Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of
the most common causes of chronic liver disease worldwide (1). NAFLD encompasses a wide spectrum of liver
diseases ranging from simple steatosis to non-alcoholic
steatohepatitis, which may progress to liver cirrhosis in ≤30%
patients, potentially leading to decompensated liver disease
(2). As liver fibrosis progresses over
a long period of time, therapies should be tolerable and safe over
decades, with good targeting to the liver and few adverse effects
on other organs.
However, no anti-fibrotic agents have yet been
approved for clinical practice (3).
Several studies have evaluated the efficacy of dipeptidyl peptidase
4 inhibitor (DPP4-I) administration in the treatment of NAFLD and
non-alcoholic steatohepatitis (NASH) patients with type 2 diabetes.
In NAFLD patients, DPP4-I administration has been shown to decrease
serum alanine aminotransferase (ALT) levels in a positive
correlation with haemoglobin A1c (HbA1c) levels (4), and ameliorate liver enzyme abnormalities
and hepatocyte ballooning in NASH patients (5). Although our previous study reported
DPP4-I as a potential new therapeutic agent against liver fibrosis
in an experimental model of liver fibrosis via suppression of
activated hepatic stellate cell (HSC) proliferation and collagen
synthesis (6), the efficacy of DPP4-I
for the prevention of NAFLD progression in clinical settings
remains to be elucidated.
The aim of the present study was to evaluate the
efficacy of DPP4-I in the prevention of NAFLD progression according
to decreases in NAFIC scores. We further hypothesize that, in a
similar manner to baseline HbA1c levels being a strong predictor of
HbA1c change, the efficacy of DPP4-I in preventing NAFLD
progression would be more significant in patients with higher HbA1c
levels compared to those with lower levels.
Materials and methods
Study design
The study was a single arm, multi-center,
non-randomized study of NAFLD patients with type 2 diabetes that
were recruited from 8 centers in Japan between June 2012 and
December 2013. All the patients received 25 mg/day of alogliptin
(Takeda, Osaka, Japan) for 12 months.
Patients
Patients enrolled were previously diagnosed with
type 2 diabetes according to the diagnostic criteria of the Japan
Diabetes Society (JDS) with ALT levels >30 IU/L, which is the
cut-off level used to screen for NAFLD (7,8). Patients
with a current daily alcohol intake of >20 g or with known liver
disease of other aetiology were excluded. Following confirmation of
eligibility, receipt of informed consent and screening procedures,
eligible patients were administered alogliptin and followed up over
a 1-year period. NAFLD was defined according to characteristic
ultrasonographic findings, such as increased hepatorenal contrast
or enhanced liver brightness (9). All
the patients provided written consent to participate in the study.
The protocols used were approved by the Ethics Committee of Nara
Medical University (Nara, Japan; UMIN000008068) and other
facilities.
Data collection
On the date of alogliptin treatment initiation,
patients underwent laboratory tests, routine medical history
inquiry and physical examinations, including age, gender and body
weight and medical history. Laboratory tests at baseline included
overnight fasting measurements of aspartate aminotransferase, ALT,
γ-glutamyltranspeptidase, total cholesterol, triglyceride,
high-density lipoprotein cholesterol, fasting plasma glucose,
HbA1c, immunoreactive insulin (IRI), ferritin, hyaluronic acid,
type IV collagen 7S and type III procollagen-N-peptide. These
parameters were measured by the standard techniques used in
clinical chemistry laboratories (SRL, Tokyo, Japan). One year after
registration, IRI, ferritin and type IV collagen 7S levels were
obtained for the estimation of NAFIC scores. HbA1c levels were
expressed in accordance with the National Glycohemoglobin
Standardization Program, as recommended by the JDS (10).
Study outcome
One year after registration, the NAFIC (NASH,
ferritin, insulin and type IV collagen 7S) score and each
individual variable was used to evaluate the efficacy of alogliptin
in the prevention of NAFLD progression in all the included
patients.
NAFIC score
The original NAFIC score is a simple clinical
scoring system allowing the differentiation of NASH from NAFLD
using a cut-off score of 2. The NAFIC score is a weighted sum of
three clinical variables: Serum ferritin ≥200 ng/ml (female) or
≥300 ng/ml (male), 1 point; serum fasting insulin ≥10 IU/ml, 1
point; and serum type IV collagen 7S ≥5.0 ng/ml, 2 points (11).
