Introduction
Ciliary neurotrophic factor (CNTF), a survival
factor for chick ciliary neurons (1),
is reported to facilitate the survival of several types of neurons
such as sympathetic, sensory and motor neurons (2). In addition, it has important roles in
triggering neurite outgrowth, preventing neuronal degeneration and
attenuating motor deficits (3). Recent
evidence suggests that CNTF is a potential activator of astrocytes,
as it could induce astrocyte hypertrophy and glial fibrillary
acidic protein (GFAP) overexpression (4,5), which is
considered as a predominant feature for the activation of
astrocytes.
Astrocytes constitute the majority of the central
nervous system (CNS), particularly in the brain. Bidirectional
communication has been reported between astrocytes and neurons. On
this basis, it is reasonable to speculate that astrocytes may
respond to the neuronal activity and be involved in the regulation
of neuron activity. Our previous study showed that CNTF-treated
astrocyte-conditioned medium (CNTF-ACM) contributed to the
elevation of the calcium current and the expression of ion channels
in cortical neurons (6). Due to the
increase of intracellular free calcium concentration
([Ca2+]i) preferentially induced by calcium
influx mediated by calcium currents, we hypothesize that CNTF-ACM
may affect the [Ca2+]i in neurons.
The present study aimed to evaluate the effects of
CNTF-ACM on the [Ca2+]i in cortical neurons
in rats. Astrocytes can secrete several types of factors, such as
nerve growth factor (NGF) (7,8) and fibroblast growth factor-2 (FGF-2).
FGF-2 (9,10) and NGF (11,12)
contribute to the expression of functional calcium channels. In
addition, the concentrations of FGF-2 and NGF were determined in
CNTF-ACM, as well as its roles in the regulation of
[Ca2+]i levels in neurons.
Materials and methods
Astrocyte culture and collection of
ACM
Pregnant Sprague-Dawley rats were obtained from the
Experimental Animal Center of Zhejiang Province (Zhengjiang,
China). Sprague-Dawley rats (1–2-day-old, n=20) were used for the
preparation of cortical astrocytes according to a previous study
(13). In brief, following
dissociation, the cortical tissue was plated on 75-cm2
poly-d-lysine-coated culture flasks in Dulbecco's modified Eagle's
medium (DMEM)/F12 (1:1) supplemented with 100 µg/ml streptomycin,
100 U/ml penicillin and 10% fetal bovine serum (Gibco, Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The cultures were
maintained in a humid incubator at 37°C in 5% CO2. After
confluence at 7–10 days in vitro (DIV), the cells were
agitated at 37°C at 250 rpm for 15–18 h for subculture. The yield
of astrocytes was >95%, as revealed by immunostaining glial
fibrillary acidic protein (GFAP; Santa Cruz Biotechology, Inc.,
Santa Cruz, CA, USA). Subsequently, the astrocytes were treated
with 50 ng/ml CNTF (PeproTechec, London, UK) for 48 h. Subsequent
to rinsing with phosphate-buffered saline (PBS) 3 times, the cells
were incubated in fresh serum-free DMEM medium (9 ml/flask) for 48
h. Following this, the CNTF-ACM was collected, centrifuged at 1,200
× g for 10 min, and filtered through a 0.2–µm filter. The untreated
ACM (UT-ACM) served as the control. Finally, the samples were
stored at −70°C until required for further analysis.
Immunoassay
Levels of FGF-2 and NGF in the CM were determined
using commercial enzyme-linked immunosorbent assay (ELISA) kits
(R&D Systems Europe, Abingdon, UK) according to the
manufacturer's protocol. The tests were performed at least in
triplicate.
Cortical neuron culture and its
treatment
For the cell culture, the mixed cells were cultured
using the same procedures for astrocytes for 24 h. Following this,
the medium was replaced with neurobasal medium supplemented with 2%
B27 supplement, 0.5 mM L-glutamine 1% antibiotic-antifungal mixture
(all from Invitrogen, Carlsbad, CA, USA). At 3 DIV, 5 µM
arabinosylcytosine C (Invitrogen) was used to inhibit the growth of
glia, followed by culturing at 37°C for 24 h to maximize the
percentage of neurons. Subsequently, the medium was replaced by
fresh medium to terminate the action of arabinosylcytosine C. At
7–10 DIV, a purity of 95% was observed by immunostaining
neuron-specific enolase (NSE; Santa Cruz Biotechnology, Inc.).
