Introduction
Brain glioma is one of the most common central
nervous system diseases, which is invariably associated with a high
mortality (1). Gliomas are
characterized by an intense local invasiveness that limits the
usefulness of preferred surgical treatment (2,3). Therefore,
reducing the invasiveness of glioma will allow for a significant
treatment option for brain tumors.
Lan et al (4)
demonstrated that aspirin is a potent antitumor agent through the
inhibition of the β-catenin signaling pathway in glioma cells.
However, the mechanism of aspirin-induced glioma invasion decrease
remains to be elucidated.
Glioma, an aggressive form of adult brain tumor, is
difficult to treat due to its invasive nature. The current
treatment for glioma is resection of the tumor, followed by
chemotherapy and radiation therapy (5,6). Even with
such radical treatments, patients with glioma suffer from recurring
tumors that arise due to the invasive nature of glioma cells. In
addition to the histological changes, several molecular changes
occur in the process of glioma genesis (7–9). Previous
studies have shown a decrease the expression of the gap junction
protein connexin 43 (Cx43) in gliomas (10–13). Cx43 is
the major gap junction protein in astrocytes; gap junctions
directly link the cytoplasm of adjacent cells, thus establishing a
glial syncytium.
Prostaglandin (PG) has been shown to promote tumor
angiogenesis and induce cell proliferation, suggesting that glioma
invasion may be associated with PG. In addition, several
experimental and human tumors synthesize prostanoids (14–16), which
can be increasingly produced during tumor development. These
cyclooxygenase (COX) metabolites may influence the
physiopathological processes associated with tumor development. The
capacity of tumors to grow, disseminate and influence host
homeostasis has, in certain cases, been associated with the
production of elevated amounts of specific prostanoids.
Aspirin, a non-steroidal anti-inflammatory drug, is
used widely to relieve pain, fever and peripheral inflammation.
Low-dose aspirin (75–150 mg/day) is recommended for long-term
prophylaxis of thrombotic events such as heart attacks and strokes,
while a higher dose (1 g) has analgesic and antipyretic effects
(17). Aspirin irreversibly inhibits
COX-1, which converts arachidonic acid (20:4n-6) to PG
endoperoxides, and thus reduces PG formation (18).
Based on the aforementioned results, we hypothesize
that aspirin could reduce the glioma invasion through regulating
the expression of Cx43, and this process is mediated by PGE2
production. To test the hypothesis, we utilized an in vitro
glioma invasion model and investigated the effects of aspirin to
reduce the glioma invasion. In addition, whether aspirin had an
effect on the expression of Cx43 at a protein level was examined by
western blot analysis, and the function of gap junction
intercellular communication (GJIC) was tested by the scrape-loading
dye transfer technique method in C6 cells.
Materials and methods
C6 cell culture
Rat C6 glioma cells (obtained from the Cell Center
Department of the Chinese Academy of Medical Sciences, Beijing,
China) were grown in Dulbecco's modified Eagle's medium (DMEM)
(Gibco, Thermo Fisher Scientific, Inc., Dreieich, Germany)
supplemented with 15% heat-inactivated fetal bovine serum (FBS;
cat. no. 10270-106; Hyclone, Thermo Fisher Scientific, Inc.,
Shanghai, China), 100 U/ml penicillin and 100 µg/ml streptomycin
under standard culture conditions. When the cells reached
confluency, the medium was aspirated and fresh serum-free medium
was added to the cells for 12 h. The cells were subsequently washed
once with sterile phosphate-buffered saline (PBS), and fresh
serum-free medium was added. The following experiments were carried
out for the cells treated with 8 mmol/l aspirin (Sigma Resources
and Technologies, Inc., Santa Clara, CA, USA) for 30, 60 and 120
min.
Measurement of PGE2
An enzyme-linked immunosorbent assay was performed
to measure the level of PGE2 expression using the appropriate kits
from HyCult Biotechnology (Uden, The Netherlands) and R&D
Systems, Inc. (Minneapolis, MN, USA), following the manufacturer's
protocol. All the assays were performed in triplicate, and data are
shown as mean ± standard error of the mean.
Cx43 protein extraction and western
blot analysis
The effect of aspirin on Cx43 protein expression was
analyzed using western blot analysis. Protein homogenates of the C6
cells samples were prepared by rapid homogenization in 10 volumes
of lysis buffer. Samples were centrifuged at 17,000 × g for 1 h.
