Introduction
Chronic suppurative otitis media (CSOM) is
characterized by a perforated tympanic membrane with recurrent or
persistent drainage from the middle ear for ≥8 weeks (1). Suppurative inflammatory reaction of the
mucous membrane, periosteum and bone of the middle ear mastoid
cavity involves a variety of cells, including neutrophils,
eosinophils, lymphocytes, mononuclear macrophages and plasma cells.
Purulent secretions consist mainly of white blood cells,
macrophages and bacterium.
The most prominent pathological feature of CSOM is
the presence of different degrees of granulation tissues (2,3). A nodular
inflammatory lesion arises from the attic region of the middle ear
mastoid cavity with consistent stimulation of the mucous membrane.
Immature connective tissues are enriched with capillaries, which
are primarily composed of compact mononuclear phagocytes and
fibroblasts. Granulation tissues began to appear after 2–3 days of
tissue injury, filling wounds or organizing foreign bodies. In the
early stages of granulation formation, macrophages infiltrate into
tissues and release a variety of inflammatory mediators such as
platelet-derived growth factor, fibroblast growth factor,
transforming growth factor-β, tumor necrosis factor (TNF) and
interleukin-1 (IL-1); and further recruit and stimulate hyperplasia
of fibroblasts and capillaries. Gradually, granulation tissues
become mature as indicated by the absorbed moisture, reduction and
disappearance of inflammatory cells, and capillary occlusion or
reduction; and eventually the maturity of connective tissues and
scar tissues. The present study investigated the potential
involvement of T cells, and the associated immune response with
granulation tissue formation in CSOM.
Materials and methods
Patients
A total of 15 patients (8 males and 7 females, aged
17–77 years old) with CSOM were included in the study. The duration
of CSOM ranged from 6 months to >30 years (Table I). Nine patients were self-reported and
diagnosed with ear discharge within the last 6 months (active
phase), while 6 cases had no ear discharge within the last 6 months
(stationary phase) (4). All the
patients underwent surgery in the Second Affiliated Hospital of
Xi'an Jiaotong University (Xi'an, China). Bacterial culture was
performed prior or during surgery. Informed consent was obtained
from all the patients, and the study protocol was approved by the
Ethics Committee of Xi'an Jiaotong University.
 | Table I.Patient characteristics, disease
course and status of granulation tissues. |
Table I.
Patient characteristics, disease
course and status of granulation tissues.
| Patient no. | Gender | Age, years | Disease course | Status of granulation
tissues |
|---|
| 1 | F | 45 | 5
years | Mature |
| 2 | F | 17 | 15 years | Mature |
| 3 | M | 32 | 6 months | Mature |
| 4 | F | 55 | 30 years | Fresh |
| 5 | M | 60 | 30 years/17
yearsa | Mature |
| 6 | M | 77 | 13 years | Fresh |
| 7 | M | 58 | 13 years | Mature |
| 8 | F | 47 | 16 years | Fresh |
| 9 | M | 36 | 28 years | Mature |
| 10 | F | 70 | 30 years | Fresh |
| 11 | F | 39 | 10 years/1 year | Mature |
| 12 | M | 21 | 8 years | Fresh |
| 13 | F | 55 | 14 years/1 month | Fresh |
| 14 | M | 22 | 10 years/1 month | Mature |
| 15 | M | 68 | 20 year | Mature |
Granulation tissue sampling
Granulation tissue specimens were collected from the
sinus, mastoid cavity or attic during surgery. Immediately
following excision, granulation tissues were immersed in 4%
formalin; and subsequently washed with Tris-buffered saline with
Tween-20 (TBST; pH 7.4), dehydrated, embedded in paraffin wax and
cryostat sectioned into slices.
Hematoxylin and eosin (H&E)
staining
Paraffin-embedded granulation tissue sections were
stained with H&E according to standard protocols. Histology was
determined by two pathologists.
