Introduction
Traumatic optic nerve injury is one of the
complications of head trauma, and is also the main cause of head
trauma causing permanent vision loss. The optic nerve is located at
the back of the eyes. Head or orbital trauma can cause optic canal
fracture fragments, which may cut off or oppress the optic nerve.
Optic nerve sheath membrane hemorrhage due to the fracture of the
skull base can cause subdural or subarachnoid hemorrhage, and optic
nerve avulsion can be caused by orbital trauma. All these can cause
traumatic optic nerve injury (1).
Visual evoked potential examination has an extremely important
value for the diagnosis of traumatic optic neuropathy. Currently,
it is a widely used clinical objective evaluation method for visual
function following injury. In the present study, through an
established animal model of indirect optic nerve injury, visual
evoked potential and the morphological changes of the optic nerve
tissue were observed following injury, as well as the decompression
changes at different times of the trauma model, in order to study
the mechanism of traumatic optic nerve injury and the association
between the surgical time and curative effect, to offer a
theoretical foundation for the selection of a suitable surgical
time for clinical treatment of traumatic optic neuropathy.
Materials and methods
Experimental materials and groups
A total of 30 healthy adult male New Zealand white
rabbits, with a body weight of 2.0–3.0 kg, were provided by the
Animal Experiment Center of Nanjing General Hospital of Nanjing
Command (Nanjing, China). Upon examination, all rabbits had normal
refractive media, equal and round pupils, and normal reaction to
light, without fundus oculi abnormalities. All the rabbits were
randomly divided into five groups (A-E), with 6 rabbits in each
group, representing the normal control, 48-h decompression, 1-week
decompression, 2-week decompression (1) and non-decompression groups, respectively.
The left eye of each animal was selected for analysis.
Establishment of the optic nerve crush
injury model
Rabbits were anesthetized by subcutaneous
administration of 50 g/l ketamine (100 mg/kg). The Tenon's capsule
was incised from the supraorbital rim, the superior and medial
rectus were separated and clipped, and the optic foramen was
separated and exposed along the temporal sclera. To block the optic
nerve and lead to a crush injury of the optic nerve, a conical soft
silica gel with a 2 mm diameter at the thin end was tucked in the
optic hole. Two days after surgery, rabbits with pupils >4.5 mm,
with disappearance of direct light reflex and no clear extruded
eyeballs were regarded as success models. Optic nerve decompression
surgery was carried out to remove the silica gel (2).
Pattern reversal visual evoked
potentials (P-VEP)
All the examinations were performed using a NeuroMax
1004 machine (Xltek, Oakville, ON, Canada) in a dark room at
18–25°C, with all ketamine rabbits anesthetized. Recording
electrodes were placed ~5 cm away and back from the midpoint of the
ears, the reference electrode was subcutaneously placed at the
midpoint of the eyes, and the ground electrode was placed at the
tip of the ipsilateral ear. The eyes were 30 cm away from the
screen, to flip stimulate (alternate light and shade of the
stimulus) the full-field monocular vision with an all wild
checkerboard pattern, whilst the contralateral eye was masked.
Stimulation parameters were as follows: The checkerboard was of
size 30′ (half light and half shade), with an average luminance 50
CD/m2, 90% contrast, and the flip frequency was 1.1 Hz.
Stimulus-caused signals were filtered (with a passband of 0.5–100
Hz), amplified (2×104 times) and input onto a
microcomputer for superposition. Superposition was for 128×, and
analysis for 300 msec. The P-VEP waveforms of 1 h pre- and
post-trauma, 1 h pre-decompression, and 2 weeks post-decompression
were recorded, and the latency and amplitude of the main wave
(P-wave) were determined, with each stimulus repeated ≥3×. For the
normal group, a set of data were collected only as a control, and
data of the non-decompression group were collected in accordance of
the check time of the 2 weeks decompression group. The data and
waveform were collected and statistical processed by a
microcomputer.
The P-VEP observation indexes included the absolute
incubation period and amplitude of the NPN contours main wave
(P-wave). The absolute incubation period is the time from the
beginning of stimulation to the peak of the P-wave, and the
irregular P-wave shape can be measured from the intersection of an
extension cord of the rise and fall branch of the P-wave peak.
Absolute volatility is the range from a previous negative peak to
the amplitude of the P-wave. The latent period and amplitude
mentioned in the following are all absolute incubation period and
amplitude.