Finally, the [Ca2+]i in the neurons was
determined following treatment with UT-ACM, CNTF-ACM or CNTF-ACM
containing 20 ng/ml of anti-rat FGF-2 monoclonal neutralizing
antibody (αFGF-2; cat. no. MAB233; R&D Systems Europe) for 48
h.
Measurement of
[Ca2+]i
To measure the level of
[Ca2+]i, cortical neurons were loaded for 45
min at 37°C in 10 µM fura-2/acetoxymethyl ester in
N-(2-hydroxyethyl) piperazine-N'-2-ethanesulfonic acid
(HEPES)-buffered Hank's balanced salt solution (20 mM HEPES, 137 mM
NaCl, 1.3 mM CaCl2, 0.4 mM MgSO4, 0.5 mM
MgCl2, 0.4 mM KH2PO4, 0.6 mM
Na2H2PO4, 3.0 mM NaHCO3
and 5.6 mM glucose) containing 0.5% bovine serum albumin. LAMBDA
DG-4 (Sutter Company, Novato, CA USA) was used to measure the
cytosolic calcium in UT-ACM- and CNTF-ACM-treated cortical neurons.
Fluorescence intensities were recorded at excitation wavelengths of
340 and 380 nm, and an emission wavelength of 510 nm. Fluorescent
signals at excitation wavelengths of 340 and 380 nm were analyzed.
The fluorescence intensity ratio (R) was calculated by the formula
of F340/F380. The level of cytosolic calcium
was measured with the formula of [Ca2+]i = b
× Kd × (R - Rmin)/(Rmax - R)
according to a previous study (14),
where Kd is the dissociation constant for fura-2 (224 nM) and b is
the ratio of the fluorescence of fura-2 at 380 nm excitation in the
presence of minimum calcium and saturating calcium.
Statistical analysis
SPSS 11.5 (SPSS, Inc., Chicago, IL, USA) software
was used for the data analysis. Two-way analysis of variance and