Immunohistochemistry and cell
counting
Paraffin sections were placed in an oven at 60°C
overnight and dewaxed in xylene three times; subsequently the
sections were rehydrated through a graded alcohol series (100, 95,
90, 75 and 50%), washed with distilled water, and finally, washed
with TBST three times. These sections were treated with 3%
H2O2 for 10 min at room temperature to
eliminate endogenous peroxidase. The sections were placed in a
pressure kettle with 0.01 mol/l citrate buffer for antigen
retrieval. Subsequently, nonspecific protein was blocked with 5%
goat serum for 30 min, followed by reaction with cluster of
differentiation 4 (CD4; cat. no. IS649; 1:180; monoclonal mouse
anti-human), CD8 (cat. no. IS623; 1:40; monoclonal mouse
anti-human) (both Dako, Glostrup, Denmark), forkhead box P3 (FOXP3;
1:20; monoclonal), OX40 (1:50; monoclonal) (both eBioscience, Inc.,
San Diego, CA, USA) and granzyme B (Novocastra, Newcastle upon
Tyne, UK) antibodies at 4°C overnight. After washing three times
with TBST for 5 min each time, sections were subsequently incubated
with the appropriate secondary antibodies (Envision; Dako), stained
with diaminobenzidine (DAB; alkaline phosphatase was used for FOXP3
staining), and counterstained with hematoxylin. Slides were
observed under a microscope (CH20BIMF200; Olympus, Tokyo,
Japan).
Five fields were randomly observed (magnification,
×200) in each tissue section to count the positively stained cells,
and the average was calculated. The cytomembrane of CD8+
T cells and CD4+ T cells stained brown-yellow. Granyzme
B-positive cells were brown-yellow granule stained in cytoplasma,
while OX40+ T cells were brown-yellow granule stained in
the cytomembrane and cytoplasma. The nucleus of FOXP3+
regulatory T (Treg) cells stained blue.
Statistical analysis
Data were analyzed using SPSS 18.0 software (SPSS,
Inc., Chicago, IL, USA) and are expressed as mean ± standard
deviation. Two-sample t-test was used for statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Granulation tissue status has no
correlation with CSOM disease course
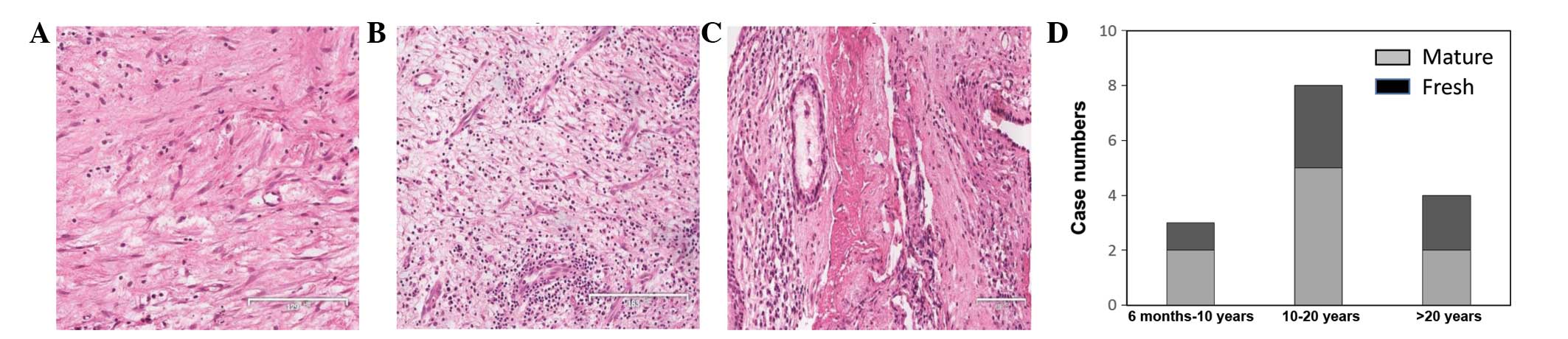
Granulation tissue status was first identified by
H&E staining. As shown in Fig. 1A,
a patient with 6 months of CSOM showed increased fibroblast growth,
capillary blockage and inflammatory cell infiltration (mature
granulation tissues). A large number of capillaries and
inflammatory cell infiltration were also observed in 1 patient with
30 years of CSOM (mature granulation tissue; Fig. 1B). Two cases with 10–20 years of CSOM
had experienced a recurrence within the past month. In 1 case,
H&E staining revealed decreased capillaries and fibroblasts at
the center surrounded by fresh granulation tissues (Fig. 1C). Notably, fresh granulation tissues,
mature granulation tissues and even necrotic tissues could be
simultaneously observed in a specimen. Characterization of
granulation tissues in H&E-stained specimens from other
patients revealed that there was no correlation between the
deteriorative status of granulation tissues with the duration of
the disease (disease course) (Fig.