Optic nerve morphology
All the experimental animals of the post-trauma
decompression group were sacrificed 2 weeks after the
decompression, and the optic nerves were fixed in 10% formaldehyde
solution, stained with haematoxylin and eosin, and observed under a
light microscope. The non-decompression group was sacrificed 4
weeks after trauma, with the same treatment as before. The optic
nerve specimens in the normal control group were collected as the
control. Morphological observations included swelling of the optic
nerve, axonal degeneration, myelin depigmentation, hyperplasia of
astrocytes, and vascular lesions within the optic nerve.
Statistical analysis
The data are presented as the mean ± standard
deviation. All statistical analyses were performed using SPSS 10.0
(SPSS, Inc., Chicago, IL, USA). Independent sample t-test was used
in self-comparison of pre- and post-decompression, and one-way
analysis of variance was used to compare the P-VEP of multiple
groups 2 weeks post-decompression. Differences between groups were
compared using the least significant difference test when
homoscedasticity was assumed. P<0.05 was considered to indicate
a statistically significant difference.
Results
P-VEP changes
The P-VEP of each healthy rabbit revealed typical
NPN contours. The NPN contours in the injured rabbits were low and
flat. The latent period of the P contour was lengthened and the
amplitude was reduced (Tables I and
II). The differences between the
latent period and amplitude pre- and post-trauma were significant
statistically (P<0.05). The comparisons between P-VEP pre- and
post-decompression were as follows: Comparisons of the amplitude
and latent periods prior and subsequent to decompression in the i)
48-h decompression group were significantly different (P<0.05),
ii) in the 1-week decompression group exhibited no differences
(P>0.05), and iii) comparisons of amplitude prior and subsequent
to decompression in the 2-week decompression group exhibited no
differences (P>0.05), while the latent period was significantly
different (P<0.05).
 | Table I.Latent period of the P-wave in the
different periods. |
Table I.
Latent period of the P-wave in the
different periods.
| Groups | Pre-trauma, msec | 1 h post-trauma,
msec | 1 h
pre-decompression, msec | 2 weeks
post-decompression, msec |
|---|
| A | 63.67±4.74 | NA | NA | NA |
| B | 62.03±8.79 | 71.77±7.96 |
86.47±14.28 | 71.25±8.51 |
| C |
65.91±12.78 | 80.58±9.38 | 94.72±7.13 | 106.43±11.79 |
| D | 63.50±7.69 | 74.02±6.14 | 116.35±17.13 | 158.73±15.16 |
| Ea | 63.80±7.60 | 75.85±5.75 | 114.42±19.57 | 169.75±16.25 |
 | Table II.Amplitude of the P-wave in the
different periods. |
Table II.
Amplitude of the P-wave in the
different periods.
| Groups | Pre-trauma, µV | 1 h post-trauma,
µV | 1 h
pre-decompression, µV | 2 weeks
post-decompression, µV |
|---|
| A | 5.35±1.17 | NA | NA | NA |
| B | 5.93±1.09 | 4.82±0.50 | 4.42±0.42 | 5.25±0.78 |
| C | 5.58±1.47 | 4.05±0.82 | 3.05±0.71 | 2.88±0.76 |
| D | 5.00±0.85 | 3.85±0.62 | 2.53±0.78 | 1.83±0.96 |
| Ea | 5.31±0.83 | 4.20±0.81 | 2.60±0.92 | 1.58±0.63 |
The comparisons of P-VEP 2 weeks post-decompression
were as follows: i) The differences of the latent period between
two groups among B, C and D were significantly different
(P<0.01), those of amplitude between groups B against C and D
were significant (P<0.01), while those between groups C and D
were significant (P<0.05). ii) The differences of the latent
period and amplitude between groups B and C with group E were
significant (P<0.01), while those between groups D and E
exhibited no significance (P>0.05). iii) There was no
significance in the differences of amplitude and latent period
between groups A and B (P>0.05), while there were significant
differences among group A and groups C-E (P<0.01).