Statistical analyses
All the variables are expressed as medians and inter
quartile ranges. Differences between the groups were evaluated
using the unpaired Student's t-test for normally distributed
variables and the Mann-Whitney U test for variables with skewed
distributions. The trend test was used to evaluate differences
between category variables. Relative risk regression analyses were
performed to estimate relative risks [95% confidence interval (CI)]
of persistence of NAFIC scores of >2 points at the end of the
study (i.e. possible persistence of NASH) in comparison with the
lowest tertile of HbA1c levels as a reference group. All the
reported P-values were 2-sided and P<0.05 was considered to
indicate a statistically significant difference. All the analyses
were performed using Stata/MP version 13.0 (Stata Corporation,
College Station, TX, USA).
Results
Patients
A total of 59 patients were enrolled in the study
between June 2012 and December 2013. A total of 20 patients were
excluded due to drop out prior to completion (5 patients) and
absence of blood examination results (15 patients). The remaining
39 patients met the inclusion criteria and were included in the
analysis. Table I shows the patient
demographics and laboratory data according to the NAFIC scores. The
median patient age, HbA1c level and body mass index were 61 years,
6.8% (53.0 mmol/mol) and 28.6 kg/m2, respectively. In 16
patients (41.0%), the NAFIC score was >2 points, indicating the
presence of NASH in 41.0% of patients with type 2 diabetes with
ultrasonographic fatty liver and ALT levels of >30 U/l.
 | Table I.Baseline characteristics of the
participants stratified by the NAFIC score. |
Table I.
Baseline characteristics of the
participants stratified by the NAFIC score.
| Characteristics | Total (n=39) | NAFIC score 0 to 1
(n=23) | NAFIC score 2 to 4
(n=16) |
|---|
| Age (years) | 61 (52–66) | 60 (52–64) | 62 (49–66) |
| Body mass index,
kg/m2 | 28.6 (26.9–31.2) | 28.2 (21.9–31.2) | 30.1 (27.7–33.9) |
| Female, % | 51.3 | 52.2 | 50.0 |
| PLT, 104
µl | 19.5 (17.1–21.8) | 19.6 (17.4–25.0) | 18.7 (11.1–21.4) |
| AST, IU/l | 40 (29–53) | 31 (27–46) | 46 (37–61) |
| ALT, IU/l | 49 (38–66) | 47 (34–64) | 55 (42–68) |
| γGTP, IU/l | 55 (38–86) | 50 (32–77) | 72 (44–106) |
