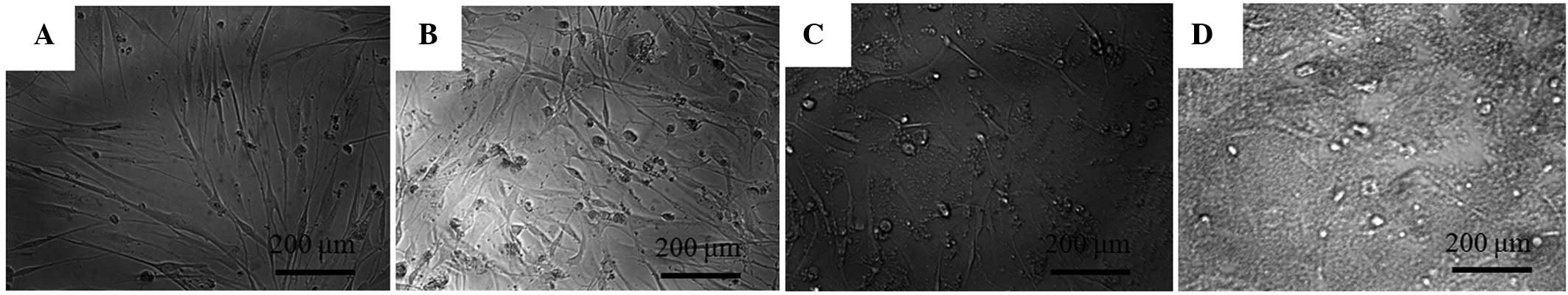
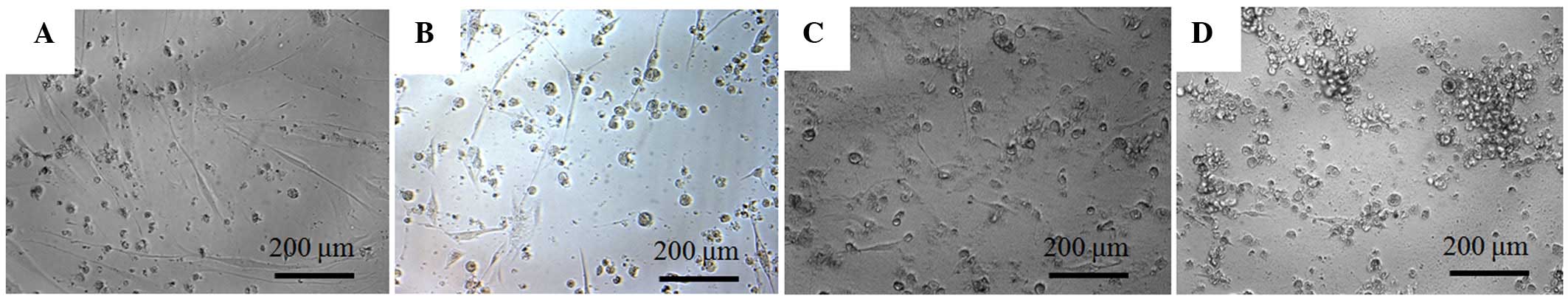
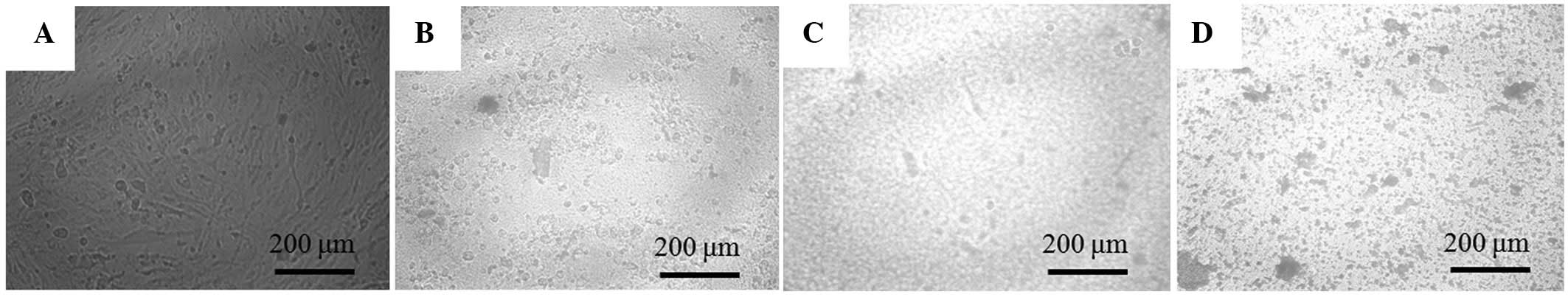
The stem cells were plated at a density of 2.0×103 cells/well in 96-well plates. The cells were incubated in MEM-α (composed of 15% FBS, 10 mM ascorbic acid 2-phosphate, 200 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin) and in the presence of the risedronate at final concentrations of 0 (untreated control), 1, 5 and 10 µM. The morphology of the cells was viewed under an inverted microscope (Leica DM IRM; Leica Microsystems, Wetzlar, Germany) on days 2, 4 and 7. The images were saved as JPEG files.
