Introduction
Bisphosphonates are pyrophosphate analogues with a
high affinity to hydroxyapatite crystals. These compounds can be
classified into nitrogen-containing bisphosphonates (including
alendronate, ibandronate, risedronate and zoledronate) and
non-nitrogen-containing bisphosphonates (including etidronate and
clodronate) (1). Risedronate has been
approved for the prevention and treatment of postmenopausal and
corticosteroid-induced osteoporosis (2). The drug is reported to reduce bone
turnover and decrease resorption, chiefly through the effects on
osteoclasts, with no undesirable effect on cortical porosity,
thickness or on cancellous bone volume (3).
Previous studies have reported that risedronate
reduces the risk for vertebral and non-vertebral fractures in
postmenopausal women with osteoporosis (2). A 24-month treatment with risedronate (5
mg/day) was associated with the prevention of bone loss at the
spine and hip, with reduced bone resorption and with significantly
reduced concentrations of urinary type I collagen cross-linked
N-terminal telopeptide and serum bone-specific alkaline phosphatase
(4). Risedronate reduced the number,
formation, vitality and activity of circulating osteoclast
precursors in cultures and cytokines, including the receptor
activator of the nuclear factor κB ligand; risedronate also reduced
tumor necrosis factor-α production (5).
Mesenchymal stem cells are characterized by the
osteogenic, adipogenic and chondrogenic differentiation
capabilities (6). Risedronate has been
shown to exert a variety of actions on mesenchymal stem cells. A
previous study reported that risedronate positively affected the
osteogenic differentiation of human mesenchymal stromal cells
(1). However, another study showed
that risedronate suppressed the osteoblast differentiation of
mesenchymal stem cells (7).
The aim of the present study was to evaluate the
effects of risedronate on the morphology and viability of human
stem cells derived from the gingiva. Mesenchymal stem cells from
the gingiva can be isolated from a minimally-invasive procedure. To
the best of our knowledge, this investigation is the first to
elucidate the effect of risedronate on stem cells derived from the
gingiva.
Materials and methods
Materials
Minimum essential medium-α (MEM-α), fetal bovine
serum (FBS) and trypsin/EDTA solution were Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Unless otherwise stated, all
the other chemicals and reagents were obtained from Sigma-Aldrich
Co. (St. Louis, MO, USA).
Isolation and culture of the stem
cells derived from gingiva
Gingival tissues removed during clinical
crown-lengthening procedures were collected from healthy patients.
The study was reviewed and approved by the Institutional Review
Board of Seoul St. Mary's Hospital College of Medicine (Catholic
University of Korea, Seoul, Republic of Korea), and informed
consent was obtained from the participants.
The tissues were de-epithelialized, minced into
fragments, and digested with dispase (1 mg/ml) and collagenase IV
(2 mg/ml). The cells were incubated at 37°C in a humidified
incubator with 5% CO2 and 95% O2.
Evaluation of stem cell
morphology
The stem cells were plated at a density of
2.0×103 cells/well in 96-well plates. The cells were
incubated in MEM-α (composed of 15% FBS, 10 mM ascorbic acid
2-phosphate, 200 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml
streptomycin) and in the presence of the risedronate at final
concentrations of 0 (untreated control), 1, 5 and 10 µM. The
morphology of the cells was viewed under an inverted microscope
(Leica DM IRM; Leica Microsystems, Wetzlar, Germany) on days 2, 4
and 7. The images were saved as JPEG files.
Determination of cell viability
The analysis of cell viability was performed on days
2, 4 and 7. Viable cells were identified using a cell counting
kit-8, (CCK-8; Dojindo, Tokyo, Japan) assay. The spectrophotometric
absorbance of the samples at 450 nm was measured using a microplate
reader (BioTek, Winooski, VT, USA), and the analysis was performed
in triplicate.
Statistical analysis
The data are presented as mean ± standard deviation.
