Introduction
RNA-binding proteins are the proteins that have
similar characteristics and intracellular distribution, and are
termed heterogeneous nuclear ribonucleoproteins (hnRNPs) (1). Their role is in sharp contrast with the
roles of small nuclear ribonucleoproteins (snRNPs) and mRNA
proteins (mRNPs). Thus far, ~20 types of hnRNPs have been
identified, ranging from A1 to U. A large number of studies have
shown that these proteins have a significant role in the
progression of gene regulation, including DNA repairing, telomerase
extending, signal transduction, and transcriptional and
translational levels (2). Of which,
hnRNP K is one type of DNA and RNA-binding protein involved in
various regulatory progressions by means of protein-protein
interaction (3).
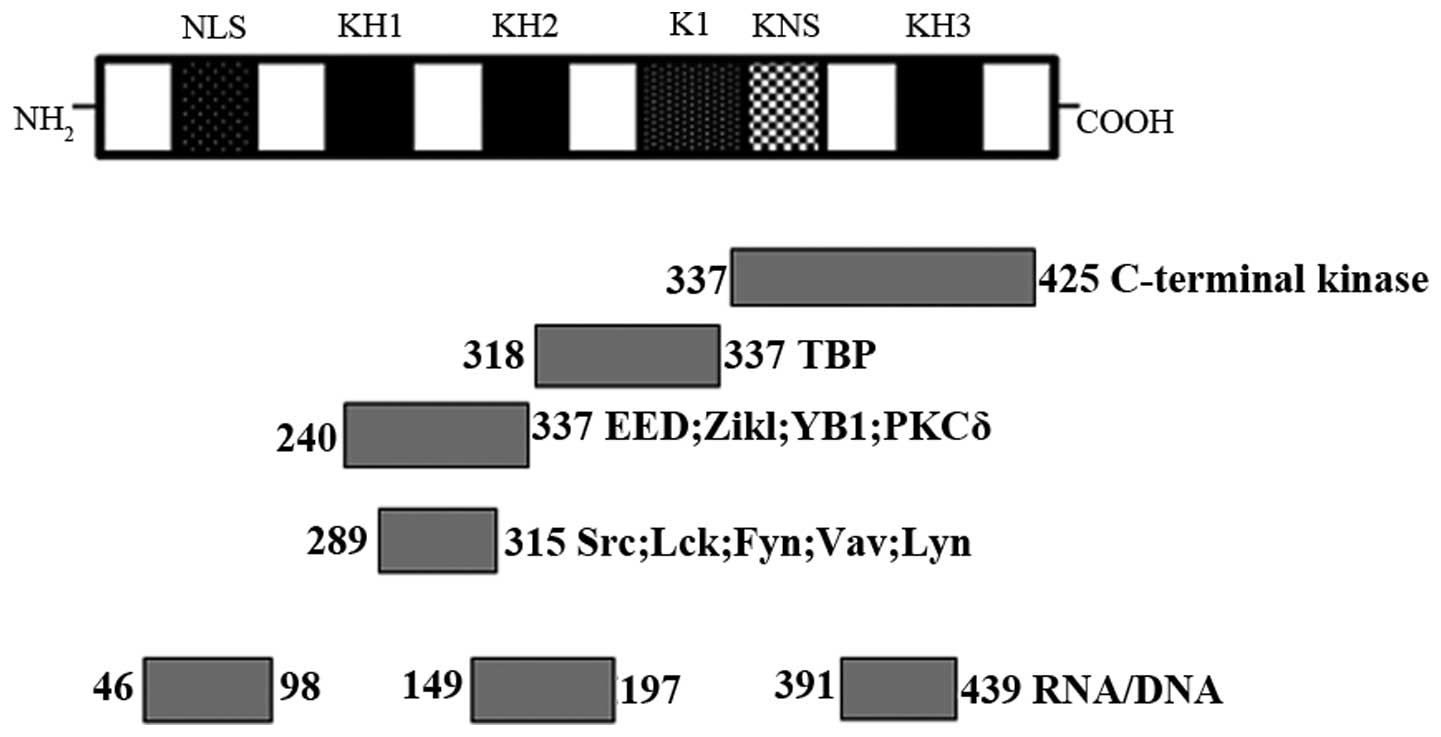
The relative molecular weight of hnRNP K is ~66 kDa,
which is comprised of three DNA-RNA binding homology domains (KH1,
KH2 and KH3), a K-protein-interactive region (KI) and a C-terminal
protein kinase-binding domain (4).
Each of these three KH domains contains 65–70 amino acids, two of
them located at the N-terminus, and the remaining one at the
C-terminus. KH domains have evolutionarily conserved features
making the KH domain with the same number of amino acids or the
same amino acid sequence exhibit similar functions in different
tissues. The typical function of KH domains is to recognize and
bind to RNA and single-stranded DNA. The KI domain lies between KH2
and KH3, which specifically exists in hnRNP K. This domain is
responsible for regulating the interaction between hnRNP K and
other proteins in the nucleus and cytoplasm. The KI region contains
the proline-rich docking sites, such as RXXPXXP and PXXPXR, which
interact particularly with SH3 domains of the Src-family signals.
