Introduction
Lung cancer is a highly invasive malignancy that has
a strong tendency to metastasize at early stages. Therefore, the
interaction of the lung cancer cells with the extracellular matrix
(ECM) in the way of cell adhesion, migration, proliferation and
metastasis has been observed. Laminin, one of the major structural
components of the basement membrane, is a strong promoter of cell
adhesion, migration, differentiation and proliferation by means of
integrins and other cell surface receptors (1–4). Increased
laminin expression was also detected in lung cancer cell lines with
increased lung cancer cell proliferation and metastatic potential
(1–4).
Although there are increasing data regarding the
role of laminin in various malignancies, only a few studies of
laminin expression in lung cancer have been reported (1–3). However,
the current available findings have been provided from preclinical
tissue or cell-based trials. As no clinical study has investigated
the laminin level in serum and/or plasma in lung cancer patients,
the significance of the serological level of laminin in this group
of patients remains to be elucidated.
Therefore, the soluble serum laminin levels in
patients with lung cancer, were evaluated, and their association
with prognosis, various known clinical variables and response to
chemotherapy were assessed, so as to elucidate whether this
biomarker may be useful in making the diagnosis and in the
assessment of the prognosis.
Materials and methods
Patients
A total number of 80 patients with histologically or
cytologically confirmed non-small cell lung cancer (NSCLC) and SCLC
treated and followed up in the Institute of Oncology (Istanbul
University, Istanbul, Turkey) were enrolled in the study. The
patients had bidimensionally measurable disease without a history
of chemo/radiotherapy in the last 6 months. The metastatic diseases
were staged with various imaging modalities such as computed
tomography, magnetic resonance imaging and positron emission
tomography/computed tomography scan. The pathological diagnosis of
lung cancer was established according to the revised World Health
Organization classification of lung tumors (5,6) and staged
relying on the revised tumor-node-metastasis staging for lung
cancer.
The clinical history, physical examination, series
of biochemistry tests and complete blood cell counts were used as
the pretreatment evaluation. Those with Eastern Cooperative
Oncology Group performance status ≤2 and appropriate blood
chemistry tests received a platinum-based chemotherapy with/without
radiotherapy depending on the stage of disease. The response to
chemotherapy was evaluated radiologically after 2–3 cycles of
chemotherapy according to revised Response Evaluation Criteria in
Solid Tumors criteria (7). The
non-responders to chemotherapy and patients with recurrent diseases
were treated with second-line chemotherapy provided when they had a
good performance status. Chemotherapy was discontinued when disease
progression or unacceptable toxicity occurred.
A total of 30 healthy age- and gender-matched
controls were included in the analysis. The study was approved by
the ethics committee of the Institute of Oncology. Written informed
consent was obtained from all the patients.
Measurement of serum laminin
levels
Serum samples were drawn from patients and healthy
controls by venipuncture and clotted at room temperature on first
admission prior to the treatment. The sera were collected following
centrifugation at room temperature for 10 min at 4,000 rpm and
frozen immediately at −20°C until analysis.
Serum laminin levels were measured by the
solid-phase sandwich enzyme-linked immunosorbent assay (ELISA)
method that used double-antibody sandwich ELISA. Serum samples and
standards were added to the wells that were pre-coated with human
laminin monoclonal antibody (cat. no. E0082h; Wuhan EIAab Science
Co., Ltd., Wuhan, China). Following incubation, laminin antibodies
labeled with biotin and combined with streptavidin-horseradish
peroxidase were added to form an immune complex and incubated for 1
h. Unbound material was washed away and subsequently the chromogen
solution was added for the conversion of the colorless solution to
a blue solution, the intensity of which was proportional to the
amount of laminin in the sample. The color changed to yellow as a
result of the acidic stop solution. The colored reaction product
was measured using an automated ELISA reader (ChroMate, 4300
Microplate Reader; Awareness Technology, Inc., Palm City, FL, USA).
The results are expressed as ng/ml.
Statistical analysis
Median values were used to classify the variables
and the Mann-Whitney U test was used to compare the clinical and
laboratory parameters. Survival was calculated from the first
admission date to the date of fatality from any cause or to the
last contact with the patient or any family member. Kaplan-Meier
test was used to estimate the survival and the differences in
survival were evaluated by the log-rank statistics. P≤0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was carried out using SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 80 consecutive patients with
pathologically confirmed diagnosis of lung cancer were enrolled in
the study. Baseline histopathological and demographic data of
patients are listed in Table I. The
median age of patients was 58.5 years (range, 36–80 years), and
males constituted the majority of the group (n=72, 90%). The
majority of the patients had NSCLC (n=68, 85%) and metastatic
disease (n=45, 56%).
 | Table I.Patient characteristics and disease
status. |
Table I.