Determination of cell viability
The analysis of cell viability was performed on days 2, 4 and 7. Viable cells were identified using a cell counting kit-8, (CCK-8; Dojindo, Tokyo, Japan) assay. The spectrophotometric absorbance of the samples at 450 nm was measured using a microplate reader (BioTek, Winooski, VT, USA), and the analysis was performed in triplicate.
Statistical analysis
The data are presented as mean ± standard deviation. Test of normality and one-way analysis of variance with post hoc test were performed to determine the differences between the groups with a commercially available program (SPSS 12 for Windows; SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Evaluation of cell morphology
The morphology of the stem cells on day 2 is shown in Fig. 1. The untreated control group showed spindle-shaped, fibroblast-like morphology. The shapes of the cells in the 1 and 5 µM groups were similar to those of the control group. However, the 10 µM group showed significant differences when compared to the control group. The shapes of the cells in the 10 µM group were rounder and fewer cells were present.
The morphology of the cells on day 4 is shown in Fig. 2. The shapes of the cells in the 1 µM group were similar to the shapes of those in the control group on day 4. Significant alterations in cytoskeletal organization were noticed in the 5 and 10 µM groups. Compared to the control group, the 5 and 10 µM groups had fewer, rounder cells.
The morphology of the cells on day 7 is shown in Fig. 3. Compared with the cells in the untreated control group, those in the 1, 5 and 10 µM groups were rounder and markedly differed.
Cell viability
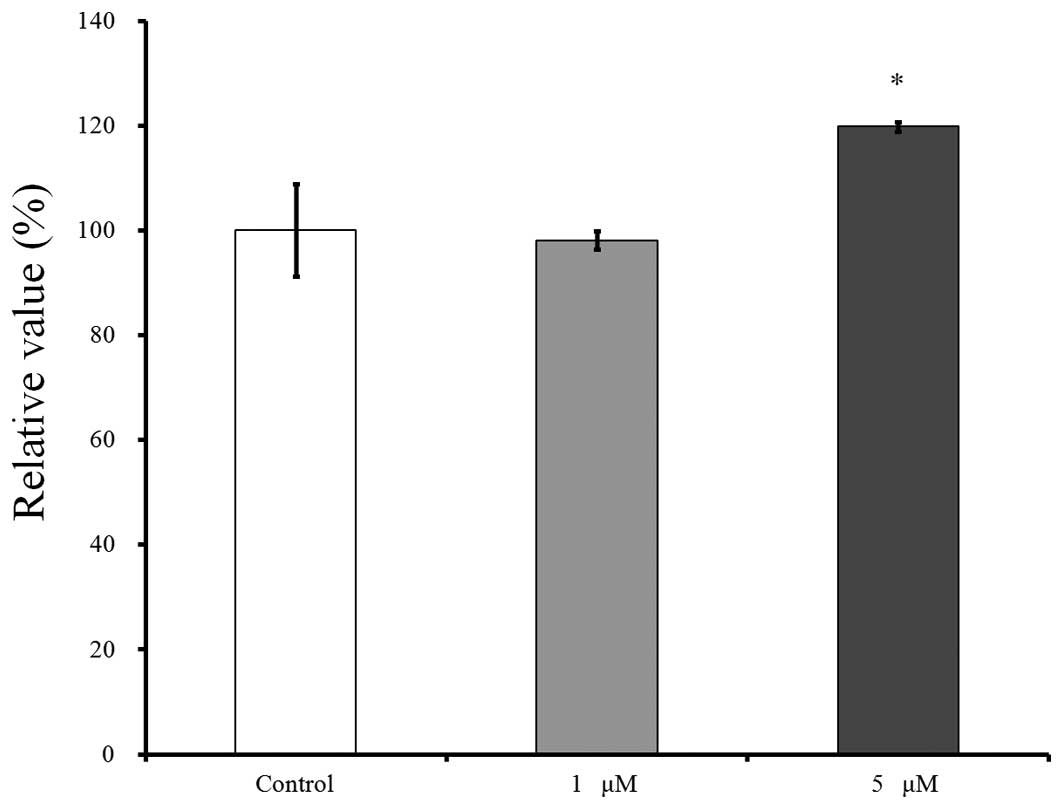
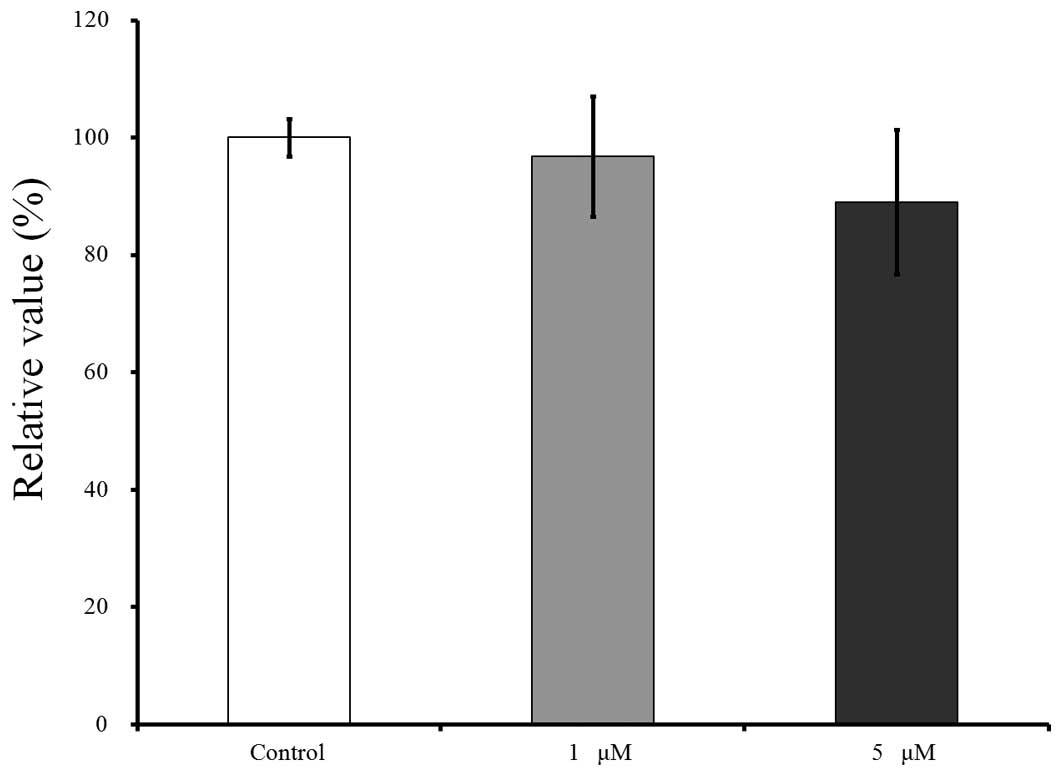
The cell viability results for days 2, 4 and 7 are shown in Figs. 4–6, respectively. CCK-8 values are shown as a ratio of the CCK-8 result of the untreated control group. The cultures that were growing in the presence of risedronate on day 2 showed an increase in the CCK-8 value at 5 µM (Fig 4). The relative value of the CCK-8 assays at 1 and 5 µM of risedronate were 98.1±1.7 and 119.8±0.9%, respectively when the CCK-8 result of the untreated control group on day 2 was considered 100% (100.0±8.8%).
The results for day 4 are shown in Fig. 5. Compared to this value, growing in the presence of risedronate at concentrations of 1 and 5 µM resulted in decreases in the CCK-8 values, to 89.3±11.5 and 87.1±9.6%, respectively, however, there were no significant differences (P>0.05).