| HbA1c, % | 6.8 (6.4–7.9) | 6.8 (6.4–7.5) | 7.1 (6.3–8.0) |
| FPG, mg/dl | 130 (115–159) | 130 (112–154) | 138 (122–172) |
| Fasting IRI,
µU/ml | 12.6 (6.5–19.3) | 10.4 (5.8–16.5) | 17.3 (12.0–25.9) |
| HOMA-IR | 4.4 (2.5–7.7) | 2.8 (1.9–5.2) | 7.0 (4.2–9.9) |
| TG, mg/dl | 176 (123–227) | 183 (125–213) | 172 (114–256) |
| HDL-C, mg/dl | 46 (41–53) | 45 (41–53) | 51 (42–54) |
| TC, mg/dl | 186 (168–207) | 182 (167–202) | 193.5 (170–211) |
| Ferritin, ng/ml | 155.0
(47.0–342.0) | 99.3
(43.3–179.0) | 314.0
(52.1–380.0) |
| Hs-CRP | 1,010
(568–1,680) | 1,135
(471–2,110) | 938 (680–1,585) |
| Hyaluronic acid,
ng/ml | 57 (24–86) | 34 (24–81) | 75 (28–113) |
| P-3-P, U/ml | 0.53 (0.49–0.65) | 0.50 (0.43–0.59) | 0.63 (0.51–0.72) |
| Type 4 collagen 7S,
ng/ml | 3.7 (3.1–5.2) | 3.3 (2.6–3.7) | 5.4 (4.5–6.7) |
| OADs use, % |
|
|
|
|
Sulfonylurea | 5.1 | 0.0 | 12.5 |
|
Thiazolidinedione | 5.1 | 6.3 | 4.4 |
|
Metformin | 2.6 |
4.4 |
0.0 |
|
α-glucosidase inhibitor |
2.6 |
0.0 |
6.3 |
| Hypertension,
% | 18.0 | 21.7 | 12.5 |
| Dyslipidemia,
% | 18.0 | 13.0 | 25.0 |
| Smoker, % | 15.4 | 13.0 | 18.8 |
Changes in NADIC scores
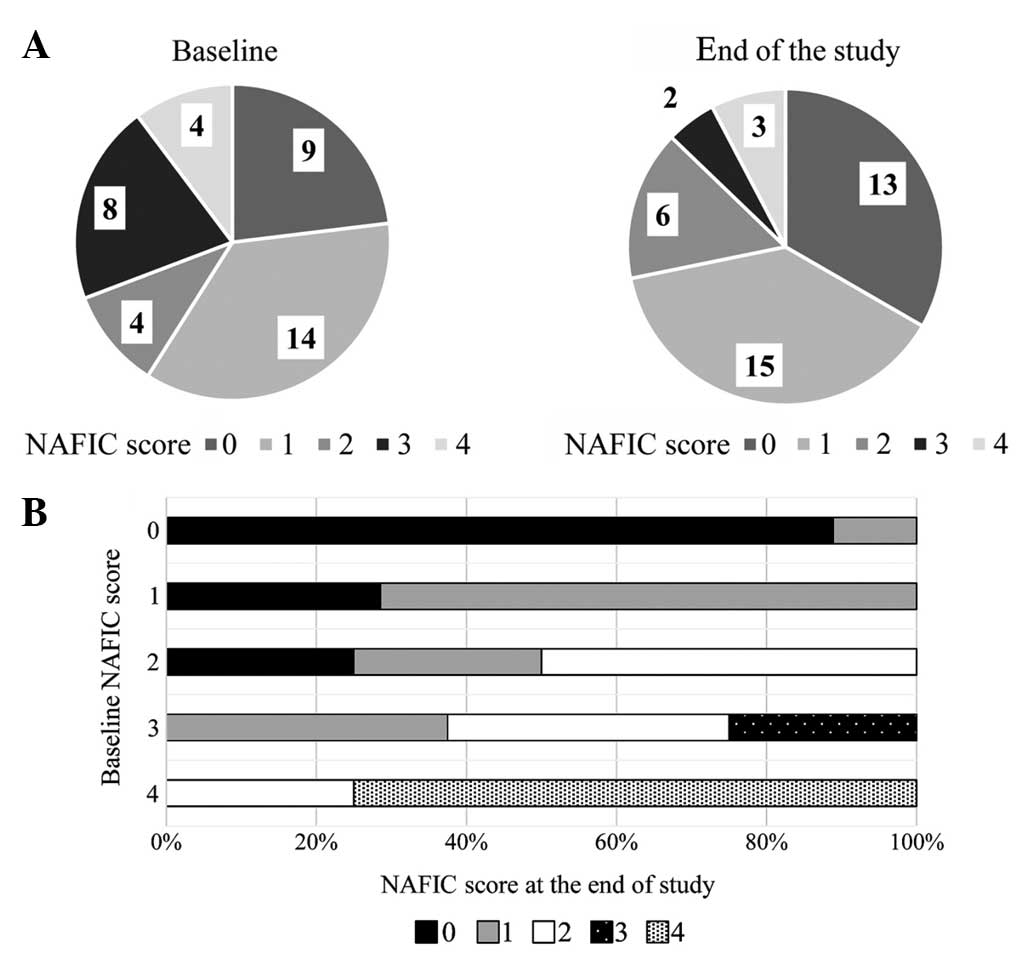
Fig. 1A shows the
changes in the NAFIC scores following treatment with alogliptin.
NAFIC scores were significantly decreased at 52 weeks after the
initiation of DPP4-I therapy. Fig. 1B
shows NAFIC score changes according to baseline NAFIC scores. NAFIC
scores decreased in 13 patients and remained >2 points in 10
patients, indicating NASH had possibly persisted in these patients.
NAFIC scores had increased in only 1 patient at the end of the
study compared to baseline scores.
Subsequently, the individual components of the NAFIC
score at the baseline and the end of the study were evaluated,
stratified according to the baseline HbA1c tertile (Table II). No significant changes in fasting
IRI or type 4 collagen 7S levels were observed; however, ferritin
levels were significantly decreased after 52 weeks administration
of DPP4-I. Stratified analysis according to the HbA1c tertile
demonstrated significant changes in the ferritin levels in the
lowest HbA1c category only.
 | Table II.Changes in fasting IRI, type 4
collagen 7S and ferritin stratified according to baseline HbA1c
levels. |
Table II.