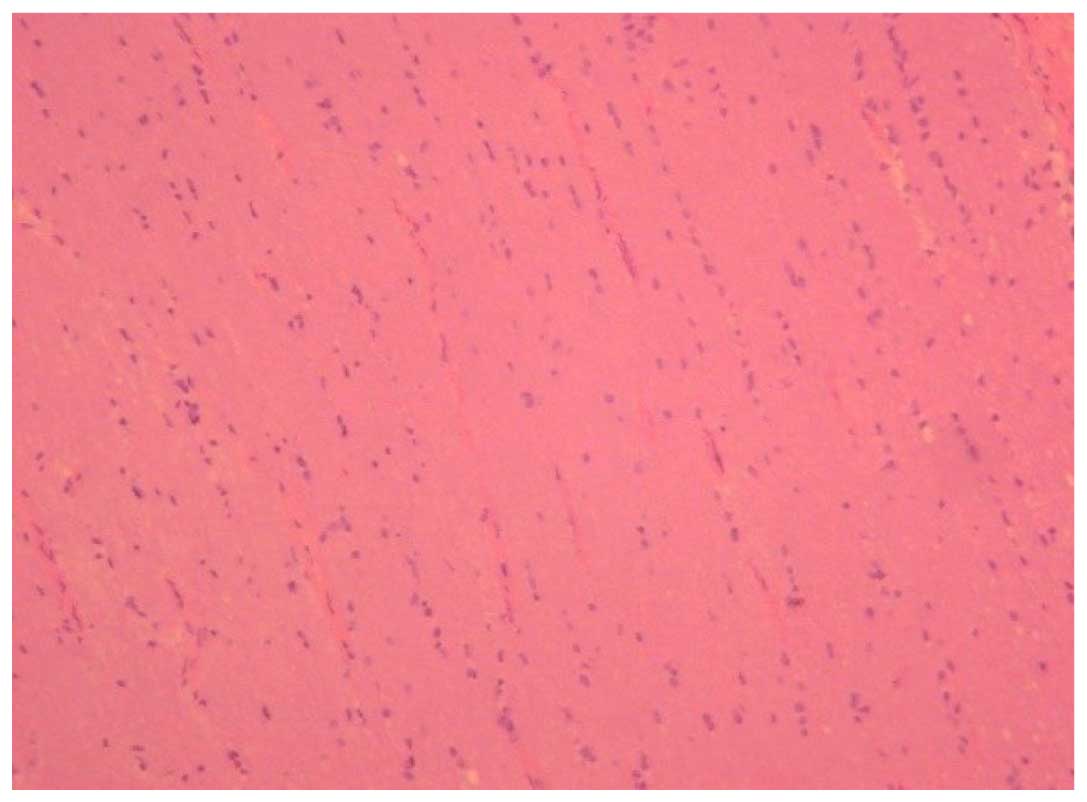
Optic nerve morphological changes
In the normal control group, the astrocytes of the
optic nerve exhibited a cylindrical form and were arranged evenly
on vertical section (Fig. 1) the
neural fibers were arranged neatly and were stained evenly, and the
cross section showed a normal configuration of the blood
vessel.
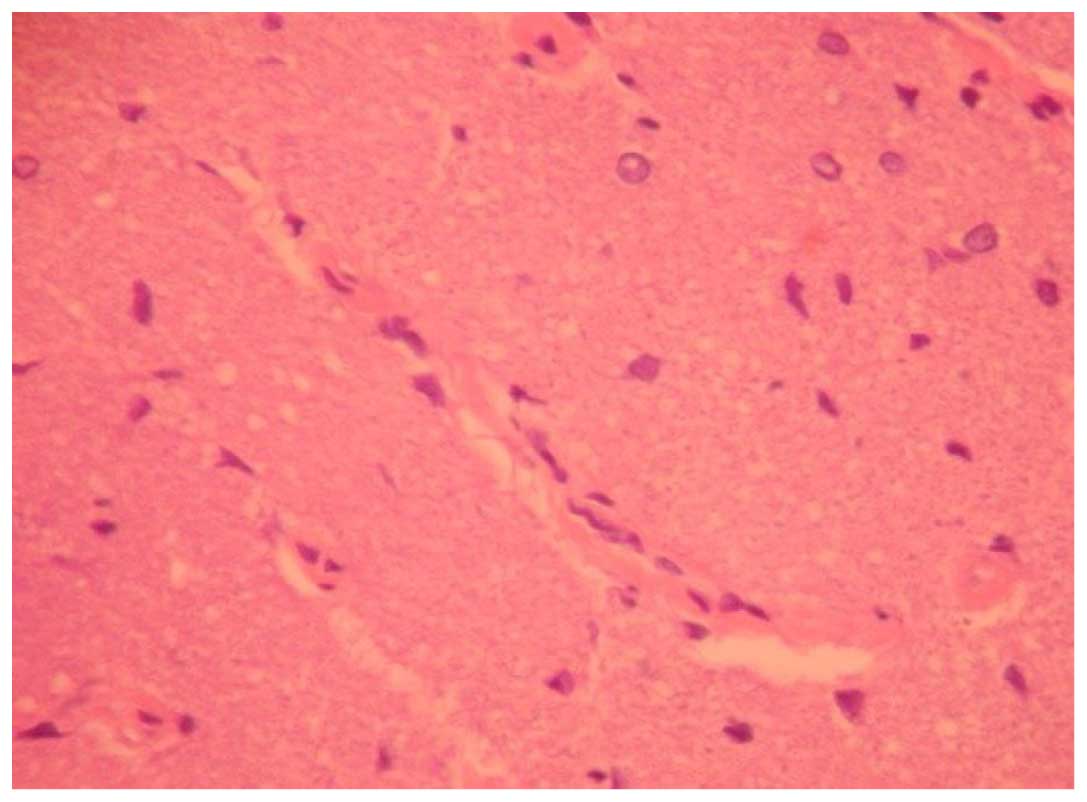
For the 48-h decompression group, the arrangement of
the astrocytes was even on the vertical section, with vacuoles,
slight swelling of the nerve, exudation around the blood vessel and