Patient characteristics and disease
status.
| Variables | Total, n |
|---|
| No. of patients | 80 |
| Age of patients,
years |
|
| ≥60 | 37 |
|
<60 | 43 |
| Gender |
|
| Male | 72 |
|
Female | 8 |
| Histology |
|
|
NSCLC | 68 |
|
Adenocarcinoma | 33 |
|
Squamous cell | 27 |
|
Undifferentiated | 8 |
| SCLC | 12 |
| Stage |
|
| II | 4 |
| III | 30 |
| IV | 34 |
|
Limited | 1 |
|
Extended | 11 |
| Response to
chemotherapy |
|
| Yes | 41 |
| No | 30 |
Serum laminin
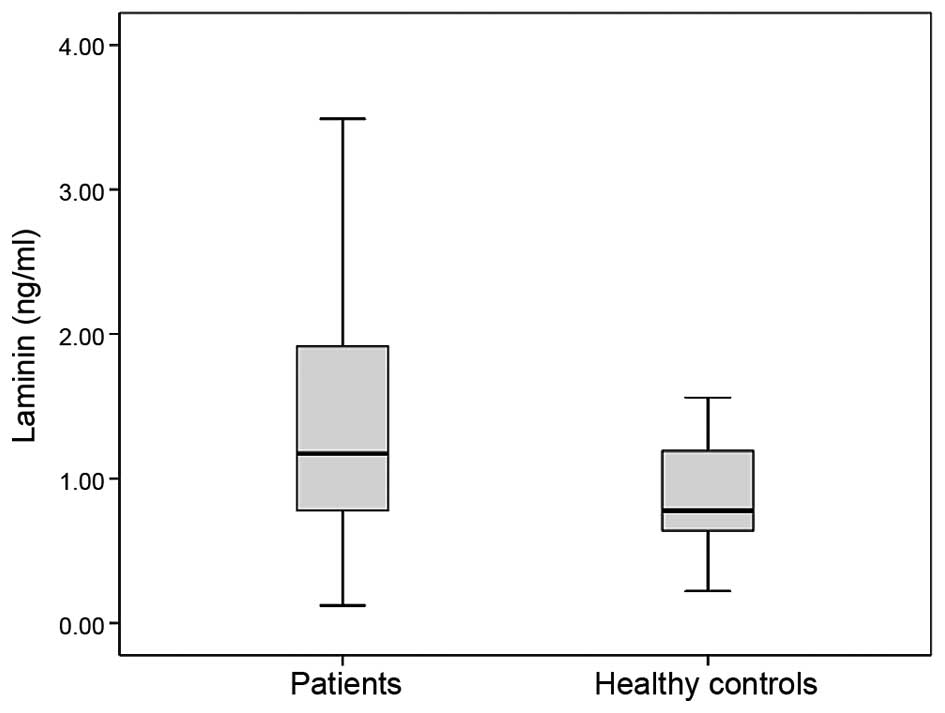
The levels of serum laminin in patients and healthy
controls are shown in Table II. The
baseline serum laminin concentrations of the lung cancer patients
were significantly higher than those in the control group (median
values 1.17 vs. 0.78 ng/ml, P=0.033) (Fig.
1).
 | Table II.Values of serum laminin levels in the
lung cancer patients and healthy controls. |
Table II.
Values of serum laminin levels in the
lung cancer patients and healthy controls.
|
| Median laminin
(range), ng/ml |
|
|---|
|
|
|
|
|---|
| Assay | Patients, n=80 | Controls, n=30 | P-value |
|---|
| Serum laminin
level | 1.17 (0.12–310) | 0.78 (0.22–1.56) | 0.033 |
Correlation between serum laminin
levels and the clinicopathological variables
Table III shows the
correlation between the serum laminin levels and
clinicopathological variables. Known clinical variables including
age of patient, gender, histology, stage of disease and response to
chemotherapy were not correlated with serum laminin concentrations
(P>0.05).
 | Table III.Distribution and survival comparisons
of the serum laminin levels on various clinical parameters in
patients with lung cancer. |
Table III.