Test of normality and one-way analysis of variance with post hoc
test were performed to determine the differences between the groups
with a commercially available program (SPSS 12 for Windows; SPSS
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Evaluation of cell morphology
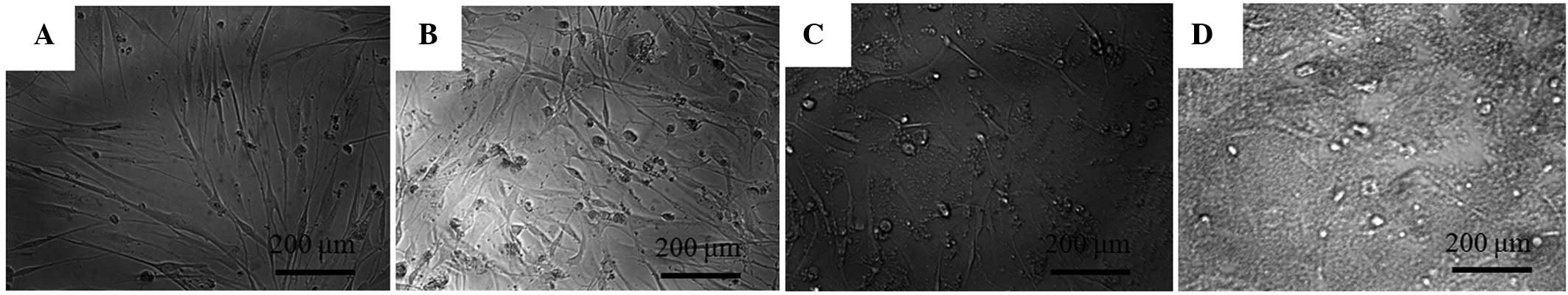
The morphology of the stem cells on day 2 is shown
in Fig. 1. The untreated control group
showed spindle-shaped, fibroblast-like morphology. The shapes of
the cells in the 1 and 5 µM groups were similar to those of the
control group. However, the 10 µM group showed significant
differences when compared to the control group. The shapes of the
cells in the 10 µM group were rounder and fewer cells were
present.
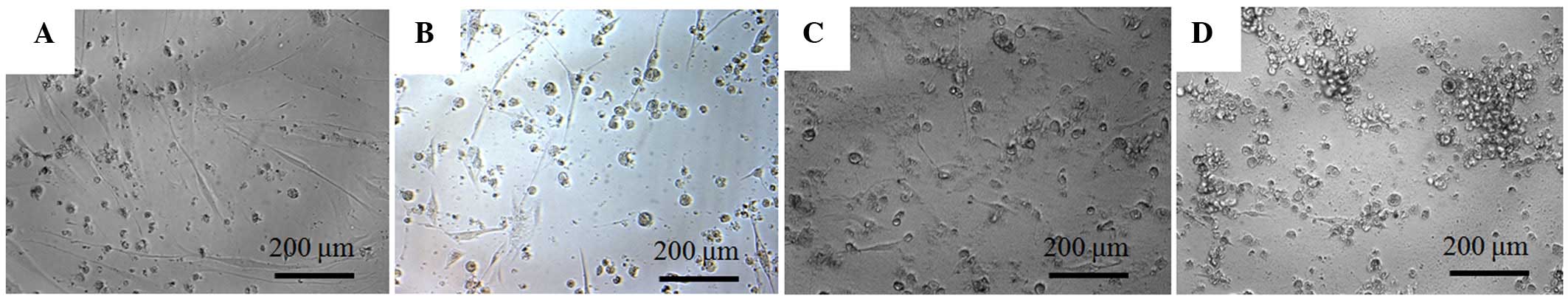
The morphology of the cells on day 4 is shown in
Fig. 2. The shapes of the cells in the
1 µM group were similar to the shapes of those in the control group
on day 4. Significant alterations in cytoskeletal organization were
noticed in the 5 and 10 µM groups. Compared to the control group,
the 5 and 10 µM groups had fewer, rounder cells.
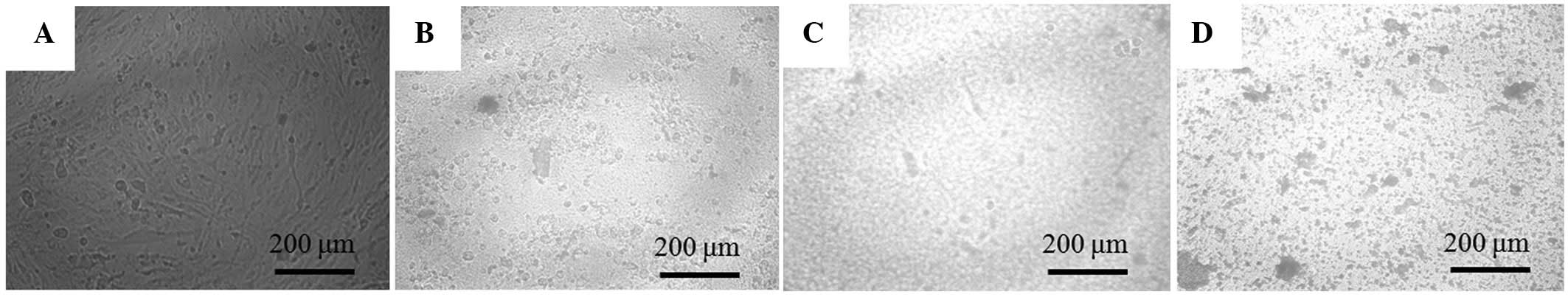
The morphology of the cells on day 7 is shown in
Fig. 3. Compared with the cells in the
untreated control group, those in the 1, 5 and 10 µM groups were
rounder and markedly differed.