a small amount of astrocytes hyperplasia observed in the damaged
area (Fig. 2).
In the 1-week group, the arrangement of the
astrocytes was inordinate on the vertical section. Numerous
vacuoles of different sizes, demyelination of the nerve, uncovered
axons, clear exudation around the blood vessel and evident
astrocytes hyperplasia were observed in the majority of areas.
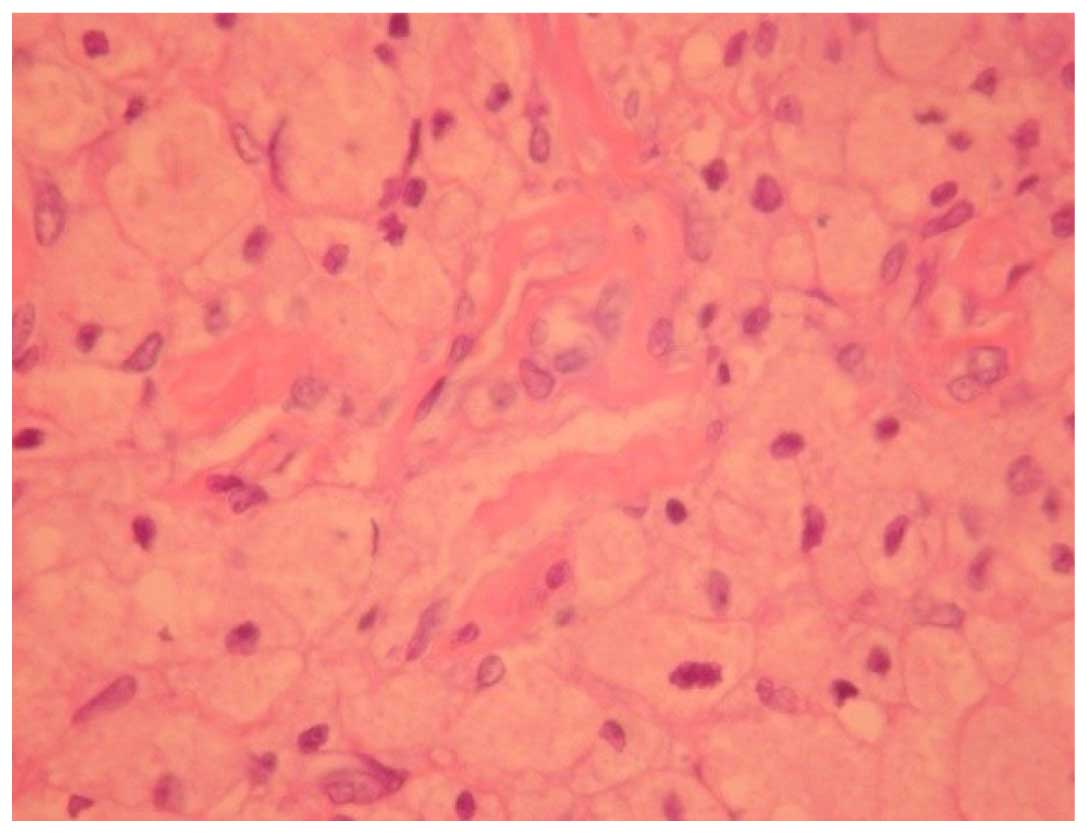
In the 2-week group, the cylindrical arrangement of
the astrocytes disappeared completely on the vertical section.