Furthermore, hnRNP K contains a nuclear-localization signal with
the function of mediating its transport from the cytoplasm to the
nucleus (5,6). Therefore, it acts as a nucleocytoplasmic
shuttling protein to regulate gene expression by the nuclear pore
complex with the help of a nuclear shuttling domain (7) (Fig. 1).
According to previous results, there is a close
association between tumors and hnRNP K; it often shows a high
expression state in a variety of tumors, such as prostate cancer,
colon cancer, nasopharyngeal cancer, oral squamous cell carcinoma,
leukemia and breast cancer. hnRNP K is able to interact with
multiple molecular partners and is involved in a number of gene
regulation steps (7–9) (Table I).
hnRNP K is specific for hnRNP family members, and compared with
other hnRNP proteins, has different structural characteristics (KH
domain and DNA binding sites), so that it can participate in
numerous cellular processes in the nucleus and cytoplasm. Of note,
in addition to having the same functions with other hnRNPs, such as
mRNA splicing and the cytoplasmic transport of mRNA, it can
regulate DNA transcription, RNA processing and RNA translation,
particularly with regards to the process of oncogene expression
(2). All these features make it
exhibit multiple roles in the cell cycle, inhibition of apoptosis
and tumor metastasis. The present review assessed certain studies
from the perspective of the role and molecular mechanisms of hnRNP
K in promoting tumors, providing a more in-depth and comprehensive
understanding of the function of hnRNP K, and information for
future investigations to further explore its role in the tumor
progression.
 | Table I.hnRNP K interacts with diverse groups
of molecular partners to regulate gene expression and signal
transduction. |
Table I.
hnRNP K interacts with diverse groups
of molecular partners to regulate gene expression and signal
transduction.
| Process | Protein
partner | Regulated gene |
|---|
| Transcription | General factors:
TBP and HMGB1 Activators: Pura, Sox10 and C/EBPb Repressors: Zik1,
Kid1 and MZF-1 | c-Myc, c-Src,
thymidine kinase eIF4E, CHRNA4 and CD43 |
| Chromatin
remodeling | Eed,
DNA-methyltransferase, scaffold attachment factor B and MARs | AR |
| RNA processing | hnRNP E2, I, K, L
and U 9G8, SRp20, YB-1 and Sam68 | β-tropomyosin,
renin |
| Translation | EF-1α | c-Myc,
15-lipoxygenase, human papilloma virus type 16, eIF4E and p21 |
| Signal
transduction | Src, Lyn, Fyn, Lck,
Itk, PKCα, PKCδ, PKCε, ERK1/2, JNK, Vav and PRMT1 |
|
hnRNP K as a transcription factor to promote
tumors
hnRNP K can be a transcription factor to promote the
expression of certain oncogenes (10,11), which
combines the upstream pyrimidine-rich regions of promoters. In
vivo it is able to interact directly with transcription
machinery-related factors, such as the TATA box-binding protein
(TBP), a subunit of the eukaryotic transcription factor TFIID, the
RNA polymerase and others (12). These
factors act synergistically to promote the transcription process by
the way of protein-protein interaction.
There are CT repetitive sequences in the promoter
region of c-myc, known as the CT element (13). It is comprised of four consecutive
repeated CCCTCCCCA sequences and a fifth repeat sequence, which is
separated by a 9-base pair long sequence located downstream of the
first four sequences. Pioneer studies have shown that the
N-terminus of hnRNP K contains 35-amino acid residues that are
necessary for transactivating the CT element. When hnRNP K
recognizes the CT element of the c-Myc promoter region in a
specific-binding manner, it can recruit and interact with TBP and
RNA polymerases to upregulate the expression of c-Myc. For example,
it was found that c-myc and hnRNP K simultaneously increased in
breast cancer (14). Following further
exploration of hnRNP K, hnRNP K promoted transcription of c-myc in
a CT element-dependent manner in these tumors, and subsequently
c-Myc stimulated cell proliferation and inhibited apoptosis during
the progression of malignant transformation.
Activation or overexpression of c-Src, a
non-receptor tyrosine kinase of numerous signal pathways, has been
associated with a host of malignant cancers (15,16). c-Src
expression is regulated by the housekeeping-like SRC1A
promoter in numerous tissues (17).
There are three substantial polypurine/polypyrimidine (TC1, TC2 and
TC3) tracts within this promoter that have a role in enhancing
transcriptional activity. In addition, hnRNP K was shown to
regulate the SRC1A promoter cooperatively with the
transcription factor Sp1 (18,19). The study by Ritchie et al
(20) proposed that hnRNP K recognizes
and binds to TC1 and TC2 of the promoter region at first, which
facilitates double strands to separate and become a single strand,
leading to the affinity of hnRNP K with the increase in
single-stranded DNA, followed by hnRNP K recruiting the basal
transcriptional machinery, TBP and TFIID. The intact TC3 tract is
capable of binding the single-stranded form with a high affinity to
retain promoter activity. This series of processes promotes the
transcription complex formation, so as to upregulate the expression
of src.