The protein concentration of soluble materials was determined by
the Coomassie G250 binding method. The protein lysates (12 µg per
lane for each sample) were fractioned on 12% SDS-polyacrylamide
gels, followed by transfer to nitrocellulose membranes (Merck
Millipore, Darmstadt, Germany). The membranes were blocked in
blocking buffer (5% non-fat dairy milk dissolved in Tris-buffered
saline with Tween-20) overnight at 4°C. The blots were subsequently
incubated with rabbit polyclonal antibody anti-Cx43 (dilution
1:400; cat. no. sc-9059; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) for 2 h. The Cx43 protein bands on these immunoblots
were visualized using the enhanced chemiluminescene kit (Boster
Inc., Wuhan, China). The Cx43 protein bands and β-actin bands were
scanned using the Bio-Rad Gel Doc™ XR+ Gel imaging system (Bio-Rad,
Berkeley, CA, USA), and integrated density values (IDVs) were
calculated by the Quantity One software and normalized with that of
β-actin.
GJIC activity
The scrape-loading dye transfer technique is a
method to evaluate GJIC activity by calculating the number of cells
containing the dye or measuring the distance of dye permeation
through the gap junctions. Glioma cells were digested into single
cells in 10 mg/ml collagenase I at 37°C overnight and 0.25%
trypsin-EDTA at room temperature for 2 min. Glioma cells were
cultured in DMEM/medium supplemented with 10% FBS and an antibiotic
mixture (penicillin 100 U/ml and streptomycin 100 µg/ml) at 37°C in
a humidified atmosphere containing 5% CO2. Aspirin was
added to the culture medium for 30, 60 and 120 min when the cell
densities had reached ~70% confluence. Subsequent to washing the
cells with PBS three times, 10 scrapes were made through the
confluent cells with a sterile pipette tip in the presence of warm
PBS containing 1 mg/ml lucifer yellow. The cells were further
incubated at 37°C and with 5% CO2 for 5 min. Following
this, lucifer yellow was removed, the cells were washed with PBS
three times and they were fixed with 4% paraformaldehyde in PBS.
The diffusion length of fluorandiol in gliomas was measured under a
laser scanning confocal microscope (Olympus, Tokyo, Japan). GJIC
activity was expressed as the mean diffusion length.
In vitro invasion assay
The C6 cells were resuspended in DMEM containing 15%
fetal calf serum to obtain a concentration of 107 cells/ml and
seeded (106 cells/well) in the upper compartments for 120 min; the
C6 cells that migrated to the lower compartment were counted under
a light microscope (Olympus). The experiments were performed in
triplicate, and migration was determined by calculating the number
of migrating cells.
Statistical analysis
Results are presented as the mean ± standard
deviation. One-way analysis of variance (ANOVA) was used to compare
the group differences in the measurements of Cx43 protein.
Dunnett's post hoc tests were applied to compare specific group
differences when the ANOVA revealed a significant difference. For
other measurements, the data were assessed using paired Student's
t-test.
Results
Relative levels of PGE2 from C6
cells
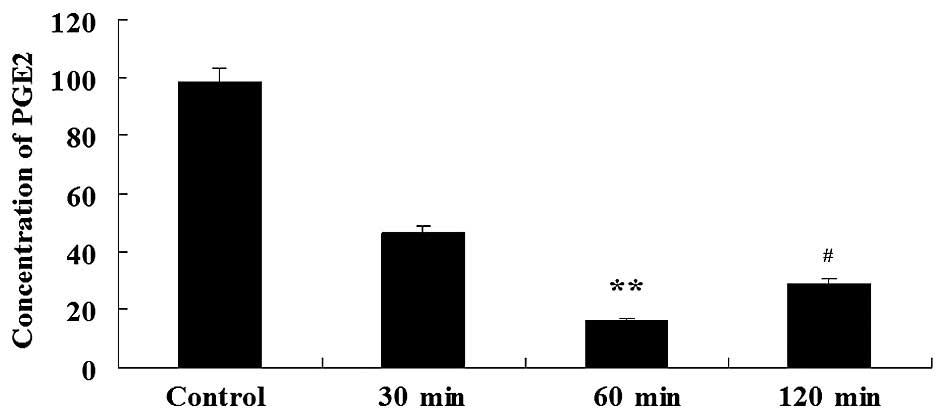
The content of PGE2 in the experimental groups was
2.12–6.05–fold lower compared to the control group; therefore,
aspirin significantly enhanced the downregulation of PGE2.