1D). Patients with a longer history of CSOM may not have mature
granulation tissues, while patients with a shorter history of CSOM
may have mature granulation tissues. The status of granulation
tissues in each patient is shown in Table
I.
Immunohistochemistry and T-cell
subtypes in granulation tissues
Five fields in each specimen were randomly selected
at high magnification. The mean positive cell count of these five
fields was considered as the mean number of positive cells of the
specimen. In all 15 specimens, the mean number of CD8+ T
cells was 27.0±30.0, while the mean number of FOXP3+
Treg cells was 22.6±22.5; and these results were significantly
higher compared to CD4+ T cells (7.1±5.7), granzyme
B-positive cells (3.1±6.7), and OX40+ T cells (2.1±2.0)
(Table II).
 | Table II.T-cell subtypes in granulation tissues
of chronic suppurative otitis media. |
Table II.
T-cell subtypes in granulation tissues
of chronic suppurative otitis media.
| T-cell subtypes | Lymphocytes, no. |
|---|
| CD4+ | 7.1±5.7 |
| CD8+ | 27.0±30.0 |
| Granzyme B
positive | 3.1±6.7 |
| Forkhead box
P3+ | 22.6±22.5 |
|
OX40+ | 2.1±2.0 |
T-cell subtypes in granulation tissues
of CSOM patients in stationary or active phases
Patients were grouped into stationary (without ear
discharge within the last 6 months, n=6) and active (with ear
discharge within the last 6 months, n=9) phases, according to the
guidelines for diagnosis and treatment of CSOM in China (2012) and
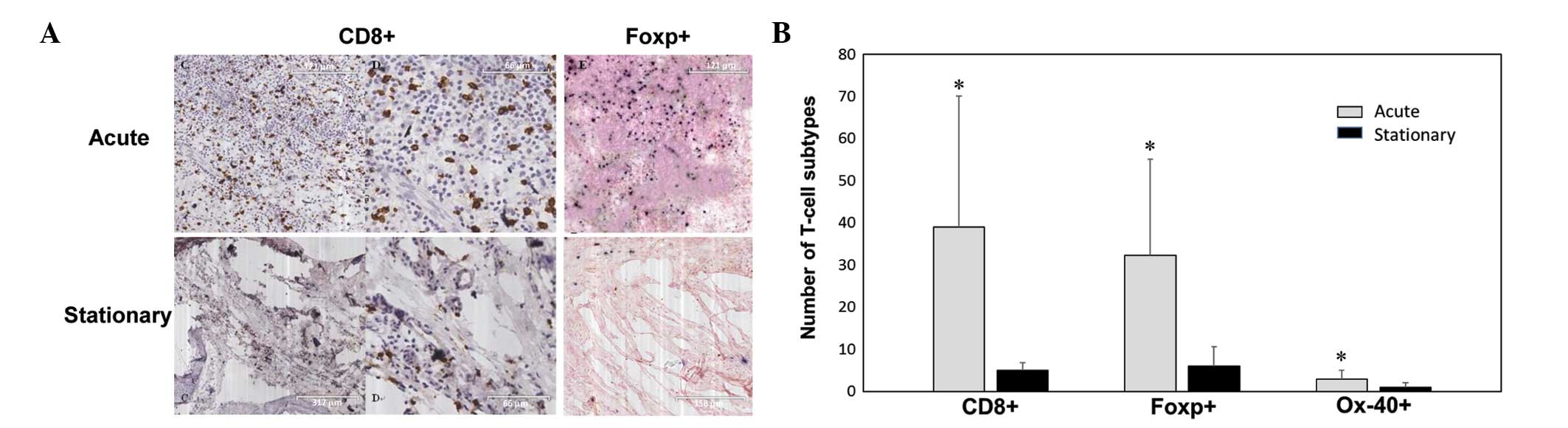
electron microscopy identification of CSOM (4). Fig. 2A shows
the CD8 and FOXP immunohistochemistry staining of granulation
tissues from a patient with 30 years of CSOM and recent ear
discharge (acute phase), and another patient with a 10-year history
of CSOM and without ear discharge in the last 6 months.