hnRNP K interaction with nuclear matrix
proteins to promote tumors
Nuclear matrix (NM) is a fibrin protein-based grid
system present in the eukaryotic nucleus, excluding the nuclear
membrane, laminin, chromatin and nucleolus. This dynamic complex
mainly contains a variety of proteins and a small amount of RNA and
DNA. NM has an important role in gene regulation process, such as
chromatin remodeling, DNA replication and transcription and RNA
processing (21). hnRNP K activates at
the chromatin level, exhibiting a transient recruitment to multiple
sites within each of the inducible gene loci, including the
promoter and transcribed regions (22). hnRNP K is abundant in the NM, which has
a role in stabilizing the NM network. Furthermore, hnRNP K as one
type of NM protein can bind to the NM attachment region (MAR)
sequences, and is located in interchromatin granule clusters
(23). MAR is a class of DNA sequence,
which exists in eukaryotic cellular chromatins and specifically
recognizes the NM (24,25). When MAR binds to NM, it creates a
position segmentation effect and maintains each transcription unit
relatively independent from each other to be free of interaction
with the surrounding chromatins. As a consequence of the anchoring
of MAR sequences to NM, chromatin fibers are organized into
topologically isolated loops to regulate the progression of gene
transcription and translation, and removal of gene silencing
resulted from the position effect. It is the position of a gene
within the loop that determines its activity (26). As hnRNP K is the constituent of NM,
chromatin remodeling and the transcription process of gene
expression will be affected accordingly if the NM internal
structure is altered or the interaction between NM and MAR
sequences is repressed, with the original normal regulatory process
affected as well. In prostate cancer cells (27), phosphorylated AKT can promote the
phosphorylation of hnRNP K. The effect of hnRNP K stabilizing AR
will be weakened in succession, which is co-located with the AR in
the NM at first. In turn, phosphorylated hnRNP K inhibits the
expression of AR after it recognizes DNA-MAR sequences in the
nucleus, which makes the androgen-sensitive prostate cancer cells
convert to androgen-insensitive cancer cells and increases the risk
of a poor prognosis in patients who have received
androgen-deprivation therapy.
Involvement of hnRNP K in RNA alternative
splicing (AS) to promote tumors
AS is an essential mechanism in post-transcriptional
regulation, which is a crucial step of the gene expression process
in eukaryotes (28). It is a major
cause for protein diversity and has critical roles in
differentiation, development and disease. Thus, a gene may encode a
variety of proteins. Therefore, its regulation is associated with
cancer. It has been confirmed that hnRNP K is involved in certain
important splicing process by interacting with Sam68, TAF15, YB1,
9G8 and SRp20 (12). Of note, it
participates in the expression of apoptosis-related genes by AS to
promote the tumor formation. The mammalian B-cell lymphoma 2
(Bcl-2) family can be classified into the multi-motif Bcl-2
proteins that bear multiple BH motifs with pro-survival (Bcl-2,
Bcl-xL, Bcl-w, myeloid leukemia-1, A1 and Bcl-B) and pro-apoptotic
(Bcl-xS, Bcl-2-associated X protein and Bcl-2 homologous
antagonist/killer) activity (29).
hnRNP K can regulate the Bcl-2 AS process and inhibit Bcl-xS
generation, which results in a reduction of apoptosis in tumor
cells (30). In the event of AS, U1
snRNA identify the pre-mRNA 5′ splicing site in a nucleotide
complementary manner while U2AF recognizes and combines the
upstream pyrimidine-rich region of the 3′ splicing site and
promotes U2 snRNP and the U4, U5 and U6 snRNP trimer to bind
together to form a 60S spliceosome where a transesterification
reaction occurs, leading to the generation of different isoforms at
different sites (31,32). Furthermore, there is a B1
splicing-regulatory region existing in the 5′ splicing site of
Bcl-xS. hnRNP K can bind to the pyrimidine-rich region of B1 to
inhibit the production of the Bcl-xS isoform. Simultaneously, hnRNP
K is able to interact with Sam68, which has a role in upregulating
Bcl-xS expression to weaken its upregulation capacity. As a result,
the apoptosis pathway is blocked, so that cancer cells survive to
escape from the apoptotic signals. Due to this advantage condition,
tumor cells can be maintained in a safe environment and proliferate
rapidly.
Involvement of hnRNP K in RNA translation to
promote tumors
The translation mechanisms of hnRNP K action are the
most intensively studied. It has been confirmed that hnRNP K can
affect the tumor growth and development at the translational level
as well. Bomsztyk et al (7)
found that hnRNP K have a direct interaction with the translation
elongation factor 1α, confirming its role in translational
regulation. Following this, it was also found that hnRNP K could
bind to the polypyrimidine sequence of translation initiation
factor eIF4E (4EBE) to upregulate oncoprotein expression and
promote certain malignant phenotype formations (33). In addition, hnRNP K can interact with
the CU-rich region of p21 mRNA 3′ untranslated region (UTR) to
inhibit p21 translation and promote cell proliferation (34). When chronic myelogenous leukemia
converts from the chronic phase to the acute phase (35), the expression product of B-cell surface
receptor (BCR)/ABL, p210, can activates the tyrosine kinase
activity of mitogen-activated protein kinase
(MAPK)Erk1/2 in a dose-dependent manner in the bone
marrow and lymphocytes cells with the BCR/ABL gene. Subsequently,
activated MAPKErk1/2 induces hnRNP K expression and
stability increased. Stable hnRNP K binds to the myc mRNA internal
ribosome entry site to stimulate translational activation and
expression upregulation (3). The
increased myc protein will facilitate leukocyte cell proliferation,
colony formation and stimulate the occurrence of leukemia.