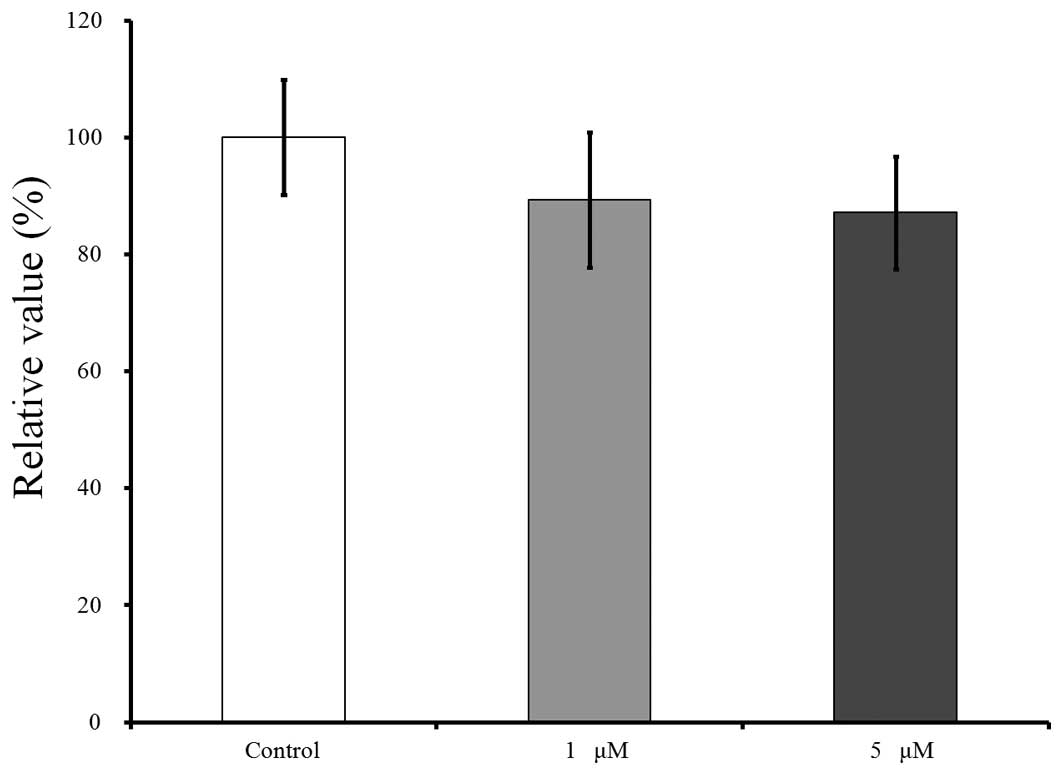
The results for day 7 are shown in Fig. 6. Relative to this value, growing in the presence of risedronate at concentrations of 1 and 5 µM resulted in decreases in the CCK-8 values to 96.7±10.3 and 89.0±12.3%, respectively.
Discussion
The present study evaluated the effects of risedronate on the morphology, cell viability and mineralization under predetermined concentrations (1–10 µM). The morphology of the cells exposed to risedronate produced fewer, rounder cells and alterations in the cytoskeletal organization. Risedronate clearly reduced the viability of mesenchymal stem cells.
Various application protocols for risedronate have been developed in order to optimize patient adherence and persistence; this was possible due to the long terminal exponential half-life of risedronate (8,9). Risedronate treatment (5 mg/day) increased bone mineral density at the lumbar, femoral neck and trochanter skeletal sites in patients recently initiated on or receiving long-term corticosteroid therapy (10). Development of a 35 mg tablet for once-a-week administration has been suggested (8,11). An alternative risedronate treatment of 75 mg on two consecutive days each month was not inferior to the 5 mg daily treatment in terms of efficacy or safety after 12 months, leading to a similar benefit (12).
The concentration of serum risedronate has been investigated in previous studies (13,14). Plasma concentration-time profiles of risedronate were evaluated following a single oral administrations at different dose times in association with breakfast, using healthy volunteers and a dose of 5 mg (13). Plasma concentration varied depending on dosage timing; the maximum concentration was 2.85 ng/ml when fasting without breakfast, 2.11 ng/ml at 30 min before breakfast, 0.19 ng/ml at 30 min after breakfast and 0.38 ng/ml at 3 h after breakfast (13). Another study showed that the dose-adjusted (dose-normalized to 1 mg) maximum concentration of a single dose with the oral administration of a 30 mg tablet was 0.16 ng/ml (14). It should be taken into account that the actual concentrations at the niche surrounding the mesenchymal stem cell and preosteoblasts in the bone marrow could be higher than the average concentration in the serum due to the affinity of risedronate for the bone matrix (1).
The effects of risedronate on mesenchymal stem cells may differ between the studies. Risedronate at concentrations of 0.3–10 µM suppressed the formation of mineralized nodules and the expression of osteoblast gene markers, including bone sialoprotein and osteocalcin (7). The addition of risedronate resulted in a significant downregulation of gene sets for osteoblast differentiation and proliferation in endothelial progenitor cells (15). Conversely, risedronate at 0.01 and 0.001 µM positively affected the osteogenic differentiation of human mesenchymal stem cells (1). Estrogen-deficient rats treated with a low dose (0.24 µg/kg) of risedronate showed enhanced osteoblast differentiation (16). The morphological changes and the reduced viability shown in the present study may be due to higher doses of risedronate. These contrasting effects of risedronate may be attributed to the differences in culturing conditions, culturing periods, the type of cells and the system model (1,17).