Distribution and survival comparisons
of the serum laminin levels on various clinical parameters in
patients with lung cancer.
|
| Serum laminin level,
P-value |
|---|
| Parameters | Distribution | Survival |
|---|
| Age of patients,
years | 0.16 | 0.43 |
|
≥60/<60 |
|
|
| Gender | 0.11 | 0.75 |
|
Male/female |
|
|
| Histology | 0.83 |
0.004 |
|
NSCLC/SCLC |
|
|
| Histology in
NSCLC | 0.24 | 0.81 |
|
Adeno/epidermoid |
|
|
| Clinical stage | 0.24 |
0.005 |
|
Non-metastatic
(I–III)/metastatic (IV) |
|
|
| Response to
chemotherapy | 0.48 |
0.009 |
|
Yes/no |
|
|
| Serum laminin
level | – | 0.68 |
| Median,
< or ≥ |
|
|
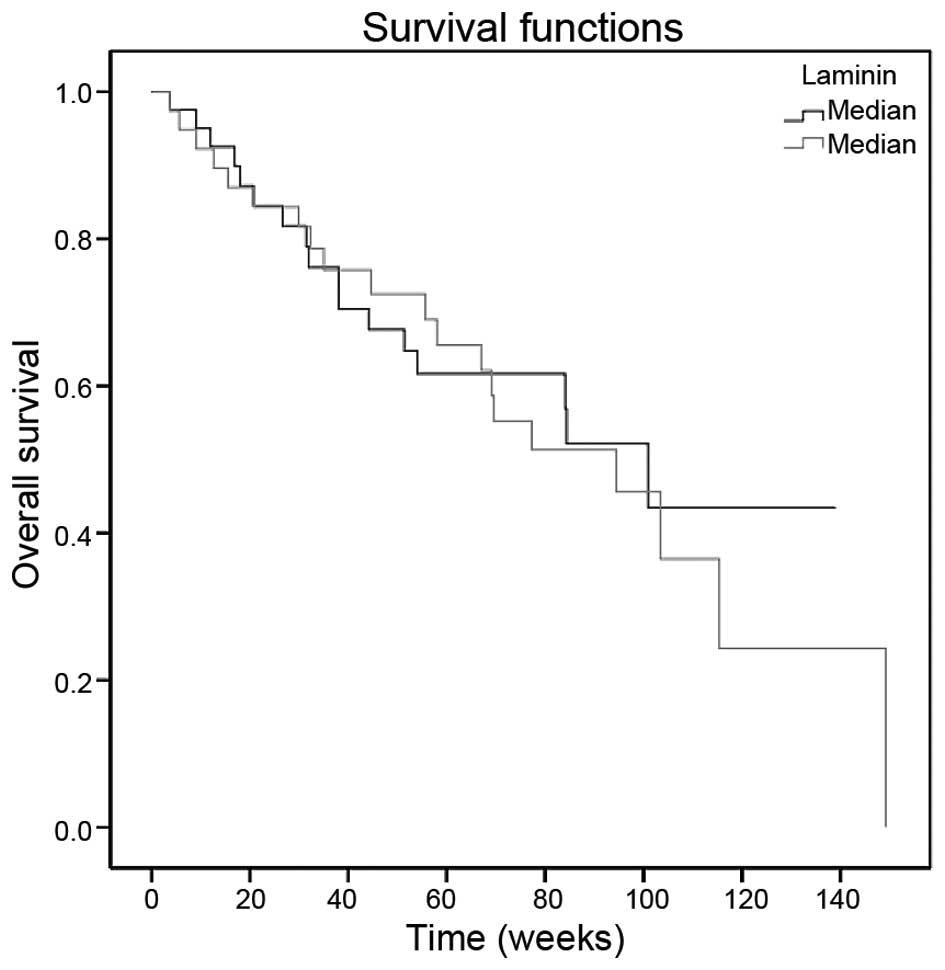
Survival
The median follow-up time was 58 weeks (range,
3.7–149.3 weeks). The median survival was 94.4 weeks (95%
confidence interval: 73.1–115.7). The 1- and 2-year overall
survival rates were 68.5 and 40.6%, respectively. Histology
(P=0.004), metastasis (P=0.005) and response to chemotherapy
(P=0.009) had prognostic factors on survival (Table III). However, the serum laminin level
was not associated with survival (P=0.68) (Table III, Fig.
2).
Discussion
There are limited studies on the role of laminin in
lung cancer that are limited to paraffin-embedded materials and
cell line trials; therefore, all the available data have been
provided from tissue cultures. However, the present study was
carried out in serum instead of tissue, which to the best of our
knowledge is a first.
Using monoclonal antibodies, laminin expression as a
basement membrane component and keratin intermediate filament
protein in normal human bronchial epithelium and 56 lung carcinomas
was assessed by Wetzels et al (1). In normal lung tissues, laminin was
located around the alveoli and beneath the epithelial basal cell
layer in bronchi and bronchioles. Laminin was stained in all the
lung cancer subtypes, such as squamous cell carcinoma,
adenocarcinoma, small cell lung carcinomas and carcinoids.