hnRNP K interacts with signaling molecules
to promote tumors
hnRNP K can cooperate with the Src tyrosine kinases
family, tryptophan/threonine kinase PKCδ, Erk1/2, Vav and other
molecules to regulate its interaction with the target proteins or
gene sequences. As combination factors vary, the effect of the
production of different signaling molecules is also significantly
different (36). For example, Jeon
et al (37) have demonstrated
that hnRNP K can bind to the signal transducer protein Vav to
become involved in the BCR signaling pathway. Interaction of the
Vav proto-oncogene product with hnRNP K regulates and promotes the
process of cell transformation by the SH3 domain (38,39). In
hepatocellular carcinoma (40), it can
increase the expression of the protein kinase inhibitor CFLP
(cellular FLICE-like inhibitory protein) that prevents
pro-caspase-8 activation and X-linked inhibitor of apoptosis
protein and maintain them at a high level to inhibit the classic
caspase apoptosis pathway activation. In breast cancer (41), the epidermal growth factor receptor
family can increase the expression of hnRNP K following activation
by exogenous growth signals, and subsequently, the upregulated
hnRNP K binds to and activates the c-myc promoter region to improve
the expression of c-myc to accelerate the tumor formation process.
In prostate cancer, hnRNP K participates in the AKT/hnRNP
K/AR/β-catenin signaling pathway (42), which has a crucial impact on converting
prostate cancer into a hormone-insensitive neuroendocrine (NE)
differentiation phenotype. The presence of this phenotype indicates
a poor prognosis for patient. Phosphorylated AKT is present at
prostate cancer cells in three pathways mainly; following promotion
of GSK3β phosphorylation, the phosphorylated GSK3β will be
transported from the cytoplasm into the nucleus; the second pathway
promotes the intracytoplasm AR to be phosphorylated and
subsequently degraded by the proteasome pathway; the last promotes
hnRNP K phosphorylation and enters into the nucleus. GSK3β and
phosphorylated hnRNP K of common positioning within the nucleus
bind to the AR sequence and repress AR expression, while increasing
the expression of NE differentiation phenotype markers,
neuron-specific enolase (NSE), simultaneously, which causes
hormone-sensitive prostate cancer to become hormone-insensitive and
NSE-independent prostate cancer phenotype, ultimately resulting in
ineffective androgen-withdrawal therapy. In the cytoplasm of tumor
cells, the activation of Ras and MEK can also make hnRNP K stably
exist in the cytoplasm (43).
Stabilized hnRNP K is able to activate ERK to promote upregulation
of matrix metalloproteinase 3 (MMP3) and MMP10. These factors have
an important role in promoting tumor metastasis. A succession of
studies are now providing a mechanistic basis, highlighting and
reinforcing that specific MMPs are key in tumor invasion and
metastasis through their catalytic and non-catalytic roles,
including modulating tumor cell motility, promoting invadopodia
formation, interactions of MMPs with pro-invasive pathways, sensing
matrix stiffness and induction and maintenance of
epithelial-mesenchymal transition (44). Therefore, hnRNP K simultaneously
provided favorable conditions for tumor invasion and metastasis
when upregulating MMPs expression.
hnRNP K interacts with non-coding RNAs
(ncRNAs) to promote tumors
In the human genome, ~90% is transcribed into
ncRNAs. ncRNAs are diverse RNA transcripts that are not transcribed
into proteins but have been shown to regulate the transcription,
stability or translation of protein-coding genes (45,46).
According to their size, they can be divided into long ncRNAs and
short ncRNAs (microRNAs). ncRNAs are associated with numerous
diseases, including a variety of tumors (47). In addition, there is a close
association between hnRNP K and ncRNAs, which may indicate that
there is contact between hnRNPK and ncRNAs in tumors. Recently,
Gumireddy et al (48)
identified that a translational regulatory ncRNA (treRNA) was
highly expressed in metastatic breast cancer and primary colon
cancer through genome-wide computational analysis. It interacted
with hnRNP K to promote tumor invasion and metastasis. treRNA can
combine with hnRNP K, FXR1, puf60, SF3B3 and other factors to
facilitate the formation of the treRNA-associated protein complex.
This complex is able to directly or indirectly bind to the
E-cadherin mRNA 3′UTR, and reduce translation efficiency of
E-cadherin mRNA, and therefore E-cadherin expression decreased.