two-tailed Student's t-test were used for the inter-group
comparison. P<0.05 was considered to indicate a statistically
significant difference. All the data are expressed as mean ±
standard error of mean.
Results
Analysis of the levels of calcium in
the different treatment medium
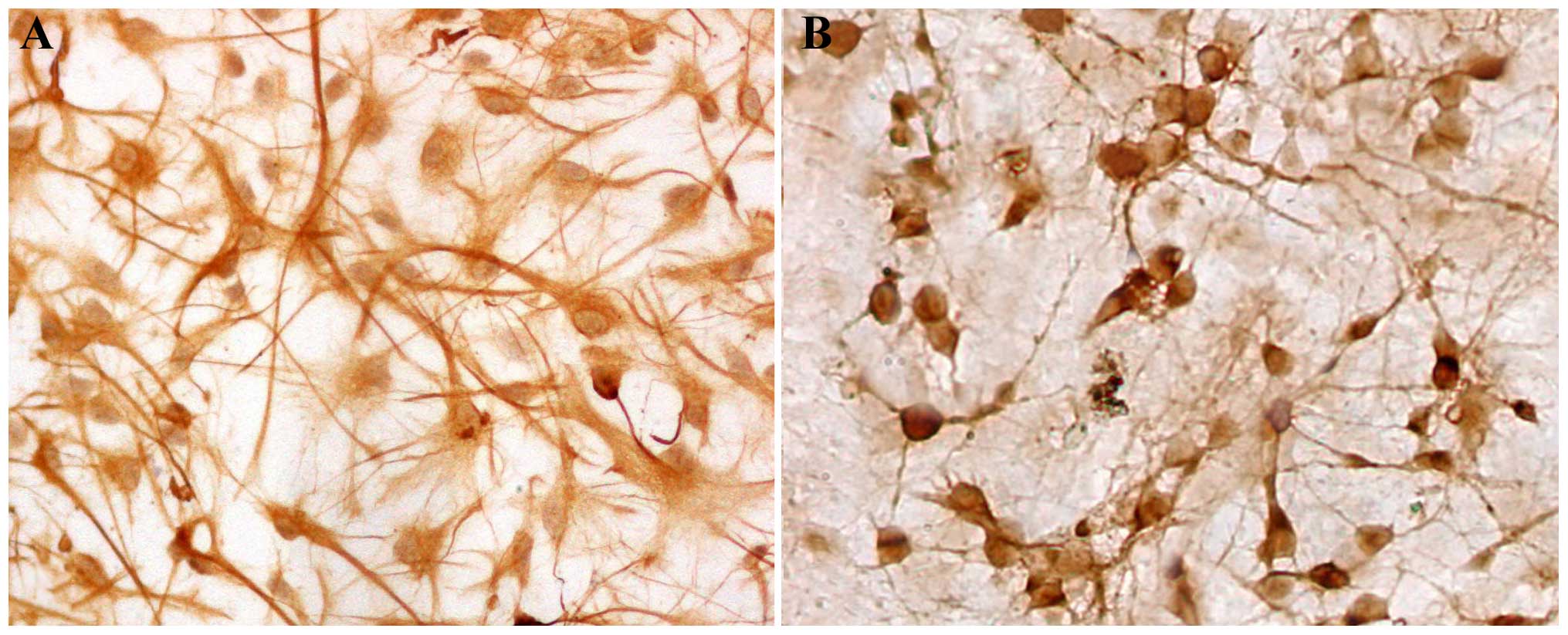
The mixed glial cultured from cerebral cortices of
neonatal rat were purified to >95% astrocytes (Fig. 1A) or neurons (Fig. 1B), as determined by immunostaining with
an antibody against GFAP or NSE, respectively. The neurons were
incubated with UT-ACM or CNTF-ACM for 48 h, and the cytosolic
digital calcium imaging was performed to test the influence of
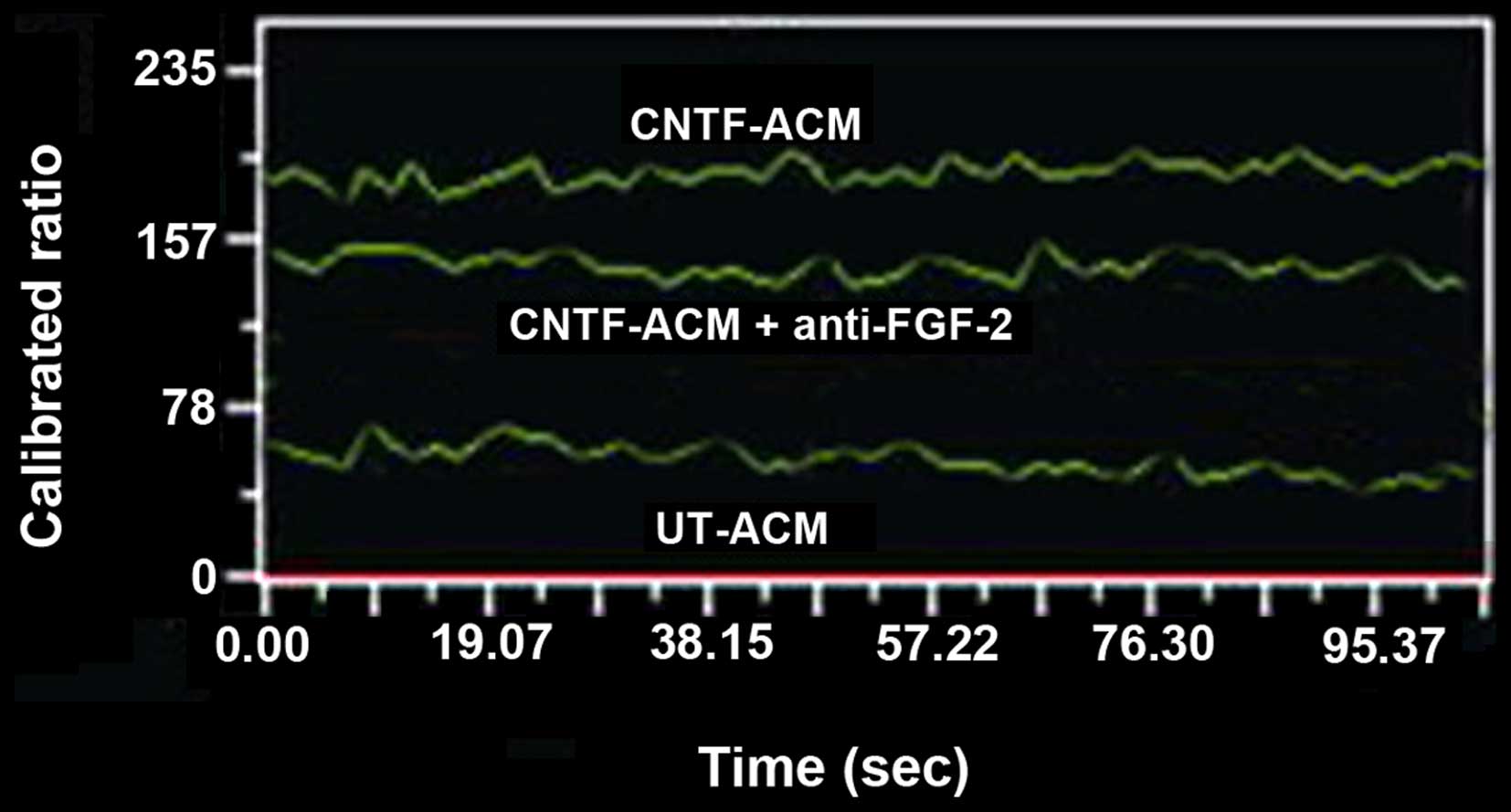
CNTF-ACM on [Ca2+]i in neurons. CNTF-ACM
resulted in significant induction of fluorescence intensity in
neurons compared to that of UT-ACM. The cytosolic free
Ca2+ concentrations were 67±7 nmol/l in neurons treated
with UT-ACM and 213±17 nmol/l in neurons treated with CNTF-ACM,
respectively (Fig. 2). Compared with
the control group, the level of cytosolic free Ca2+ was
clearly elevated in the CNTF-ACM (P<0.01).
Concentrations of FGF-2 and NGF in the
different treatment medium
In addition, these factors could augment the
amplitude of calcium currents in neurons. To investigate which
component in CNTF-ACM is involved in the upregulation of
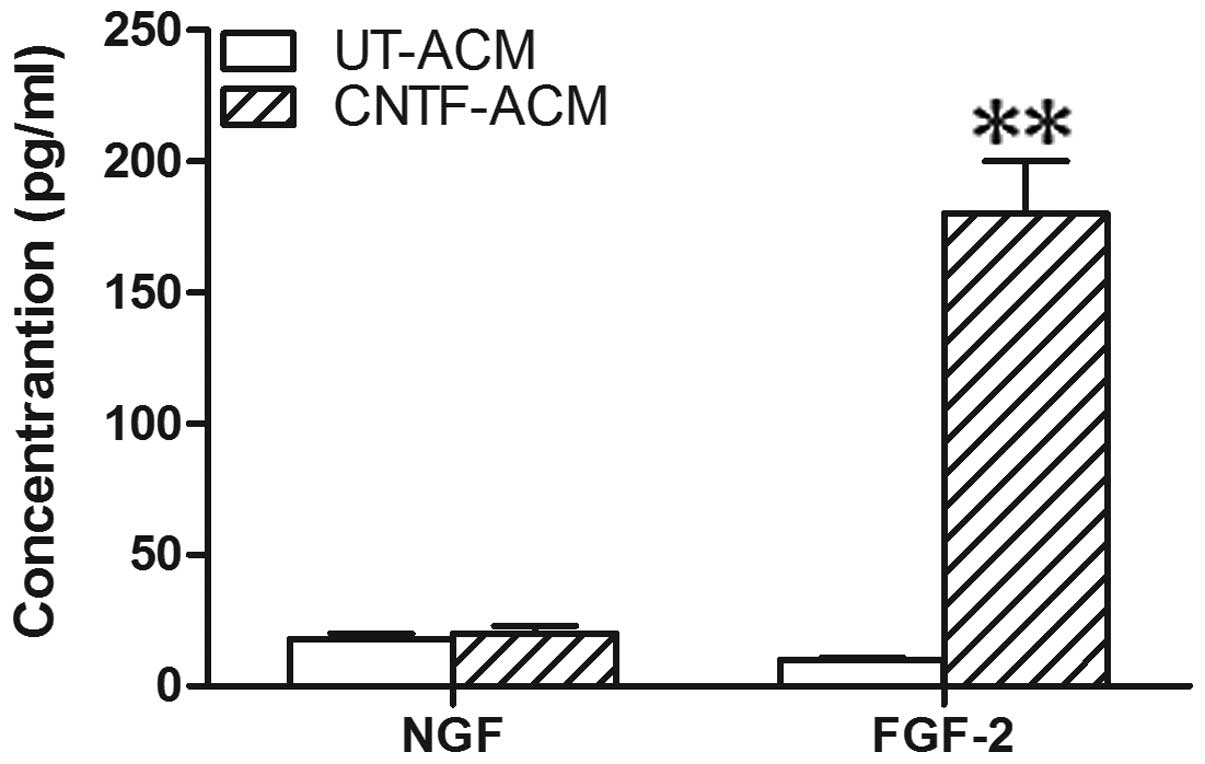
[Ca2+]i in neurons, the concentrations of
FGF-2 and NGF were determined in the CM using the ELISA assay. The