Specifically, aspirin decreased PGE2 after 60 min of treatment. The
relative levels of PGE2 in the control group, and the 30, 60 and
120 min aspirin groups were 98.24±1.23, 46.21±1.32, 16.47±2.31 and
29.05±1.98 µg/l, respectively (Fig.
1). This demonstrated that aspirin has a direct inhibitory
effect on the PGE2 level in glioma.
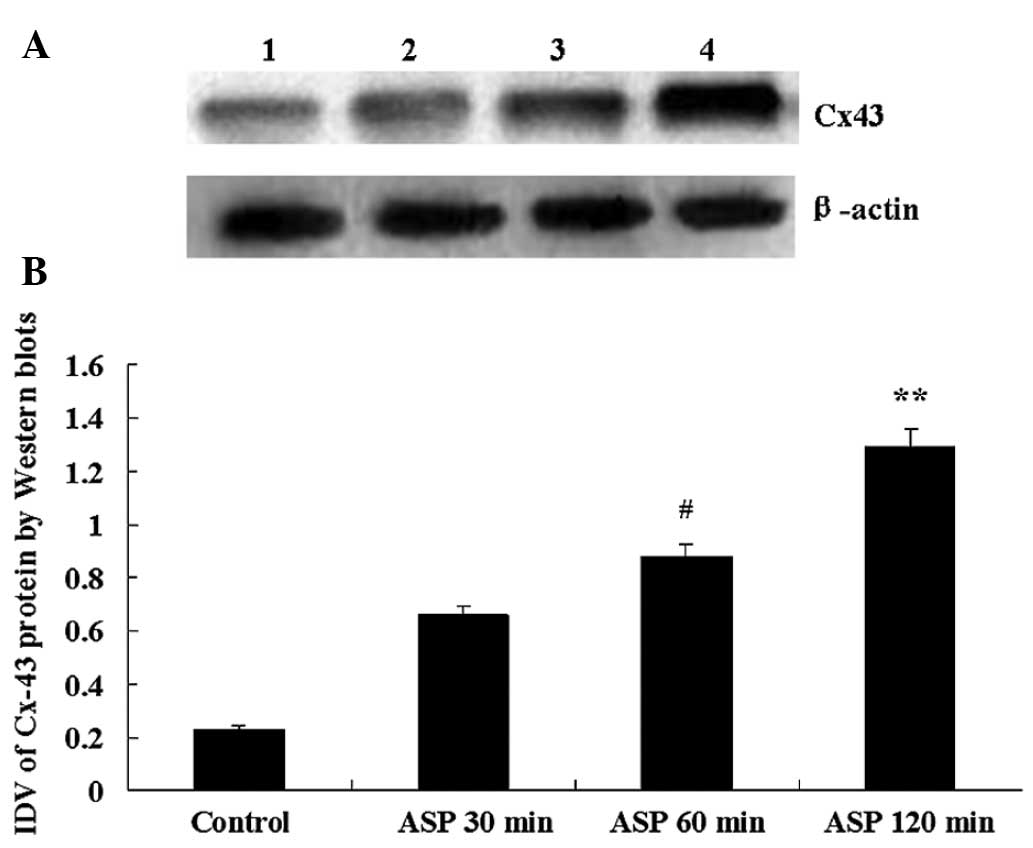
Aspirin induces overexpression of Cx43
proteins in C6 cells
In C6 cells infused with sterile saline (control
group), the level of Cx43 expressed was low. Compared with the
control group, the expression of Cx43 protein was markedly
increased at three time-points following aspirin treatment. The
IDVs of Cx43 at control group, 30, 60 and 120 min group were
0.238±0.058, 0.669±0.055, 0.886±0.065 and 1.292±0.048,
respectively. The largest IVD was at 120 min after aspirin
treatment (Fig. 2).