Cell viability
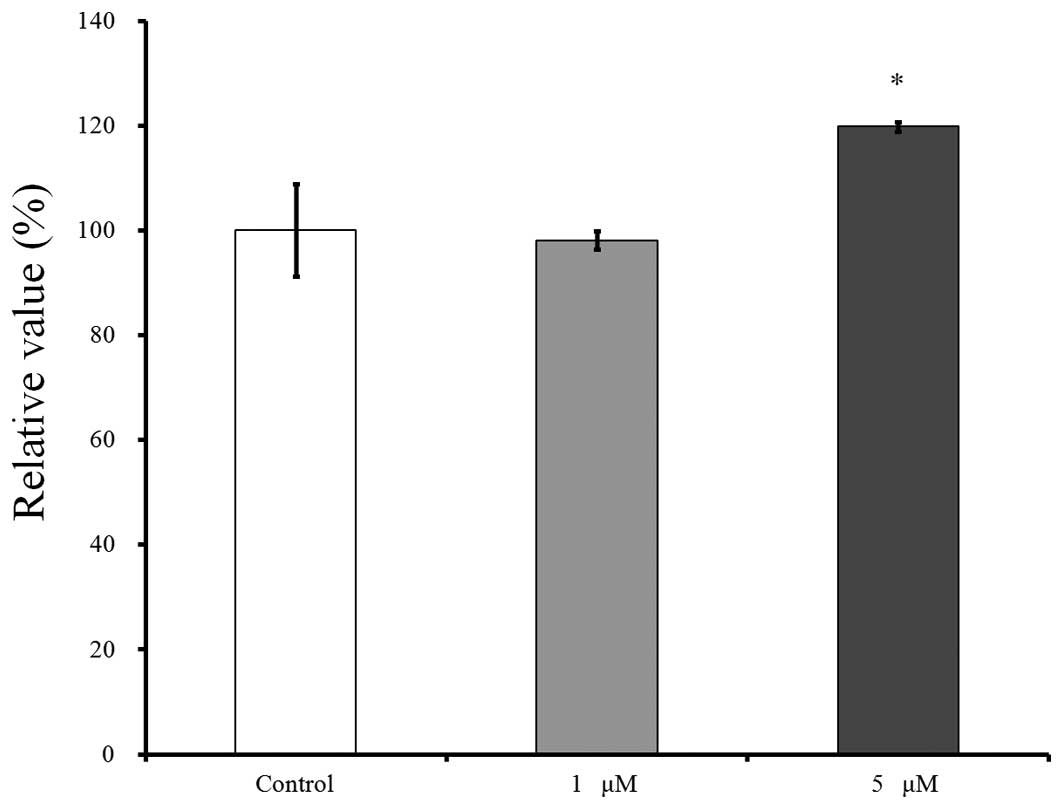
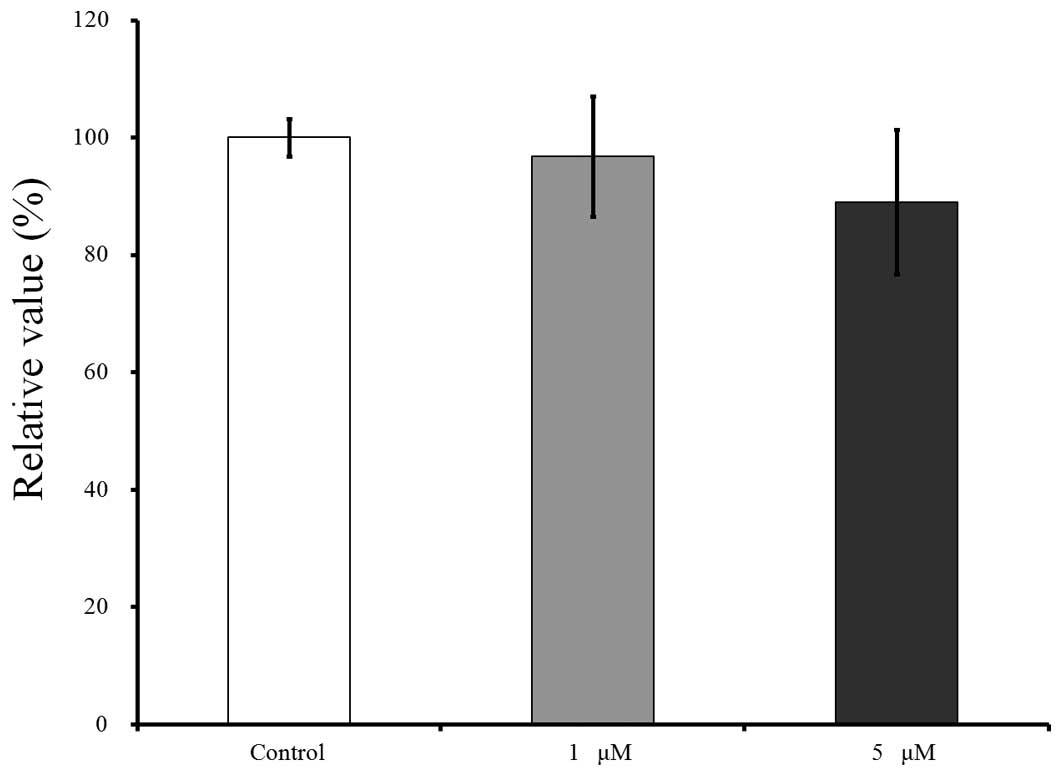
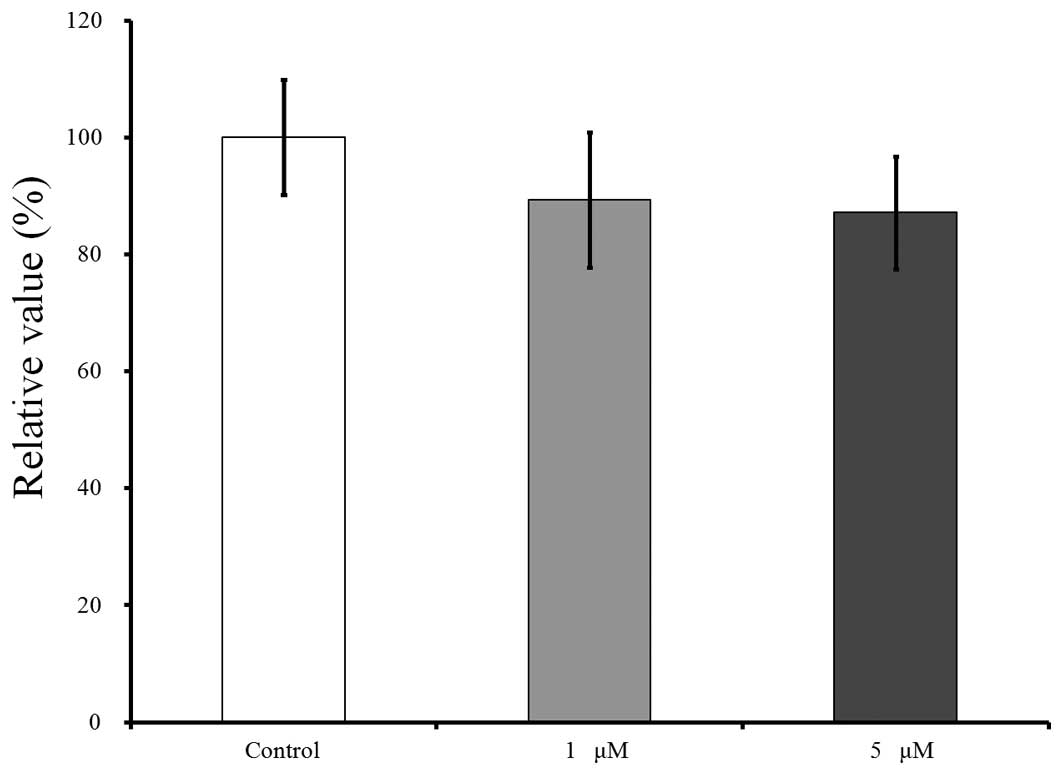
The cell viability results for days 2, 4 and 7 are
shown in Figs. 4–6, respectively. CCK-8 values are shown as a
ratio of the CCK-8 result of the untreated control group. The
cultures that were growing in the presence of risedronate on day 2
showed an increase in the CCK-8 value at 5 µM (Fig 4). The relative value of the CCK-8 assays
at 1 and 5 µM of risedronate were 98.1±1.7 and 119.8±0.9%,
respectively when the CCK-8 result of the untreated control group
on day 2 was considered 100% (100.0±8.8%).