concentration of FGF-2 showed clear elevation in the CNTF-ACM
compared with the UT-ACM (Fig. 3).
However, no statistical changes were observed in the level of NGF
in the CNTF-ACM compared with the UT-ACM. Based on these findings,
FGF-2 may be involved in the upregulation of
[Ca2+]i, as mediated by CNTF-ACM in
neurons.
Assessing the effects of FGF-2 on
neurons in CNTF-AMC
Following this, 20 ng/ml αFGF-2 was used to
attenuate the potential effects of FGF-2 on neurons in CNTF-AMC.
Subsequent to treatment with CNTF-ACM (containing αFGF-2) for 48 h,
[Ca2+]i in the cortical neurons was measured.
The level of [Ca2+]i in neurons was lower
than that treated with CNTF-ACM (152±11 vs. 213±17 nmol/l;
P<0.05). However, the level was higher than that in the neurons
treated with UT-ACM (152±11 vs. 67±7 nmol/l; P<0.05) (Fig. 2).
Discussion
Astrocytes are capable of secreting abundant
neuroactive substances, such as neurotransmitters, cytokines and
metabolites, which regulate the neuronal activity by direct binding
to the receptors and ion channels in neurons (15). ACM, a liquid medium with soluble
substances released from astrocytes, constructs a microenvironment
that supports certain astrocyte-neuron interaction. Notably, the
effects of astrocytes on neurons may be mimicked partially by
exposing neurons to ACM in vitro. For example, ACM is
involved in the protection of neurons against damage and
contributes to the survival of neurons (16,17). Thus,