Downregulated E-cadherin leads to a direct result of adhesion
activity decrease between tumor cells. As a consequence, tumor
cells shed from the primary tumor into the circulation system, and
position in the new site. In addition, Qin et al (49) showed that hnRNP K is a target of
miR-205. miR-205 has a complex regulatory role in tumor initiation
and growth processes. It can inhibit or promote tumor formation
depending on its binding targets and microenvironment. Furthermore,
it was found that miR-205 can bind to the 3′UTR of hnRNP K to
reduce hnRNP K expression (50).
However, miR-205 is downregulated in prostate cancer, so its
inhibition for hnRNP K is derepressed, which leads to promoting the
state of tumors (51).
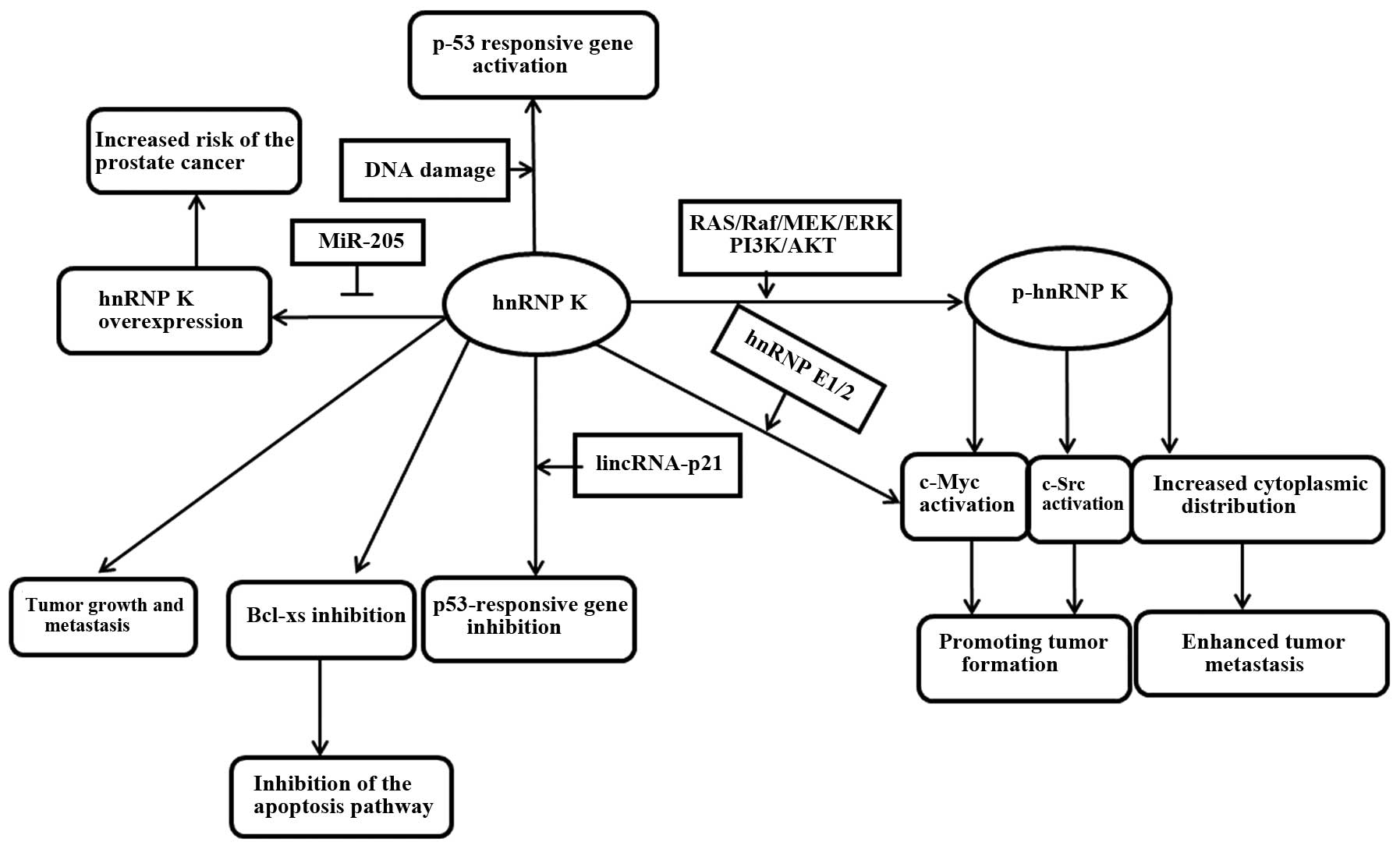
Conclusion
hnRNP K is an RNA/DNA-binding protein that is a
target of multiple kinases or recruits factors involved in signal
transduction and gene expression. Its abnormal expression can make
the tumor formation risk increase significantly. In several tumors,
the hnRNP K expression level progressively increases from normal to
hyperplasia to carcinoma tissue, and it is often associated with
tumor stage, indicating an asociation between hnRNP K expression
and tumors progression (52,53). Inoue et al (54) have proved that hnRNP K has an important
role in tumor invasion. They identified that hnRNP K is involved in
tumor cell metastasis, and its cytoplasmic localization is
essential for cell invasion and metastasis. Recently, it was also
proved that if hnRNP K is overexpressed, cell malignancy and
metastatic ability would be improved in vitro and in
vivo. Furthermore, Hope and Murray (55) demonstrated in colon cancer that hnRNP K
in addition to the high expression appeared with an abnormal
cytoplasmic localization, and it correlated with lymph node
metastasis, suggesting that it is a poor prognostic markers. Gao
et al (43) have demonstrated
that hnRNP K could induce the expression of certain genes involved
in the cell extracellular matrix, cell motility and angiogenesis by
cDNA microarray analysis and signaling pathway analysis. Therefore,
regardless of the tumor type, the abnormal increase or cytoplasmic
localization of hnRNP K may be regarded as a valid marker of poor
prognosis (Table II). In summary,
hnRNP K is involved in multiple cellular functions relevant to
cancer development and progression (Fig.