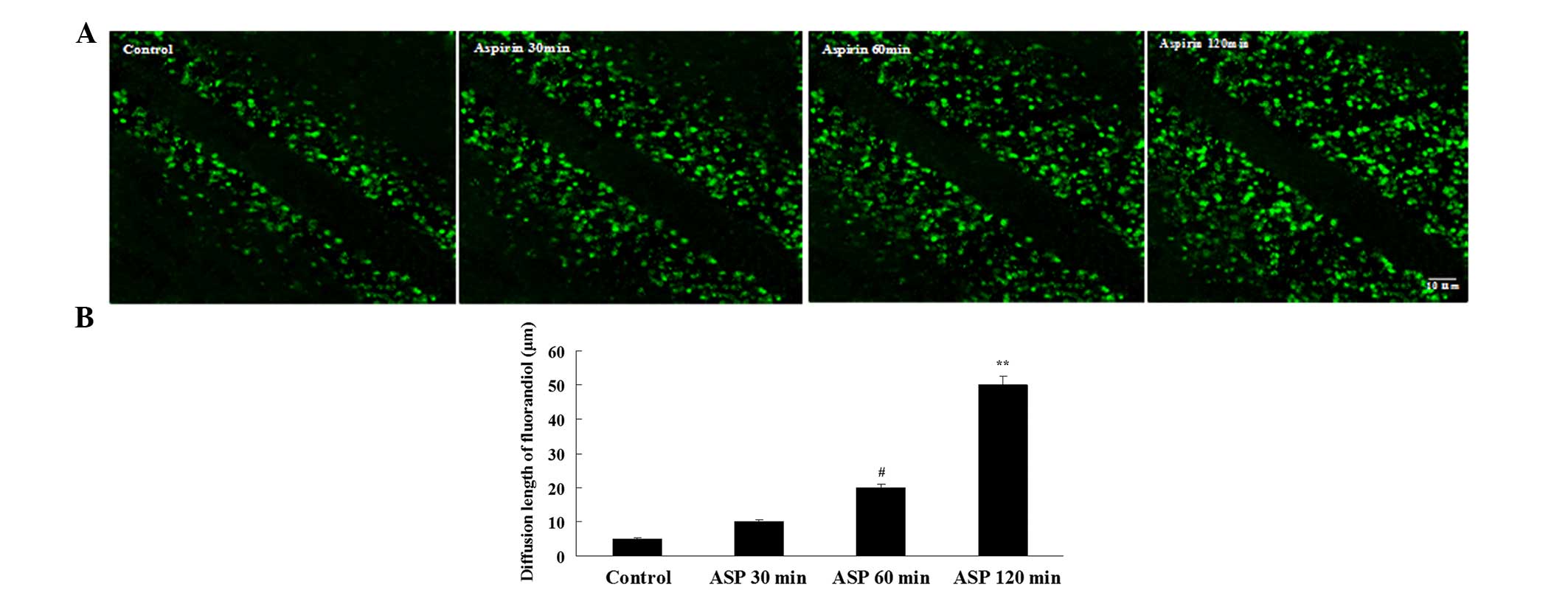
Aspirin enhances GJIC activity in C6
Cells
Dye transfer is a commonly used method to evaluate
GJIC activity by calculating the number of dye-labeled cells or
measuring the gap junction dye permeation. To determine if the
increases in Cx43 protein are associated with the changes in GJIC
activity, the cells were treated with aspirin for 30, 60 and 120
min, respectively, and the dye transfer between neighboring cells
was evaluated. Aspirin increased the fluorescent yellow diffusion
distance and the peak value at 120 min, suggesting that aspirin can
enhance the communication function of the gap of glioma cells
(Fig. 3).
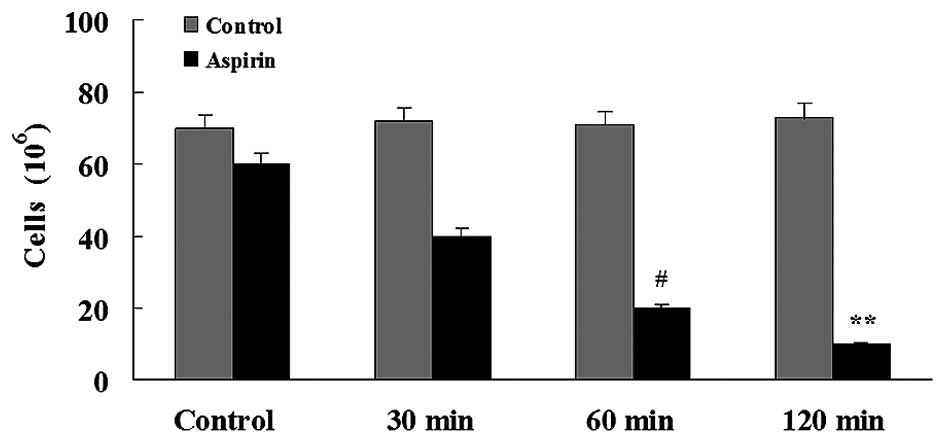
Glioma invasion
The percentage of migrating cells was 1.6- to
4.1-fold lower in the aspirin-treated groups compared to the
control group. Aspirin significantly reduced the number of
migrating C6 cells after 120 min (Fig.