Xu et al (2)
studied the expression of ECM proteins in 57 formalin-fixed,
paraffin-embedded human NSCLC specimens and compared them with 23
normal lung tissues. The positive expression rate of laminin was
higher in NSCLC stroma compared to that in normal lung tissue, 54.4
and 26.1%, respectively (P<0.05). In 19.3% of the tumors,
laminin was stained intracellularly as well. The expression rates
of laminin in well- and moderately differentiated NSCLC were higher
compared to those in poorly differentiated cancers (P<0.05).
Additionally, laminin was more frequently observed in squamous cell
carcinoma compared to adenocarcinoma (P<0.05) and a higher
expression rate of laminin was associated with node-negative
disease (P<0.05). However, there was no correlation between the
expression of laminin and the stage of disease.
Szelachowska et al (3) analyzed the hypothesis that the level of
intracellular laminin was of prognostic importance in NSCLC by
designing an immunohistochemical (IHC) study. The increased level
of intracellular laminin and the presence of laminin in >50% of
cells caused a significantly reduced patient survival. Thus, the
increased laminin accumulation is associated with the progression
of the malignancy.
These conflicting results may be attributable to
several factors in that, for example, no consensus exists on which
tumors and methods should be used to test the expression of
laminin. Recently, IHC has been increasingly used as an adjunctive
method in diagnostic histopathology. However, there are limitations
with IHC, the most important of which are a lack of assay
standardization and variance in the interpretation of the IHC
staining. In a number of cases, the studies were performed on a
relatively small sample size, which may have been insufficient to
illustrate significant differences.
Proteases degrade laminin into several fragments.
Laminin P1 is one such fragments that is chemically a
pepsin-resistant soluble peptide (4).
A sensitive radioimmunoassay for the laminin P1 fragment showed
that laminin had cross-reactivity with its fragment P1. Nakano
et al (4) measured the serum
laminin P1 level in 43 lung cancer patients, in individuals with
benign lung disease and in normal subjects with a radioimmunoassay
that was sensitive for the laminin P1 fragment, and detected the
cross-reactivity between laminin and its fragment (4). The level of serum laminin P1 was elevated
in 58.9 and 11.5% of SCLC and NSCLC patients, respectively. The
values of laminin P1 in SCLC patients were significantly higher
than those in the NSCLC patients (P<0.01) and in the patients
with respiratory infection (P<0.01), and also those in healthy
individuals (P<0.01). Similarly, serum laminin P1 in SCLC was
correlated with the chemotherapeutic response. However, no
correlation was identified between the laminin P1 level and
clinical stage of SCLC.
A total of 80 patients with different histology and
stages of lung cancer were enrolled in the present study. The serum
laminin levels of the lung cancer patients were significantly
higher than those in the control group. However, clinical
variables, such as age, gender, site of lesion, histology, stage,
serum LDH levels and response to chemotherapy, were not correlated
with the serum laminin concentrations. Additionally, laminin did
not have a prognostic role in survival in lung cancer.
In conclusion, the present study concurs with the
previous studies that have investigated laminin in tissue, and
serum laminin levels may have a diagnostic role in lung cancer.
Additionally, laminin had no predictive and prognostic values.
Although the small sample size and the short follow-up time are
limitations and may have influenced the results, this study
contributes significant information in that it was carried out with
serum instead of tissue and it evaluated all stages of the disease.
Larger scale studies in larger patient populations are required to
determine the exact role of serum laminin in lung cancer
patients.
References
|
1
|
Wetzels RHW, Schaafsma HE, Leigh IM, Lane
EB, Troyanovsky SM, Wagenaar SSC, Vooijs GP and Ramaekers FCS:
Laminin and type VII collagen distribution in different types of
human lung carcinoma: Correlation with expression of keratins 14,
16, 17 and 18. Histopathology. 20:295–303. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Y, Zhao Y, Su B, Chen Y and Zhou C:
Expression of collagen IV, fibronectin, laminin in non-small cell
lung cancer and its correlation with chemosensitivities and
apoptosis. Chinese-German J Clin Oncol. 5:58–62. 2006. View Article : Google Scholar
|
|
3
|
Szelachowska Y, Jelen M and Kornafel J:
Prognostic significance of intracellular laminin and Her2/neu
overexpression in non-small cell lung cancer. Anticancer Res.
26:3871–3876. 2006.PubMed/NCBI
|
|
4
|
Nakano T, Iwahashi N, Maeda J, Hada T and
Higashino K: Serum laminin P1 in small cell lung cancer: a valuable
indicator of distant metastasis? Br J Cancer. 65:608–612. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brambilla E, Travis WD, Colby TV, Corrin B
and Shimosato Y: The new World Health Organization classification
of lung tumours. Eur Respir J. 18:1059–1068. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D and
Verweij J: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|