Evident astrocytic hyperplasia filled the field of view, and clear
nerve demyelination and local necrosis was observed, while
exudation around the blood vessel was reduced, with inflammatory
infiltration in some of the blood vessels (Fig. 3).
In the non-decompression group, large areas of
necrosis, evident nerve demyelination, serious exudation around the
blood vessel and astrocytic hyperplasia were observed.
Discussion
Optic nerve injury is a type of severe head trauma
combined injury, and numerous studies have established a series of
optic nerve injury animal models, such as optic nerve transverse,
tractive and crush injury (3–5); each has its advantages and disadvantages.
Optic nerve transection injury is established by cutting off the
optic nerve at any position prior to the optic chiasma, leading to
all retinal optic ganglion cell axons to rupture completely, which
can guarantee all experimental animals at the same degree of
wounding and is convenient for a control study. The common
characteristics of the optic nerve crush animal model are to crush
the optic nerve at any position prior to optic chiasma with
different instruments, to maintain the integrity of the epineurium.
This model is simple, easy to operate, and can exactly establish
the optic nerve injury with a high injury rate, as exposure of the
optic nerve from the conjunctival approach results in lighter
trauma to the experimental animals, with a low animal mortality,
improved repeatability, and is similar to clinical practice,
therefore, it is widely used. However, the injury degree is
operator dependent, as the optic nerve damage is variable under
different operators, and the damage degree is difficult to
quantitate precisely. The optic nerve tractive injury animal model
includes parallel and vertical optic canal axial traction. The
former can exactly lead to diffuse axonal injury, the latter is
similar to optic canal fracture caused by a cutting injury. The
optic nerve crush injury animal model can imitate the optic nerve
damage that extrudes the surrounding tissue of the orbital neoplasm
and clinical indirect optic nerve injury. This simple surgery is
easy to operate, can cause exact optic nerve damage in experimental
animals with less trauma, and postoperative nursing and further
observation is simple. All the aforementioned models have
limitations on quantifying the injuries. In the present study, a
circular cone soft silicone was adopted to block the optic nerve to
establish an optic nerve crush injury, and the size of the smaller
end and the depth of extrusion were controlled. The P-VEP and
pathological results showed that the optic nerve crush injury was
incomplete, and the injury degree was consistent.
Clinically, indirect optic nerve injury caused by
head trauma is difficult to examine and evaluate. Visual
electrophysiological examination is an objective visual function
assessment method, and can clinically improve early diagnosis
ability of indirect optic nerve injury (6,7). VEP can
develop from visual information through the visual nerve system. At
the later stages of visual formation are the mass responses to
visual stimuli that are recorded from electrodes placed on the
cerebral cortex, which can reflect the total functional status from
the retinal ganglion cells to the visual cortex. VEP, mainly on
behalf of the electrical activity complicated within the process of
visual production in the 10–20° of the central vision, from the
synapse, axon, optic nerve to the occipital visual cortex, can
sensitively reflect the integrity and function of the neuron axon
and myelin sheath in each optic nerve district. It has been shown
that following all wild stimulation in one eye of the rabbit, the
occurrence rate of the P-wave in the NPN contours recorded from the
cortex was 100%, and the latent period was the most stable, with a
higher amplitude and relatively variable N-wave (8). Therefore, in the present experiment, only
the wave latency and amplitude of the P-wave were used for
analysis. There are numerous types of visual evoked cortical
potential. As a stable waveform with small individual variation,
P-VEP is widely used to evaluate the optic nerve diseases. In optic
nerve tractional injury, Bain et al (9) reported that the electrophysiological
changes began to appear when the optic nerve stretch to 5.5 mm,
while the morphology change appeared following a stretch to 6.8 mm,
suggesting that electrophysiological changes are more sensitive
compared to morphology. Therefore, P-VEP was used as the main
observation index in the present study.