The results for day 4 are shown in Fig. 5. Compared to this value, growing in the
presence of risedronate at concentrations of 1 and 5 µM resulted in
decreases in the CCK-8 values, to 89.3±11.5 and 87.1±9.6%,
respectively, however, there were no significant differences
(P>0.05).
The results for day 7 are shown in Fig. 6. Relative to this value, growing in the
presence of risedronate at concentrations of 1 and 5 µM resulted in
decreases in the CCK-8 values to 96.7±10.3 and 89.0±12.3%,
respectively.
Discussion
The present study evaluated the effects of
risedronate on the morphology, cell viability and mineralization
under predetermined concentrations (1–10 µM). The
morphology of the cells exposed to risedronate produced fewer,
rounder cells and alterations in the cytoskeletal organization.
Risedronate clearly reduced the viability of mesenchymal stem
cells.
Various application protocols for risedronate have
been developed in order to optimize patient adherence and
persistence; this was possible due to the long terminal exponential
half-life of risedronate (8,9). Risedronate treatment (5 mg/day) increased
bone mineral density at the lumbar, femoral neck and trochanter
skeletal sites in patients recently initiated on or receiving
long-term corticosteroid therapy (10). Development of a 35 mg tablet for
once-a-week administration has been suggested (8,11). An
alternative risedronate treatment of 75 mg on two consecutive days
each month was not inferior to the 5 mg daily treatment in terms of
efficacy or safety after 12 months, leading to a similar benefit
(12).
The concentration of serum risedronate has been
investigated in previous studies (13,14). Plasma
concentration-time profiles of risedronate were evaluated following
a single oral administrations at different dose times in
association with breakfast, using healthy volunteers and a dose of
5 mg (13). Plasma concentration
varied depending on dosage timing; the maximum concentration was
2.85 ng/ml when fasting without breakfast, 2.11 ng/ml at 30 min
before breakfast, 0.19 ng/ml at 30 min after breakfast and 0.38
ng/ml at 3 h after breakfast (13).
Another study showed that the dose-adjusted (dose-normalized to 1
mg) maximum concentration of a single dose with the oral
administration of a 30 mg tablet was 0.16 ng/ml (14). It should be taken into account that the
actual concentrations at the niche surrounding the mesenchymal stem
cell and preosteoblasts in the bone marrow could be higher than the
average concentration in the serum due to the affinity of
risedronate for the bone matrix (1).
The effects of risedronate on mesenchymal stem cells
may differ between the studies. Risedronate at concentrations of
0.3–10 µM suppressed the formation of mineralized nodules and the
expression of osteoblast gene markers, including bone sialoprotein
and osteocalcin (7). The addition of
risedronate resulted in a significant downregulation of gene sets
for osteoblast differentiation and proliferation in endothelial
progenitor cells (15). Conversely,
risedronate at 0.01 and 0.001 µM positively affected the osteogenic
differentiation of human mesenchymal stem cells (1). Estrogen-deficient rats treated with a low
dose (0.24 µg/kg) of risedronate showed enhanced osteoblast
differentiation (16). The
morphological changes and the reduced viability shown in the
present study may be due to higher doses of risedronate. These
contrasting effects of risedronate may be attributed to the
differences in culturing conditions, culturing periods, the type of
cells and the system model (1,17).
Within the limits of the present study, risedronate
in the tested concentrations produced notable alterations in cell
morphology and reduced viability of mesenchymal stem cells. Direct
application of risedronate onto oral tissues may produce adverse
effects at high doses. The concentration and application time of
risedronate should be meticulously controlled to obtain optimal
results.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, Information and
Communication Technology and Future Planning (grant no.