2). The overexpressed hnRNP K in numerous tumors, as a
multifunctional protein, may hopefully become a therapeutic target
due to its role in promoting malignant transformation and tumor
metastasis. If this hypothesis is true, reasonable drugs and
therapies can be designed to intervene with tumor growth according
to the regulatory characteristics of hnRNP K. Further
investigations are required.
 | Table II.Heterogeneous nuclear
ribonucleoprotein K expression in individual types of cancer and
its association with prognosis in different types of cancer. |
Table II.
Heterogeneous nuclear
ribonucleoprotein K expression in individual types of cancer and
its association with prognosis in different types of cancer.
| Type of cancer | Expression in tumor
tissuea | Prognostic
significance |
|---|
| Colorectal | Increased | Survival |
| Esophageal squamous
cell | Increased | Poor prognosis |
| Hepatocellular | Increased | ND |
| Lung | Increased | ND |
| Melanoma | Increased | ND |
| Nasopharyngeal | Increased | Poor prognosis |
| Oral squamous
cell | Increased | Poor prognosis |
| Prostate | Increased | Poor prognosis |
Acknowledgements
The present study was supported in part by grants
from the National Natural Science Foundation of China (no.
81172322), Science and Technology Commission of Shanghai
Municipality (no. 11ZR1421000) and Science and Technology Fund of
Shanghai Jiao Tong University School of Medicine (no. YZ1027).
References
|
1
|
Swanson MS and Dreyfuss G: Classification
and purification of proteins of heterogeneous nuclear
ribonucleoprotein particles by RNA-binding specificities. Mol Cell
Biol. 8:2237–2241. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carpenter B, MacKay C, Alnabulsi A, MacKay
M, Telfer C, Melvin WT and Murray GI: The roles of heterogeneous
nuclear ribonucleoproteins in tumour development and progression.
Biochim Biophys Acta. 1765:85–100. 2006.PubMed/NCBI
|
|
3
|
Evans JR, Mitchell SA, Spriggs KA,
Ostrowski J, Bomsztyk K, Ostarek D and Willis AE: Members of the
poly (rC) binding protein family stimulate the activity of the
c-myc internal ribosome entry segment in vitro and in vivo.
Oncogene. 22:8012–8020. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dejgaard K and Leffers H: Characterisation
of the nucleic-acid-binding activity of KH domains. Different
properties of different domains. Eur J Biochem. 241:425–431. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson SM, Datar KV, Paddy MR, Swedlow JR
and Swanson MS: Characterization of nuclear polyadenylated
RNA-binding proteins in Saccharomyces cerevisiae. J Cell Biol.
127:1173–1184. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Michael WM, Eder PS and Dreyfuss G: The K
nuclear shuttling domain: A novel signal for nuclear import and
nuclear export in the hnRNP K protein. EMBO J. 16:3587–3598. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bomsztyk K, Van Seuningen I, Suzuki H,
Denisenko O and Ostrowski J: Diverse molecular interactions of the
hnRNP K protein. FEBS Lett. 403:113–115. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ostareck-Lederer A, Ostareck DH, Cans C,
Neubauer G, Bomsztyk K, Superti-Furga G and Hentze MW:
c-Src-mediated phosphorylation of hnRNP K drives translational
activation of specifically silenced mRNAs. Mol Cell Biol.
22:4535–4543. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ostareck-Lederer A, Ostareck DH and Hentze
MW: Cytoplasmic regulatory functions of the KH-domain proteins
hnRNPs K and E1/E2. Trends Biochem Sci. 23:409–411. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Choi HS, Hwang CK, Song KY, Law PY, Wei LN
and Loh HH: Poly(C)-binding proteins as transcriptional regulators
of gene expression. Biochem Biophys Res Commun. 380:431–436. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michelotti EF, Michelotti GA, Aronsohn AI
and Levens D: Heterogeneous nuclear ribonucleoprotein K is a
transcription factor. Mol Cell Biol. 16:2350–2360. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shnyreva M, Schullery DS, Suzuki H, Higaki
Y and Bomsztyk K: Interaction of two multifunctional proteins.
Heterogeneous nuclear ribonucleoprotein K and Y-box-binding
protein. J Biol Chem. 275:15498–15503. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takimoto M, Tomonaga T, Matunis M, Avigan
M, Krutzsch H, Dreyfuss G and Levens D: Specific binding of
heterogeneous ribonucleoprotein particle protein K to the human
c-myc promoter, in vitro. J Biol Chem. 268:18249–18258.