Changes in fasting IRI, type 4
collagen 7S and ferritin stratified according to baseline HbA1c
levels.
|
| HbA1c range, % |
|---|
|
|
|
|---|
| Changes | 5.5–6.5 (n=14) | 6.6–7.2 (n=12) | 7.3–11.8
(n=13) | Total |
|---|
| Fasting IRI,
µU/ml |
|
|
|
|
| Month
0 | 12.7
(6.1–48.6) | 11.5
(6.5–17.2) | 13.6
(11.5–19.3) | 12.6
(6.5–19.3) |
| Month
12 | 9.6 (8.0–17.4) | 9.3 (6.2–23.1) | 12.1
(9.1–16.0) | 10.4
(8.0–17.7) |
|
P-value | 0.6378 | 0.4328 | 0.1330 | 0.5029 |
| Type 4 collagen 7S,
ng/ml |
|
|
|
|
| Month
0 | 3.7 (2.8–4.7) | 3.7 (2.6–4.8) | 4.8 (3.5–6.8) | 3.7 (3.1–5.2) |
| Month
12 | 3.4 (3.3–3.7) | 3.6 (3.1–4.0) | 3.7 (3.3–4.5) | 3.7 (3.3–4.5) |
|
P-value | 0.2547 | 0.5828 | 0.8887 | 0.6249 |
| Ferritin,
ng/ml |
|
|
|
|
| Month
0 | 124.7
(85.2–205.0) | 211.5
(53.0–380.0) | 155.0
(31.5–286.0) | 155.0
(47.0–342.0) |
| Month
12 | 100.7
(54.5–137.0) | 137.0
(40.3–251.5) | 102.0
(19.0–251.0) | 108.0
(36.0–191.0) |
|
P-value | 0.0035 | 0.0844 | 0.0869 | 0.0003 |
NASH and HbA1c tertiles
The association between possibly persistent NASH at
the end of the study and HbA1c tertiles are shown in Table III. The relative risks for possibly
persistent NASH was 4.92 (95% CI, 0.61–40.0) in the highest HbA1c
tertile group compared to the reference category of the lowest
HbA1c tertile. However, no statistically significant linear trend
was observed across all HbA1c categories (P=0.145).
 | Table III.Association between the possibly
persistent NASH at the end of the study and baseline glycemic
control. |
Table III.
Association between the possibly
persistent NASH at the end of the study and baseline glycemic
control.
|
| HbA1c tertiles |
|
|---|
|
|
|
|
|---|
| Possibly persistent
NASH | First (n=201) | Second (n=168) | Third (n=151) | P-value for
trend |
|---|
| RR for possibly
persistent NASH (crude) | Reference | 1.17
(0.28–4.83) | 1.79
(0.52–6.15) | 0.347 |
| RR for possibly
persistent NASH (model 1) | Reference | 1.29
(0.14–11.62) | 4.92
(0.61–40.0) | 0.145 |
Discussion
To the best of our knowledge, this is the first
clinical study to demonstrate the efficacy of administering DPP4-I
for 12 months in order to decrease serum ferritin levels. According
to the NAFIC scores, this would prevent the progression of NAFLD in
patients with type 2 diabetes. The effects were observed only among
patients in the lower HbA1c tertile at baseline, which was
different from the efficacy in order to use for lowering HbA1c
level. Therefore, we speculate that the reduction in oxidative
stress, resulting from a decrease in serum ferritin levels,
suppressed the extent of liver injury induced by inflammatory
cytokines in NAFLD patients with early type 2 diabetes. DPP4-I may
therefore be efficient in preventing the progression of NAFLD to
NASH in NAFLD patients with early type 2 diabetes.
A number of previous studies have reported the
effect of DPP4-I on liver dysfunction in NAFLD patients. Iwasaki
et al (12) first reported that
4 months of sitagliptin administration resulted in improved liver
enzyme abnormalities in NAFLD patients with type 2 diabetes. Yilmaz
et al (13) reported the effect
of DPP4-I in patients with biopsy-proven NASH with type 2 diabetes.
This study demonstrated that administration of sitagliptin for 12
months ameliorated liver enzyme abnormalities and hepatocyte
ballooning in patients whose body weight decreased during the study
period. Fukuhara et al (4)
reported that administration of sitagliptin for 12 months in
patients with biopsy-proven NAFLD with type 2 diabetes improved the
liver enzyme abnormalities in parallel with decreases in HbA1c
levels. However, the present study did not evaluate the association
between changes in body weight, HbA1c levels and NAFIC scores
during the study period. This study demonstrated that
administration of DPP4-I for 12 months significantly reduced NAFIC
scores in NAFLD patients with type 2 diabetes. Furthermore, in
patients in the highest HbA1c tertile, NAFIC scores were observed
that had remained >2 points during the study period, indicating
the persistence of NASH. Self-care activity in patients with lower
HbA1c levels was known to be higher compared to those with higher
HbA1c levels (14); therefore,
patients with lower HbA1c levels may have had ideal lifestyles and
body weights preventing worsening of HbA1c levels.