ACM is an efficacious medium to study the biological roles of
astrocytes in modulating the activity of neurons.
CNTF is a member of the interleukin-6 (IL-6) family,
which is mainly synthesized by astrocytes in the CNS. In
physiological status terms, the levels of CNTF and its receptor are
extremely low in brain parenchyma. However, significant elevation
is noticeable in their expression in the presence of brain injury
(18,19). Therefore, CNTF is considered to have
important roles in coordinating the cellular response to insult in
the CNS. As is well-known, the biological functions of CNTF are
mediated by a tripartite receptor complex constituted by a
nonsignaling subunit (CNTFRα) and two signaling subunits [i.e.
gp130 and leukemia inhibitory factor receptor β (LIFRβ)]. The
CNTFRα is exclusively expressed in neurons (20), while the CNTF functional receptors
(gp130 and LIFRβ) are mainly detected in astrocytes. This suggests
that these factors may serve as an action target of CNTF (21). According to the dose-response and
time-course experiments, the maximal stimulation of CNTF on
astrocytes was observed at 50 ng/ml and the 48-h time-point
(22). On this basis, the same
concentration and time course were adopted in the present study to
determine the activation of astrocytes induced by CNTF. To rule out
the possible roles of exogenous CNTF in CNTF-ACM, astrocytes were
rinsed with PBS 3 times following CNTF treatment. Subsequently, the
cells were cultured with fresh serum-free DMEM medium for 48 h
before ACM collection.