1993.PubMed/NCBI
|
|
14
|
Samuel SK, Spencer VA, Bajno L, Sun JM,
Holth LT, Oesterreich S and Davie JR: In situ cross-linking by
cisplatin of nuclear matrix-bound transcription factors to nuclear
DNA of human breast cancer cells. Cancer Res. 58:3004–3008.
1998.PubMed/NCBI
|
|
15
|
Biscardi JS, Tice DA and Parsons SJ:
c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res.
76:61–119. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jacobs C and Rübsamen H: Expression of
pp60c-src protein kinase in adult and fetal human tissue: High
activities in some sarcomas and mammary carcinomas. Cancer Res.
43:1696–1702. 1983.PubMed/NCBI
|
|
17
|
Bonham K, Ritchie SA, Dehm SM, Snyder K
and Boyd FM: An alternative, human SRC promoter and its regulation
by hepatic nuclear factor-1alpha. J Biol Chem. 275:37604–37611.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du Q, Melnikova IN and Gardner PD:
Differential effects of heterogeneous nuclear ribonucleoprotein K
on Sp1- and Sp3-mediated transcriptional activation of a neuronal
nicotinic acetylcholine receptor promoter. J Biol Chem.
273:19877–19883. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ritchie S, Boyd FM, Wong J and Bonham K:
Transcription of the human c-Src promoter is dependent on Sp1, a
novel pyrimidine binding factor SPy, and can be inhibited by
triplex-forming oligonucleotides. J Biol Chem. 275:847–854. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ritchie SA, Pasha MK, Batten DJ, Sharma
RK, Olson DJ, Ross AR and Bonham K: Identification of the SRC
pyrimidine-binding protein (SPy) as hnRNP K: Implications in the
regulation of SRC1A transcription. Nucleic Acids Res. 31:1502–1513.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barboro P, D'Arrigo C, Diaspro A, Mormino
M, Alberti I, Parodi S, Patrone E and Balbi C: Unraveling the
organization of the internal nuclear matrix: RNA-dependent
anchoring of NuMA to a lamin scaffold. Exp Cell Res. 279:202–218.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ostrowski J, Kawata Y, Schullery DS,
Denisenko ON and Bomsztyk K: Transient recruitment of the hnRNP K
protein to inducibly transcribed gene loci. Nucleic Acids Res.
31:3954–3962. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saitoh N, Spahr CS, Patterson SD, Bubulya
P, Neuwald AF and Spector DL: Proteomic analysis of interchromatin
granule clusters. Mol Biol Cell. 15:3876–3890. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barboro P, D'Arrigo C, Repaci E, Bagnasco
L, Orecchia P, Carnemolla B, Patrone E and Balbi C: Proteomic
analysis of the nuclear matrix in the early stages of rat liver
carcinogenesis: Identification of differentially expressed and
MAR-binding proteins. Exp Cell Res. 315:226–239. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barboro P, Repaci E, D'Arrigo C and Balbi
C: The role of nuclear matrix proteins binding to matrix attachment
regions (Mars) in prostate cancer cell differentiation. PLoS One.
7:e406172012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marenduzzo D, Faro-Trindade I and Cook PR:
What are the molecular ties that maintain genomic loops? Trends
Genet. 23:126–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barboro P, Borzì L, Repaci E, Ferrari N
and Balbi C: Androgen receptor activity is affected by both nuclear
matrix localization and the phosphorylation status of the
heterogeneous nuclear ribonucleoprotein K in anti-androgen-treated
LNCaP cells. PLoS One. 8:e792122013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen K, Dai X and Wu J: Alternative
splicing: An important mechanism in stem cell biology. World J Stem
Cells. 7:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lanave C, Santamaria M and Saccone C:
Comparative genomics: The evolutionary history of the Bcl-2 family.
Gene. 333:71–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Revil T, Pelletier J, Toutant J, Cloutier
A and Chabot B: Heterogeneous nuclear ribonucleoprotein K represses
the production of pro-apoptotic Bcl-xS splice isoform. J Biol Chem.
284:21458–21467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wahl MC, Will CL and Lührmann R: The
spliceosome: Design principles of a dynamic RNP machine. Cell.
136:701–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan Q, Shai O, Lee LJ, Frey BJ and
Blencowe BJ: Deep surveying of alternative splicing complexity in
the human transcriptome by high-throughput sequencing. Nat Genet.