In the liver, DPP4 is expressed on the surface of
HSCs and may contribute to activated HSC-induced ECM accumulation
(15). Kaji et al (6) reported that DPP4-I inhibited liver
fibrosis and production of hepatic transforming growth factor-β1
(TGF-β1), along with attenuation of α-smooth muscle actin-positive
activated HSCs. These results indicate that the suppression of
activated HSC function may underlie the anti-fibrotic effect of
DPP4-I. As high glucose levels and high insulin levels stimulate
the proliferation of activated HSCs in a dose-dependent manner
(16), DPP4-I may be more effective
against NASH progression in comparatively low glucose conditions.
These experimental studies may explain the greater efficacy of
DPP4-I in the prevention of NASH progression in patients in the
lower HbA1c category in the present study.
Reductions in serum ferritin levels were observed
only among NAFIC score components. Experimental models have
demonstrated that iron increases hepatocyte apoptosis and
contributes to the development of fibrosis directly and indirectly
via induction of TGF-β1 production by hepatocytes and macrophages
(17). By contrast, iron depletion
inhibits the pancreatic TGF signal, thus inhibiting the
phosphorylation of Smad2 (18).
Kajikawa et al (19)
demonstrated that eicosapentaenoic acid reduces hepatic reactive
oxygen species levels and serum ferritin in the methionine- and
choline-deficient diet rat model in parallel with hepatic TGF-β1.
DPP4-I reduced serum ferritin levels, which may lead to reduced
levels of hepatic TGF-β1 and consequent inhibition of NASH
progression.
The present study had several limitations. Firstly,
NAFLD and NASH progression were evaluated using ultrasonography and
a non-invasive scoring system. The NAFIC score was established to
differentiate NASH from NAFL in a cross-sectional study. It is not
clear whether the NAFIC score can be used to evaluate longitudinal
outcome, which may have resulted in misclassification. Although
liver biopsy is the gold standard for the diagnosis of NAFLD and
assessment of disease progression, it is unrealistic to perform
liver biopsies in all NAFLD patients with type 2 diabetes (20). Secondly, as this was a single arm study
with a small number of patients and a short observation period for
this type of study, to assessing the effect of significant
potential confounding factors, such as calorie intake and exercise
status, could not be performed.
In conclusion, DPP4-I administration resulted in
decreased NAFIC scores, demonstrating the efficacy of DPP4-I
against NAFLD progression. These results indicate that DPP4-I may
represent a potential new therapeutic strategy for the prevention
of NAFLD progression in NAFLD patients with type 2 diabetes in the
future.
Acknowledgements
Takeda Pharmaceutical Company Limited (Osaka, Japan)
supported the present study. The authors would like to thank Mr.
Yoshie Nakai and Mr. Hisayo Iino (Third Department of Internal
Medicine, Nara Medical University) for their clerical support and
Enago (www.enago.jp) for the review of the
English language.
References
|
1
|
Angulo P: Nonalcoholic fatty liver
disease. N Engl J Med. 346:1221–1231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vernon G, Baranova A and Younossi ZM:
Systematic review: The epidemiology and natural history of
non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
in adults. Aliment Pharmacol Ther. 34:274–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedman SL: Liver fibrosis - from bench
to bedside. J Hepatol. 38(Suppl 1): S38–S53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukuhara T, Hyogo H, Ochi H, Fujino H, Kan
H, Naeshiro N, Honda Y, Miyaki D, Kawaoka T, Tsuge M, et al:
Efficacy and safety of sitagliptin for the treatment of
nonalcoholic fatty liver disease with type 2 diabetes mellitus.
Hepatogastroenterology. 61:323–328. 2014.PubMed/NCBI
|
|
5
|
Yilmaz Y, Yonal O, Deyneli O, Celikel CA,
Kalayci C and Duman DG: Effects of sitagliptin in diabetic patients
with nonalcoholic steatohepatitis. Acta Gastroenterol Belg.