Compared with the normal control group, the
incubation period of the P-wave was evidently prolonged in the
early stage of trauma, with a significantly lower amplitude,
suggesting that visual function is not completely lost in the
direct mechanical effect caused partly by axon injury.
Subsequently, the incubation period continued to prolong, and the
amplitude continued to reduce. Following direct injury, the blood
circulation obstacle and tissue edema of the surrounding
environment caused secondary axonal injury leading to the further
decline in visual function. The 2-week post-decompression P-VEP
results showed that when decompression was implemented 48-h after
injury, the incubation period will be significantly shorter
compared to pre-decompression, and can return to normal levels,
with no evident difference to group A. When decompression occurs 1
or 2 weeks after injury, there were no evident improvements on the
incubation period, and the incubation period of the 2-week
post-decompression group was markedly prolonged compared to the
2-week pre-decompression. These results indicate that following
optic nerve injury, early decompression can protect the visual
function better than later decompression, and to a certain extent
it can reverse the damage on visual function, as early
decompression (1 week) can prevent axon secondary injury, avoiding
further visual function decline, whereas decompression 2 weeks
after is not ineffective. From 108 cases of clinical superciliary
arch fractures, Matteini et al (1) concluded that the best surgical
intervention must be carried out within 7 days, and <12 days,
which is consistent with the present experimental results.
Swelling, myelinoclasis and axon changes were the
main observations following indirect optic nerve injury. In the
present experiment, due to the difference of decompression time
after injury, the optic nerve morphology change was different at 2
weeks after decompression. In the 48-h decompression group,
vacuoles, slight swelling of the nerve, exudation around the blood
vessel and a small amount of astrocytes hyperplasia were observed
in the damaged area, whereas the arrangement of astrocytes was even
on the vertical section. As the decompression time prolonged,
myelinoclasis aggravated gradually, accompanied by necrosis of the
damaged area, and inordinate arrangement and evident hyperplasia of
the astrocytes. The present results showed that as the
decompression time prolonged, the optic nerve crush injury appeared
to exhibit mild to severe local pathological changes, and evident
myelinoclasis changes and astrocytes proliferation appeared in the
non-decompression trauma group 4 weeks after injury. Certain
studies (10,11) quantitatively analyzed retinal optic
ganglion cells (RGCs) following optic nerve crush injury and
reported that RGCs reduced rapidly in the first 2 weeks, reduce to
~50% at the end of 2 weeks, and decreased slowly at the latter 2
weeks. Thus, direct optic nerve damage can lead to part of the axon
injury and induce a series of changes, such as RGC acute loss,
impaired RGC release cytotoxic compounds, causing intracellular
calcium overload, free radicals and glutamic acid increase,
followed by an intact axon or the original RGCs on the damage edge
in axons suffered from secondary degeneration (12). Therefore, positive and effective
measures following optic nerve injury can reduce and delay the
secondary injury of RGCs, otherwise, the RGCs will eventually
degenerate (13).
The pathogenesis of traumatic optic nerve injury
remains to be elucidated. Maxwell et al (14) proposed that due to space limitations of
the optic canal, progressive blood circulation disorder and
hematoma of the indirect optic nerve injury may turn into a vicious
cycle, which leads to further loss of the original recovery
potential of the injured optic fiber. The optic nerve secondary
damage theories are the theoretical basis of optic nerve
decompression. There are controversies on the treatment of
traumatic optic nerve injury, and the choice of clinical treatment
is largely based on personal experience (15). Goldberg and Steinsapir (16) proposed that optic nerve decompression
is beneficial to the recovery of indirect optic nerve injury
regardless of whether there are optic canal fractures. Cook et
al (17) retrospectively analyzed
the treatment results of 244 patients reported in 46 studies, and
identified that the treatment group recovered better than the no
treatment group. No significant difference was apparent between the
curative effect of surgical decompression, hormone therapy and
surgery plus hormone treatment, and visual recovery was associated
with the extent of the initial injury. Recovery of patients without
fractures was better than with fractures, and anterior ethmoid
fracture was better than posterior ethmoid fracture.