Compared to the level of
[Ca2+]i in the UT-ACM group, a stable
increase was noticed in [Ca2+]i in cultured
neurons from rat cortex treated by CNTF-ACM. This is consistent
with our previous study, indicating that CNTF can upregulate the
activity of L-type calcium channel in neurons (6). Additionally, the ELISA assay revealed
that the concentration of FGF-2 in CNTF-ACM was increased markedly
compared with that in UT-ACM, which were in accordance with
previous studies (23,24). Previously, CNTF contributed to the
elevation of NGF in astrocytes (23);
however, in the present study, no notable changes were observed in
NGF content between CNTF-ACM and UT-ACM.
In the present study, the effects of αFGF-2, a FGF-2
monoclonal neutralizing antibody, was also investigated on the
level of [Ca2+]i in neurons. The results
revealed that αFGF-2 could attenuate the increase of
[Ca2+]i in neurons, which demonstrated that
FGF-2 is responsible for the induction of
[Ca2+]i by CNTF-ACM in neurons. Notably,
αFGF-2 failed to block the effects of CNTF-ACM on
[Ca2+]i completely, which may be associated
with the presence of other active substances contained in CNTF-ACM.
To date, several cytokines have been identified from
cytokine-activated astrocytes, such as IL-1β, glia-derived
neurotrophic factor, neurotrophin-3, brain-derived neurotrophic
factor, transforming growth factor-β, interferon-γ, as well as
tumor necrosis factor-α (8). These
cytokines may participate in the upregulation of
[Ca2+]i by CNTF-ACM in neurons.
In conclusion, the present results showed that
CNTF-ACM induced an increase of the [Ca2+]i
in rat cortical neurons, which was partially attributable to the
elevation of FGF-2 in CNTF-ACM. These findings provide a new
insight into the indirect regulatory effects of CNTF on neuronal
biological function through activating astrocytes.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81171117), the
Doctor Point Foundation of Ministry of Education of China (grant
no. 44) and the National Natural Science Foundation of Anhui
Province (grant no. 1506c085014).
References
|
1
|
Adler R, Landa KB, Manthorpe M and Varon
S: Cholinergic neuronotrophic factors: Intraocular distribution of
trophic activity for ciliary neurons. Science. 204:1434–1436. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Askvig JM and Watt JA: The MAPK and PI3K
pathways mediate CNTF-induced neuronal survival and process
outgrowth in hypothalamic organotypic cultures. J Cell Commun
Signal. 9:217–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chowdhury SR, Saleh A, Akude E, Smith DR,
Morrow D, Tessler L, Calcutt NA and Fernyhough P: Ciliary
neurotrophic factor reverses aberrant mitochondrial bioenergetics
through the JAK/STAT pathway in cultured sensory neurons derived
from streptozotocin-induced diabetic rodents. Cell Mol Neurobiol.
34:643–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vigneswara V, Akpan N, Berry M, Logan A,
Troy CM and Ahmed Z: Combined suppression of CASP2 and CASP6
protects retinal ganglion cells from apoptosis and promotes axon
regeneration through CNTF-mediated JAK/STAT signalling. Brain.
137:1656–1675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seidel JL, Faideau M, Aiba I, Pannasch U,
Escartin C, Rouach N, Bonvento G and Shuttleworth CW: Ciliary
neurotrophic factor (CNTF) activation of astrocytes decreases
spreading depolarization susceptibility and increases potassium
clearance. Glia. 63:91–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Zheng H, Liu C, Zhu C, Wang W and
Li Z: Ciliary neurotrophic factor-treated astrocyte conditioned
medium regulates the L-type calcium channel activity in rat
cortical neurons. Neurochem Res. 33:826–832. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kajitani N, Hisaoka-Nakashima K, Morioka
N, Okada-Tsuchioka M, Kaneko M, Kasai M, Shibasaki C, Nakata Y and
Takebayashi M: Antidepressant acts on astrocytes leading to an
increase in the expression of neurotrophic/growth factors:
Differential regulation of FGF-2 by noradrenaline. PLoS One.