75:240–244. 2012.PubMed/NCBI
|
|
6
|
Kaji K, Yoshiji H, Ikenaka Y, Noguchi R,
Aihara Y, Douhara A, Moriya K, Kawaratani H, Shirai Y, Yoshii J, et
al: Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis
via suppression of activated hepatic stellate cell in rats. J
Gastroenterol. 49:481–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fraser A, Longnecker MP and Lawlor DA:
Prevalence of elevated alanine aminotransferase among US
adolescents and associated factors: NHANES 1999–2004.
Gastroenterology. 133:1814–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strauss RS, Barlow SE and Dietz WH:
Prevalence of abnormal serum aminotransferase values in overweight
and obese adolescents. J Pediatr. 136:727–733. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamaguchi M, Kojima T, Itoh Y, Harano Y,
Fujii K, Nakajima T, Kato T, Takeda N, Okuda J, Ida K, et al: The
severity of ultrasonographic findings in nonalcoholic fatty liver
disease reflects the metabolic syndrome and visceral fat
accumulation. Am J Gastroenterol. 102:2708–2715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
The Committee of the Japan Diabetes
Society on the diagnostic criteria of diabetes mellitus. Report of
the Committee on the classification and diagnostic criteria of
diabetes mellitus. J Diabetes Investig. 1:212–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sumida Y, Yoneda M, Hyogo H, Yamaguchi K,
Ono M, Fujii H, Eguchi Y, Suzuki Y, Imai S, Kanemasa K, et al:
Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD):
A simple clinical scoring system using ferritin, fasting insulin,
and type IV collagen 7S for predicting steatohepatitis in
nonalcoholic fatty liver disease. J Gastroenterol. 46:257–268.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwasaki T, Yoneda M, Inamori M, Shirakawa
J, Higurashi T, Maeda S, Terauchi Y and Nakajima A: Sitagliptin as
a novel treatment agent for non-alcoholic Fatty liver disease
patients with type 2 diabetes mellitus. Hepatogastroenterology.
58:2103–2105. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yilmaz Y, Atug O, Yonal O, Duman D,
Ozdogan O, Imeryuz N and Kalayci C: Dipeptidyl peptidase IV
inhibitors: Therapeutic potential in nonalcoholic fatty liver
disease. Med Sci Monit. 15:HY1–HY5. 2009.PubMed/NCBI
|
|
14
|
Mashitani T, Hayashino Y, Okamura S,
Kitatani M, Furuya M, Matsunaga S, Kuwata H, Tsujii S and Ishii H:
Patient-reported adherence to insulin regimen is associated with
glycemic control among Japanese patients with type 2 diabetes:
Diabetes Distress and Care Registry at Tenri (DDCRT 3). Diabetes
Res Clin Pract. 100:189–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levy MT, McCaughan GW, Abbott CA, Park JE,
Cunningham AM, Müller E, Rettig WJ and Gorrell MD: Fibroblast
activation protein: A cell surface dipeptidyl peptidase and
gelatinase expressed by stellate cells at the tissue remodelling
interface in human cirrhosis. Hepatology. 29:1768–1778. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaji K, Yoshiji H, Kitade M, Ikenaka Y,
Noguchi R, Yoshii J, Yanase K, Namisaki T, Yamazaki M, Moriya K, et
al: Impact of insulin resistance on the progression of chronic
liver diseases. Int J Mol Med. 22:801–808. 2008.PubMed/NCBI
|
|
17
|
George J, Pera N, Phung N, Leclercq I, Yun
Hou J and Farrell G: Lipid peroxidation, stellate cell activation
and hepatic fibrogenesis in a rat model of chronic steatohepatitis.
J Hepatol. 39:756–764. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minamiyama Y, Takemura S, Kodai S,
Shinkawa H, Tsukioka T, Ichikawa H, Naito Y, Yoshikawa T and Okada
S: Iron restriction improves type 2 diabetes mellitus in Otsuka
Long-Evans Tokushima fatty rats. Am J Physiol Endocrinol Metab.
298:E1140–E1149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kajikawa S, Imada K, Takeuchi T, Shimizu
Y, Kawashima A, Harada T and Mizuguchi K: Eicosapentaenoic acid
attenuates progression of hepatic fibrosis with inhibition of
reactive oxygen species production in rats fed methionine- and
choline-deficient diet. Dig Dis Sci. 56:1065–1074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sumida Y, Nakajima A and Itoh Y:
Limitations of liver biopsy and non-invasive diagnostic tests for
the diagnosis of nonalcoholic fatty liver disease/nonalcoholic
steatohepatitis. World J Gastroenterol. 20:475–485. 2014.
View Article : Google Scholar : PubMed/NCBI
|