4).
Discussion
Inflammation has emerged as a major factor promoting
cancer development and supporting cancer progression. However,
inflammation can also have cancer-inhibitory effects (19–22).
Inflammatory mediators can be produced by the stroma, by
tumor-infiltrating leukocytes or directly by the cancer cells
themselves. Prominent among tumor-sustaining mediators is PGE2, a
prostanoid lipid associated with enhancement of cancer cell
survival, growth and migration (23).
COX-1 and −2, enzymes that are critical for the production of PGE2,
are often overexpressed in colorectal, breast, stomach, lung and
pancreatic cancers (24). Whether
glioma similarly express abnormal levels of COX-2 remains to be
elucidated. Castelli et al (25) reported that glioma can secrete PGE2.
The present results showed that the expression of PGE2 and COX-2 in
C6 cells were significantly higher compared to those in
astrocytes.
The C6 glioma cell line has been widely used in the
cellular and molecular characterization of glial cells. One of the
well-known characteristics of astrocytes concerns the aspect of
intercellular coupling via gap junctions (26). Numerous studies have reported the
presence of gap junctions between astrocytes morphologically,
electrophysiologically and immunohistochemically (27–30). Gap
junction proteins are encoded by a family of genes, and two of the
most characterized proteins are Cx32 (31,32) and Cx43
(33). The study by Naus et al
(34) showed that the mRNAs encoding
these two proteins are readily detectable in the neonatal and adult
brain (35,36). In addition, primary cultures of
astrocytes express only the Cx43 mRNA, and the level of this mRNA
is significantly reduced in the glioma cells (35).
Dysregulation of gap junction coupling is a
phenotypic alteration commonly observed in neoplastic cells. The
studies by Bodenstine et al and Saunders et al
(37–39)
demonstrated a specific loss of homotypic and heterotypic GJIC in
metastatic cells. While the dysregulation of GJIC in neoplastic
cells is apparent, the specific signaling events and the mediators
of those signaling events between malignant cells or between
malignant cells and the surrounding stromal compartment appear to
be largely context dependent (40,41).
Furthermore, restoration of GJIC appears to reduce the metastatic
ability of cancer cells in certain cases (42,43). The
present study used the transfer dye method to detect the function
of GJIC in glioma cells, and it was found that the fluorescent
yellow diffusion distance was short, indicating that the cell gap
junction communication function is weak, which may be one of the
reasons for its aggressive nature.
The COX-2 inhibitor aspirin was used to treat the C6
cells. The results showed that the expression of COX-2 and PGE2
were significantly decreased, and the 30 and 60 min treatments
reduced the levels the most, respectively, suggesting that aspirin
may inhibit the synthesis of PGE2 by inhibiting of COX-2. The
expression of the CX43 protein was shown to be the highest at 120
min of treatment, while the GJIC function of the glioma cells was
the strongest, and the fluorescent yellow diffusion distance
increased. At this time, the glioma invasion was the lowest. This
indicates that aspirin can enhance the function of GJIC by
inhibiting the expression of PGE2, which can decrease the invasion
of glioma.
In conclusion, the biochemical mechanism of the
aspirin-induced glioma invasion decrease is complex. The present
results showed that one of the possibilities may be through the
PGE2/GJIC signal pathway. This study further proved that if the
ability of the glioma cells to synthesize PEG2 is eliminated, then
the GJIC function can effectively be enhanced. Treatment of
patients with a COX inhibitor that is similar to aspirin at surgery
may provide more potential benefits. Although the present study is
only a preliminary investigation, it indicates that the use of
aspirin may make glioma therapy more effective, and may increase
the survival and quality of life of the patients.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant nos. 81101912 and 81201048), the
Hebei Province Science and Technology Support Program (grant no.