7:e511972012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liberto CM, Albrecht PJ, Herx LM, Yong VW
and Levison SW: Pro-regenerative properties of cytokine-activated
astrocytes. J Neurochem. 89:1092–1100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katsuki H, Shitaka Y, Saito H and Matsuki
N: A potential role of Ras-mediated signal transduction for the
enhancement of depolarization-induced Ca2+ responses in
hippocampal neurons by basic fibroblast growth factor. Brain Res
Dev Brain Res. 111:169–176. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yagami T, Takase K, Yamamoto Y, Ueda K,
Takasu N, Okamura N, Sakaeda T and Fujimoto M: Fibroblast growth
factor 2 induces apoptosis in the early primary culture of rat
cortical neurons. Exp Cell Res. 316:2278–2290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hall KE, Sheng HC, Srinivasan S,
Spitsbergen JM, Tuttle JB, Steers WD and Wiley JW: Treatment of
aged rat sensory neurons in short-term, serum-free culture with
nerve growth factor reverses the effect of aging on neurite
outgrowth, calcium currents, and neuronal survival. Brain Res.
888:128–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vivas O, Kruse M and Hille B: Nerve growth
factor sensitizes adult sympathetic neurons to the proinflammatory
peptide bradykinin. J Neurosci. 34:11959–11971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCarthy KD and de Vellis J: Preparation
of separate astroglial and oligodendroglial cell cultures from rat
cerebral tissue. J Cell Biol. 85:890–902. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zsembery A, Boyce AT, Liang L,
Peti-Peterdi J, Bell PD and Schwiebert EM: Sustained calcium entry
through P2X nucleotide receptor channels in human airway epithelial
cells. J Biol Chem. 278:13398–13408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ojeda SR, Ma YJ, Lee BJ and Prevot V:
Glia-to-neuron signaling and the neuroendocrine control of female
puberty. Recent Prog Horm Res. 55:197–224. 2000.PubMed/NCBI
|
|
16
|
Lu X, Al-Aref R, Zhao D, Shen J, Yan Y and
Gao Y: Astrocyte-conditioned medium attenuates glutamate-induced
apoptotic cell death in primary cultured spinal cord neurons of
rats. Neurol Res. 37:803–808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taylor AR, Gifondorwa DJ, Newbern JM,
Robinson MB, Strupe JL, Prevette D, Oppenheim RW and Milligan CE:
Astrocyte and muscle-derived secreted factors differentially
regulate motoneuron survival. J Neurosci. 27:634–644. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang SS, Keasey MP, Arnold SA, Reid R,
Geralds J and Hagg T: Endogenous CNTF mediates stroke-induced adult
CNS neurogenesis in mice. Neurobiol Dis. 49:68–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watt JA, Bone S, Pressler M, Cranston HJ
and Paden CM: Ciliary neurotrophic factor is expressed in the
magnocellular neurosecretory system of the rat in vivo: Evidence
for injury- and activity-induced upregulation. Exp Neurol.
197:206–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ip NY, McClain J, Barrezueta NX, Aldrich
TH, Pan L, Li Y, Wiegand SJ, Friedman B, Davis S and Yancopoulos
GD: The alpha component of the CNTF receptor is required for
signaling and defines potential CNTF targets in the adult and
during development. Neuron. 10:89–102. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rudge JS, Li Y, Pasnikowski EM, Mattsson
K, Pan L, Yancopoulos GD, Wiegand SJ, Lindsay RM and Ip NY:
Neurotrophic factor receptors and their signal transduction
capabilities in rat astrocytes. Eur J Neurosci. 6:693–705. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levison SW, Hudgins SN and Crawford JL:
Ciliary neurotrophic factor stimulates nuclear hypertrophy and
increases the GFAP content of cultured astrocytes. Brain Res.
803:189–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Albrecht PJ, Dahl JP, Stoltzfus OK,
Levenson R and Levison SW: Ciliary neurotrophic factor activates
spinal cord astrocytes, stimulating their production and release of
fibroblast growth factor-2, to increase motor neuron survival. Exp
Neurol. 173:46–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Albrecht PJ, Murtie JC, Ness JK, Redwine
JM, Enterline JR, Armstrong RC and Levison SW: Astrocytes produce
CNTF during the remyelination phase of viral-induced spinal cord
demyelination to stimulate FGF-2 production. Neurobiol Dis.
13:89–101. 2003. View Article : Google Scholar : PubMed/NCBI
|