The present study established a rabbit optic nerve
crush injury animal model, and systematically observed visual
function changes following optic nerve injury in the different
periods after decompression. Neuron secondary injury is one of the
main reasons for the progressive decline of the optic nerve
function, and therefore, protecting neurons without primary damage
from secondary damage is an important aspect of optic nerve injury
research. Optic nerve decompression can reduce the indirect optic
nerve injury, and early decompression following optic nerve injury
can better protect the visual function compared to later
decompression. To a certain extent, this can avoid further decline
of visual function and reverse optic nerve function damage
(18).
Acknowledgements
The authors would like to thank Dr Y.L. Cao from the
Medical School of Nanjing University for providing the animal
model. The present study was supported by a grant (no. CNJ13C005)
from the Medical Science Foundation of Chinese People's Liberation
Army.
References
|
1
|
Matteini C, Renzi G, Becelli R, Belli E
and Iannetti G: Surgical timing in orbital fracture treatment:
Experience with 108 consecutive cases. J Craniofac Surg.
15:145–150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng Y, Luo L, Ma Z, Sun X and Hu Y: In
vivo detection of severity of optic nerve crush using
manganese-enhanced magnetic resonance imaging in rats. Chin Med J
(Engl). 127:522–527. 2014.PubMed/NCBI
|
|
3
|
Solomon AS, Lavie V, Hauben U, Monsonego
A, Yoles E and Schwartz M: Complete transection of rat optic nerve
while sparing the meninges and the vasculature: An experimental
model for optic nerve neuropathy and trauma. J Neurosci Methods.
70:21–25. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bien A, Seidenbecher CI, Böckers TM, Sabel
BA and Kreutz MR: Apoptotic versus necrotic characteristics of
retinal ganglion cell death after partial optic nerve injury. J
Neurotrauma. 16:153–163. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoles E, Wheeler LA and Schwartz M:
Alpha2-adrenoreceptor agonists are neuroprotective in a rat model
of optic nerve degeneration. Invest Ophthalmol Vis Sci. 40:65–73.
1999.PubMed/NCBI
|
|
6
|
Nakamura A, Akio T, Matsuda E and Wakami
Y: Pattern visual evoked potentials in malingering. J
Neuroophthalmol. 21:42–45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan H, Li F and Zhang L: A new and
reliable animal model for optic nerve injury. Curr Eye Res.
37:941–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polianskiĭ VB, Evtikhin DV and Sokolov EN:
The brightness components of the visual evoked potential to color
stimuli in the rabbit. Zh Vyssh Nerv Deiat Im I P Pavlova.
49:1046–1051. 1999.(In Russian). PubMed/NCBI
|
|
9
|
Bain AC, Raghupathi R and Meaney DF:
Dynamic stretch correlates to both morphological abnormalities and
electrophysiological impairment in a model of traumatic axonal
injury. J Neurotrauma. 18:499–511. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takano M: Axonal regeneration of retinal
ganglion cells. Nippon Ganka Gakkai Zasshi. 100:972–981. 1996.(In
Japanese). PubMed/NCBI
|
|
11
|
Lynch DR and Dawson TM: Secondary
mechanisms in neuronal trauma. Curr Opin Neurol. 7:510–516. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Povlishock JT and Christman CW: The
pathobiology of traumatically induced axonal injury in animals and
humans: A review of current thoughts. J Neurotrauma. 12:555–564.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoles E and Muller S: NMDA-receptor
antagonist protects neurons from secondary degeneration after
partial optic crush. J Neurotrauma. 16:153–163. 1995.
|
|
14
|
Maxwell WL, Povlishock JT and Graham DL: A
mechanistic analysis of nondisruptive axonal injury: A review. J
Neurotrauma. 14:419–440. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zimmerer R, Rana M, Schumann P and
Gellrich NC: Diagnosis and treatment of optic nerve trauma. Facial
Plast Surg. 30:518–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldberg RA and Steinsapir KD:
Extracranial optic canal decompression: Indications and technique.
Ophthal Plast Reconstr Surg. 12:163–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cook MW, Levin LA, Joseph MP and Pinczower
EF: Traumatic optic neuropathy. A meta-analysis. Arch Otolaryngol
Head Neck Surg. 122:389–392. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen F, Zuo K, Feng S, Guo J, Fan Y, Shi J
and Li H: A modified surgical procedure for endoscopic optic nerve
decompression for the treatment of traumatic optic neuropathy. N Am
J Med Sci. 6:270–273. 2014.PubMed/NCBI
|