NRF-2014R1A1A1003106).
References
|
1
|
Casado-Díaz A, Santiago-Mora R, Dorado G
and Quesada-Gómez JM: Risedronate positively affects osteogenic
differentiation of human mesenchymal stromal cells. Arch Med Res.
44:325–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crandall C: Risedronate: A clinical
review. Arch Intern Med. 161:353–360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dunn CJ and Goa KL: Risedronate. A review
of its pharmacological properties and clinical use in resorptive
bone disease. Drugs. 61:685–712. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Välimäki MJ, Farrerons-Minguella J, Halse
J, Kröger H, Maroni M, Mulder H, Muñoz-Torres M, Sääf M and Snorre
Øfjord E: Effects of risedronate 5 mg/d on bone mineral density and
bone turnover markers in late-postmenopausal women with osteopenia:
A multinational, 24-month, randomized, double-blind,
placebo-controlled, parallel-group, phase III trial. Clin Ther.
29:1937–1949. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Amelio P, Grimaldi A, Di Bella S, Tamone
C, Brianza SZ, Ravazzoli MG, Bernabei P, Cristofaro MA, Pescarmona
GP and Isaia G: Risedronate reduces osteoclast precursors and
cytokine production in postmenopausal osteoporotic women. J Bone
Miner Res. 23:373–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin SH, Lee JE, Yun JH, Kim I, Ko Y and
Park JB: Isolation and characterization of human mesenchymal stem
cells from gingival connective tissue. J Periodontal Res.
50:461–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujita H, Kurokawa K, Ogino T, Ono M,
Yamamoto M, Oka T, Nakanishi T, Kobayashi N, Tanaka N, Ogawa T, et
al: Effect of risedronate on osteoblast differentiation, expression
of receptor activator of NF-κB ligand and apoptosis in mesenchymal
stem cells. Basic Clin Pharmacol Toxicol. 109:78–84. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
White NJ and Perry CM: Risedronate once a
week. Treat Endocrinol. 2:415–420; discussion 421. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frampton JE: Risedronate on two
consecutive days per month. Drugs Aging. 26:355–362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dougherty JA: Risedronate for the
prevention and treatment of corticosteroid-induced osteoporosis.
Ann Pharmacother. 36:512–516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tauchmanovà L, De Simone G, Musella T,
Orio F, Ricci P, Nappi C, Lombardi G, Colao A, Rotoli B and Selleri
C: Effects of various antireabsorptive treatments on bone mineral
density in hypogonadal young women after allogeneic stem cell
transplantation. Bone Marrow Transplant. 37:81–88. 2006.PubMed/NCBI
|
|
12
|
Delmas PD, Benhamou CL, Man Z,
Tlustochowicz W, Matzkin E, Eusebio R, Zanchetta J, Olszynski WP,
Recker RR and McClung MR: Monthly dosing of 75 mg risedronate on 2
consecutive days a month: Efficacy and safety results. Osteoporos
Int. 19:1039–1045. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogura Y, Gonsho A, Cyong JC and Orimo H:
Clinical trial of risedronate in Japanese volunteers: A study on
the effects of timing of dosing on absorption. J Bone Miner Metab.
22:120–126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mitchell DY, Barr WH, Eusebio RA, Stevens
KA, Duke FP, Russell DA, Nesbitt JD, Powell JH and Thompson GA:
Risedronate pharmacokinetics and intra- and inter-subject
variability upon single-dose intravenous and oral administration.
Pharm Res. 18:166–170. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peris P, Atkinson EJ, Gössl M, Kane TL,
McCready LK, Lerman A, Khosla S and McGregor UI: Effects of
bisphosphonate treatment on circulating osteogenic endothelial
progenitor cells in postmenopausal women. Mayo Clin Proc. 88:46–55.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang G, Zhu Z, Lei C, Li M, Liu F, Mao Y,
Yu Z, Liu M, Zhao X and Tang T: Low-dose risedronate sodium
protects bone cells after abrupt oestrogen withdrawal. J Int Med
Res. 40:1761–1774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JB, Zhang H, Lin CY, Chung CP, Byun
Y, Park YS and Yang VC: Simvastatin maintains osteoblastic
viability while promoting differentiation by partially regulating
the expressions of estrogen receptors α. J Surg Res. 174:278–283.
2012. View Article : Google Scholar : PubMed/NCBI
|