152777189), and the Hebei Province Administration of Traditional
Chinese Medicine, (grant no. 2014195) and the Hebei Province
Department of Health and Family Planning Commission (grant no.
20150491).
References
|
1
|
Drappatz J, Schiff D, Kesari S, Norden AD
and Wen PY: Medical management of brain tumor patients. Neurol
Clin. 25:1035–1071, ix. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cahill CM, Waterman WR, Xie Y, Auron PE
and Calderwood SK: Transcriptional repression of the prointerleukin
1beta gene by heat shock factor 1. J Biol Chem. 271:24874–24879.
1996.PubMed/NCBI
|
|
3
|
Chatterjee S, Premachandran S, Sharma D,
Bagewadikar RS and Poduval TB: Therapeutic treatment with
L-arginine rescues mice from heat stroke-induced death:
Physiological and molecular mechanisms. Shock. 24:341–347. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lan F, Yue X, Han L, Yuan X, Shi Z, Huang
K, Yang Y, Zou J, Zhang J, Jiang T, et al: Antitumor effect of
aspirin in glioblastoma cells by modulation of β-catenin/T-cell
factor-mediated transcriptional activity. J Neurosurg. 115:780–788.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmed R, Oborski MJ, Hwang M, Lieberman FS
and Mountz JM: Malignant gliomas: Current perspectives in
diagnosis, treatment, and early response assessment using advanced
quantitative imaging methods. Cancer Manag Res. 6:149–170.
2014.PubMed/NCBI
|
|
6
|
Huse JT and Holland EC: Targeting brain
cancer: Advances in the molecular pathology of malignant glioma and
medulloblastoma. Nat Rev Cancer. 10:319–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakada M, Nakada S, Demuth T, Tran NL,
Hoelzinger DB and Berens ME: Molecular targets of glioma invasion.
Cell Mol Life Sci. 64:458–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phillips HS, Kharbanda S, Chen R, Forrest
WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, et
al: Molecular subclasses of high-grade glioma predict prognosis,
delineate a pattern of disease progression, and resemble stages in
neurogenesis. Cancer Cell. 9:157–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Louis DN, Holland EC and Cairncross JG:
Glioma classification: A molecular reappraisal. Am J Pathol.
159:779–786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang RP, Fan Y, Hossain MZ, Peng A, Zeng
ZL and Boynton AL: Reversion of the neoplastic phenotype of human
glioblastoma cells by connexin 43 (cx43). Cancer Res. 58:5089–5096.
1998.PubMed/NCBI
|
|
11
|
Soroceanu L, Manning TJ Jr and Sontheimer
H: Reduced expression of connexin-43 and functional gap junction
coupling in human gliomas. Glia. 33:107–117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pu P, Xia Z, Yu S and Huang Q: Altered
expression of Cx43 in astrocytic tumors. Clin Neurol Neurosurg.
107:49–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mesnil M, Crespin S, Avanzo JL and
Zaidan-Dagli ML: Defective gap junctional intercellular
communication in the carcinogenic process. Biochim Biophys Acta.
1719:125–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Honn KV, Bockman RS and Marnett LJ:
Prostaglandins and cancer: A review of tumor initiation through
tumor metastasis. Prostaglandins. 21:833–864. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levine L: Arachidonic acid transformation
and tumor production. Adv Cancer Res. 35:49–79. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bockman RS: Prostaglandins in cancer: A
review. Cancer Invest. 1:485–493. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weissmann G: Aspirin. Sci Am. 264:84–90.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vane JR: Inhibition of prostaglandin
synthesis as a mechanism of action for aspirin-like drugs. Nat New
Biol. 231:232–235. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coussens LM, Zitvogel L and Palucka AK:
Neutralizing tumor-promoting chronic inflammation: A magic bullet?
Science. 339:286–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rakoff-Nahoum S and Medzhitov R: Toll-like
receptors and cancer. Nat Rev Cancer. 9:57–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balkwill F, Charles KA and Mantovani A:
Smoldering and polarized inflammation in the initiation and
promotion of malignant disease. Cancer Cell. 7:211–217. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang D and Dubois RN: Eicosanoids and
cancer. Nat Rev Cancer. 10:181–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dannenberg AJ and Subbaramaiah K:
Targeting cyclooxygenase-2 in human neoplasia: Rationale and
promise. Cancer Cell. 4:431–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Castelli MG, Butti G, Chiabrando C, Cozzi
E, Fanelli R, Gaetani P, Silvani V and Paoletti P: Arachidonic acid
metabolic profiles in human meningiomas and gliomas. J Neurooncol.