40:1413–1415. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lynch M, Chen L, Ravitz MJ, Mehtani S,
Korenblat K, Pazin MJ and Schmidt EV: hnRNP K binds a core
polypyrimidine element in the eukaryotic translation initiation
factor 4E (eIF4E) promoter, and its regulation of eIF4E contributes
to neoplastic transformation. Mol Cell Biol. 25:6436–6453. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yano M, Okano HJ and Okano H: Involvement
of Hu and heterogeneous nuclear ribonucleoprotein K in neuronal
differentiation through p21 mRNA post-transcriptional regulation. J
Biol Chem. 280:12690–12699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Notari M, Neviani P, Santhanam R, Blaser
BW, Chang JS, Galietta A, Willis AE, Roy DC, Caligiuri MA, Marcucci
G, et al: A MAPK/HNRPK pathway controls BCR/ABL oncogenic potential
by regulating MYC mRNA translation. Blood. 107:2507–2516. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ostrowski J, Schullery DS, Denisenko ON,
Higaki Y, Watts J, Aebersold R, Stempka L, Gschwendt M and Bomsztyk
K: Role of tyrosine phosphorylation in the regulation of the
interaction of heterogenous nuclear ribonucleoprotein K protein
with its protein and RNA partners. J Biol Chem. 275:3619–3628.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeon HK, Ahn JH, Choe J, Park JH and Lee
TH: Anti-IgM induces up-regulation and tyrosine-phosphorylation of
heterogeneous nuclear ribonucleoprotein K proteins (hnRNP K) in a
Ramos B cell line. Immunol Lett. 98:303–310. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bustelo XR, Suen KL, Michael WM, Dreyfuss
G and Barbacid M: Association of the vav proto-oncogene product
with poly(rC)-specific RNA-binding proteins. Mol Cell Biol.
15:1324–1332. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Groysman M, Nagano M, Shaanan B and Katzav
S: Mutagenic analysis of Vav reveals that an intact SH3 domain is
required for transformation. Oncogene. 17:1597–1606. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao Z, Ko HL, Goh EH, Wang B and Ren EC:
hnRNP K suppresses apoptosis independent of p53 status by
maintaining high levels of endogenous caspase inhibitors.
Carcinogenesis. 34:1458–1467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mandal M, Vadlamudi R, Nguyen D, Wang RA,
Costa L, Bagheri-Yarmand R, Mendelsohn J and Kumar R: Growth
factors regulate heterogeneous nuclear ribonucleoprotein K
expression and function. J Biol Chem. 276:9699–9704. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mukhopadhyay NK, Kim J, Cinar B,
Ramachandran A, Hager MH, Di Vizio D, Adam RM, Rubin MA,
Raychaudhuri P, De Benedetti A, et al: Heterogeneous nuclear
ribonucleoprotein K is a novel regulator of androgen receptor
translation. Cancer Res. 69:2210–2218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gao R, Yu Y, Inoue A, Widodo N, Kaul SC
and Wadhwa R: Heterogeneous nuclear ribonucleoprotein K (hnRNP-K)
promotes tumor metastasis by induction of genes involved in
extracellular matrix, cell movement, and angiogenesis. J Biol Chem.
288:15046–15056. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brown GT and Murray GI: Current
mechanistic insights into the roles of matrix metalloproteinases in
tumour invasion and metastasis. J Pathol. 237:273–281. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mattick JS: The genetic signatures of
noncoding RNAs. PLoS Genet. 5:e10004592009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
47
|
Ling H, Fabbri M and Calin GA: MicroRNAs
and other non-coding RNAs as targets for anticancer drug
development. Nat Rev Drug Discov. 12:847–865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gumireddy K, Li A, Yan J, Setoyama T,
Johannes GJ, Orom UA, Tchou J, Liu Q, Zhang L, Speicher DW, et al:
Identification of a long non-coding RNA-associated RNP complex
regulating metastasis at the translational step. EMBO J.
32:2672–2684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qin AY, Zhang XW, Liu L, Yu JP, Li H, Wang
SZ, Ren XB and Cao S: miR-205 in cancer: An angel or a devil? Eur J
Cell Biol. 92:54–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Szczyrba J, Nolte E, Hart M, Döll C, Wach
S, Taubert H, Keck B, Kremmer E, Stöhr R, et al: Identification of
ZNF217, hnRNP-K, VEGF-A and IPO7 as targets for microRNAs that are
downregulated in prostate carcinoma. Int J Cancer. 132:775–784.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Szczyrba J, Löprich E, Wach S, Jung V,
Unteregger G, et al: The microRNA profile of prostate carcinoma
obtained by deep sequencing. Mol Cancer Res. 8:529–538. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Carpenter B, McKay M, Dundas SR, Lawrie
LC, Telfer C and Murray GI: Heterogeneous nuclear ribonucleoprotein
K is over expressed, aberrantly localised and is associated with
poor prognosis in colorectal cancer. Br J Cancer. 95:921–927. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roychoudhury P and Chaudhuri K: Evidence
for heterogeneous nuclear ribonucleoprotein K overexpression in
oral squamous cell carcinoma. Br J Cancer. 97:574–575; author reply
576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Inoue A, Sawata SY, Taira K and Wadhwa R:
Loss-of-function screening by randomized intracellular antibodies:
Identification of hnRNP-K as a potential target for metastasis.
Proc Natl Acad Sci USA. 104:8983–8988. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hope NR and Murray GI: The expression
profile of RNA-binding proteins in primary and metastatic
colorectal cancer: Relationship of heterogeneous nuclear
ribonucleoproteins with prognosis. Hum Pathol. 42:393–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|