Within the limits of the present study, risedronate in the tested concentrations produced notable alterations in cell morphology and reduced viability of mesenchymal stem cells. Direct application of risedronate onto oral tissues may produce adverse effects at high doses. The concentration and application time of risedronate should be meticulously controlled to obtain optimal results.
Acknowledgements
The present study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information and Communication Technology and Future Planning (grant no. NRF-2014R1A1A1003106).
References
|
Casado-Díaz A, Santiago-Mora R, Dorado G and Quesada-Gómez JM: Risedronate positively affects osteogenic differentiation of human mesenchymal stromal cells. Arch Med Res. 44:325–334. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Crandall C: Risedronate: A clinical review. Arch Intern Med. 161:353–360. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Dunn CJ and Goa KL: Risedronate. A review of its pharmacological properties and clinical use in resorptive bone disease. Drugs. 61:685–712. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Välimäki MJ, Farrerons-Minguella J, Halse J, Kröger H, Maroni M, Mulder H, Muñoz-Torres M, Sääf M and Snorre Øfjord E: Effects of risedronate 5 mg/d on bone mineral density and bone turnover markers in late-postmenopausal women with osteopenia: A multinational, 24-month, randomized, double-blind, placebo-controlled, parallel-group, phase III trial. Clin Ther. 29:1937–1949. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
D'Amelio P, Grimaldi A, Di Bella S, Tamone C, Brianza SZ, Ravazzoli MG, Bernabei P, Cristofaro MA, Pescarmona GP and Isaia G: Risedronate reduces osteoclast precursors and cytokine production in postmenopausal osteoporotic women. J Bone Miner Res. 23:373–379. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Jin SH, Lee JE, Yun JH, Kim I, Ko Y and Park JB: Isolation and characterization of human mesenchymal stem cells from gingival connective tissue. J Periodontal Res. 50:461–467. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Fujita H, Kurokawa K, Ogino T, Ono M, Yamamoto M, Oka T, Nakanishi T, Kobayashi N, Tanaka N, Ogawa T, et al: Effect of risedronate on osteoblast differentiation, expression of receptor activator of NF-κB ligand and apoptosis in mesenchymal stem cells. Basic Clin Pharmacol Toxicol. 109:78–84. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
White NJ and Perry CM: Risedronate once a week. Treat Endocrinol. 2:415–420; discussion 421. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Frampton JE: Risedronate on two consecutive days per month. Drugs Aging. 26:355–362. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Dougherty JA: Risedronate for the prevention and treatment of corticosteroid-induced osteoporosis. Ann Pharmacother. 36:512–516. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Tauchmanovà L, De Simone G, Musella T, Orio F, Ricci P, Nappi C, Lombardi G, Colao A, Rotoli B and Selleri C: Effects of various antireabsorptive treatments on bone mineral density in hypogonadal young women after allogeneic stem cell transplantation. Bone Marrow Transplant. 37:81–88. 2006.PubMed/NCBI | |
|
Delmas PD, Benhamou CL, Man Z, Tlustochowicz W, Matzkin E, Eusebio R, Zanchetta J, Olszynski WP, Recker RR and McClung MR: Monthly dosing of 75 mg risedronate on 2 consecutive days a month: Efficacy and safety results. Osteoporos Int. 19:1039–1045. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Ogura Y, Gonsho A, Cyong JC and Orimo H: Clinical trial of risedronate in Japanese volunteers: A study on the effects of timing of dosing on absorption. J Bone Miner Metab. 22:120–126. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Mitchell DY, Barr WH, Eusebio RA, Stevens KA, Duke FP, Russell DA, Nesbitt JD, Powell JH and Thompson GA: Risedronate pharmacokinetics and intra- and inter-subject variability upon single-dose intravenous and oral administration. Pharm Res. 18:166–170. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Peris P, Atkinson EJ, Gössl M, Kane TL, McCready LK, Lerman A, Khosla S and McGregor UI: Effects of bisphosphonate treatment on circulating osteogenic endothelial progenitor cells in postmenopausal women. Mayo Clin Proc. 88:46–55. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Wang G, Zhu Z, Lei C, Li M, Liu F, Mao Y, Yu Z, Liu M, Zhao X and Tang T: Low-dose risedronate sodium protects bone cells after abrupt oestrogen withdrawal. J Int Med Res. 40:1761–1774. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Park JB, Zhang H, Lin CY, Chung CP, Byun Y, Park YS and Yang VC: Simvastatin maintains osteoblastic viability while promoting differentiation by partially regulating the expressions of estrogen receptors α. J Surg Res. 174:278–283. 2012. View Article : Google Scholar : PubMed/NCBI |