5:369–375. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagy JI and Rash JE: Connexins and gap
junctions of astrocytes and oligodendrocytes in the CNS. Brain Res
Brain Res Rev. 32:29–44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gutnick MJ, Connors BW and Ransom BR:
Dye-coupling between glial cells in the guinea pig neocortical
slice. Brain Res. 213:486–492. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kettenmann H and Ransom BR: Electrical
coupling between astrocytes and between oligodendrocytes studied in
mammalian cell cultures. Glia. 1:64–73. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dermietzel R, Traub O, Hwang TK, Beyer E,
Bennett MVL, Spray DC and Willecke K: Differential expression of
three gap junction proteins in developing and mature brain tissues.
Proc Natl Acad Sci USA. 86:10148–10152. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamamoto T, Ochalski A, Hertzberg EL and
Nagy JI: On the organization of astrocytic gap junctions in rat
brain as suggested by LM and EM immunohistochemistry of connexin43
expression. J Comp Neurol. 302:853–883. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar NM and Gilula NB: Cloning and
characterization of human and rat liver cDNAs coding for a gap
junction protein. J Cell Biol. 103:767–776. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paul DL: Molecular cloning of cDNA for rat
liver gap junction protein. J Cell Biol. 103:123–134. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beyer EC, Paul DL and Goodenough DA:
Connexin43: A protein from rat heart homologous to a gap junction
protein from liver. J Cell Biol. 105:2621–2629. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Naus CC, Belliveau DJ and Bechberger JF:
Regional differences in connexin32 and connexin43 messenger RNAs in
rat brain. Neurosci Lett. 111:297–302. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Naus CC, Bechberger JF, Caveney S and
Wilson JX: Expression of gap junction genes in astrocytes and C6
glioma cells. Neurosci Lett. 126:33–36. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Belliveau DJ, Kidder GM and Naus CCG:
Expression of gap junction genes during postnatal neural
development. Dev Genet. 12:308–317. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bodenstine TM, Vaidya KS, Ismail A, Beck
BH, Cook LM, Diers AR, Landar A and Welch DR: Homotypic gap
junctional communication associated with metastasis suppression
increases with PKA activity and is unaffected by PI3K inhibition.
Cancer Res. 70:10002–10011. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bodenstine TM, Vaidya KS, Ismail A, Beck
BH, Diers AR, Edmonds MD, Kirsammer GT, Landar A and Welch DR:
Subsets of ATP-sensitive potassium channel (KATP) inhibitors
increase gap junctional intercellular communication in metastatic
cancer cell lines independent of SUR expression. FEBS Lett.
586:27–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Saunders MM, Seraj MJ, Li Z, Zhou Z,
Winter CR, Welch DR and Donahue HJ: Breast cancer metastatic
potential correlates with a breakdown in homospecific and
heterospecific gap junctional intercellular communication. Cancer
Res. 61:1765–1767. 2001.PubMed/NCBI
|
|
40
|
Kapoor P, Saunders MM, Li Z, Zhou Z,
Sheaffer N, Kunze EL, Samant RS, Welch DR and Donahue HJ: Breast
cancer metastatic potential: correlation with increased heterotypic
gap junctional intercellular communication between breast cancer
cells and osteoblastic cells. Int J Cancer. 111:693–697. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Z, Zhou Z, Welch DR and Donahue HJ:
Expressing connexin 43 in breast cancer cells reduces their
metastasis to lungs. Clin Exp Metastasis. 25:893–901. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu D, Caveney S, Kidder GM and Naus CC:
Transfection of C6 glioma cells with connexin 43 cDNA: Analysis of
expression, intercellular coupling, and cell proliferation. Proc
Natl Acad Sci USA. 88:1883–1887. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Martin W, Zempel G, Hülser D and Willecke
K: Growth inhibition of oncogene-transformed rat fibroblasts by
cocultured normal cells: Relevance of metabolic cooperation
mediated by gap junctions. Cancer Res. 51:5348–5351.
1991.PubMed/NCBI
|