There were significantly more CD8+ T
cells (39±31), FOXP3+ Treg cells (32.3±22.8), and
OX40+ cells (2.9±2.1) in the granulation tissues from
the CSOM patients in the acute phase compared to the stationary
phase (all P<0.05, Fig. 2B). There
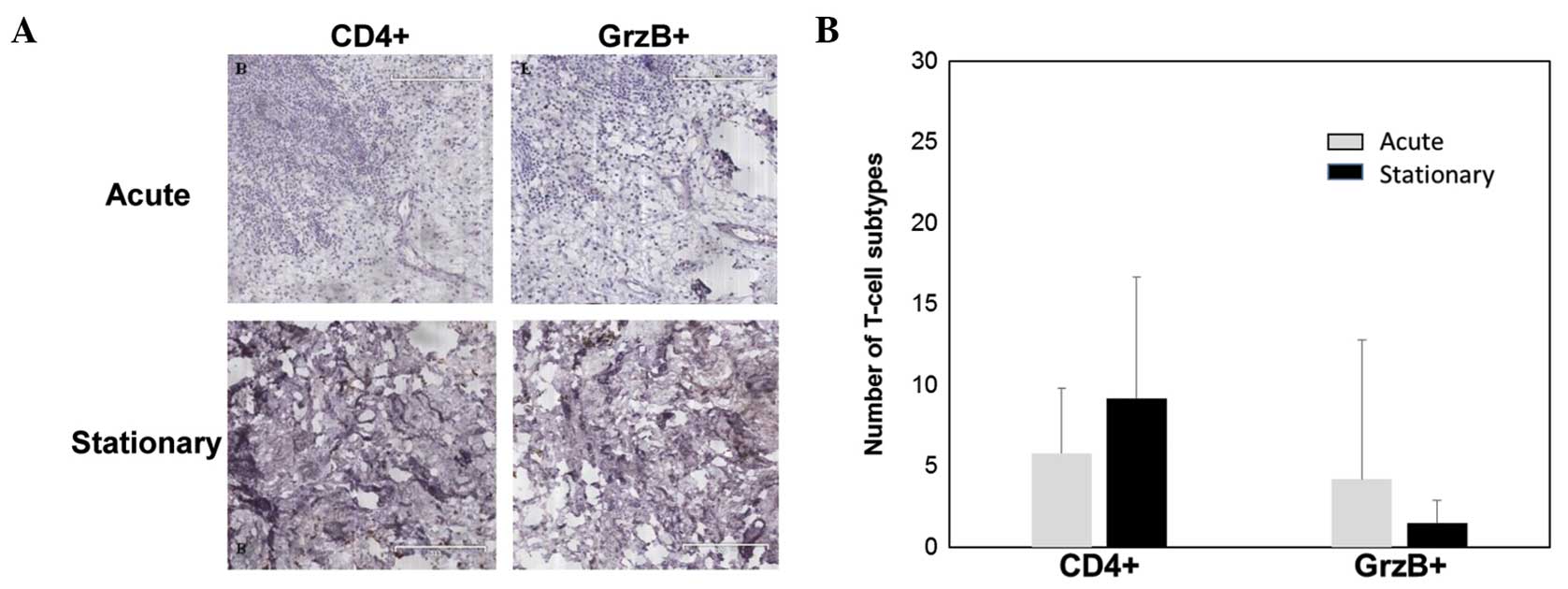
were few CD4+ T cells and granzyme B-positive cells in
the granulation tissues in the acute phase compared to
CD8+ and FOXP+ cells (Fig. 3A). No significant difference was
identified in the number of CD4+ T cells and granzyme
B-positive cells between the granulation tissues in the acute and
stationary phases (P>0.05) (Fig.
3B).
T-cell subtypes in fresh and mature
granulation tissues
As characterized by H&E staining, granulation
tissues were classified into two types. Specimens that exhibited a
large number of capillaries and inflammatory cell infiltration were
classified as fresh granulation tissues (6 cases), while the
remaining specimens were classified as mature granulation tissues
(9 cases). The number of different T-cell subtypes in fresh and
mature granulation tissues was counted. As shown in Table III, there were significantly more
CD8+ T cells (48±30) and FOXP3+ Treg cells
(41.3±22.8) in fresh granulation tissues compared to mature
granulation tissues (P<0.05). By contrast, no significant
differences were identified in the number of CD4+ T
cells, granzyme B-positive cells and OX40+ T cells
between the two groups (P>0.05).
 | Table III.T-cell subtypes in fresh and mature
granulation tissues of chronic suppurative otitis media. |
Table III.
T-cell subtypes in fresh and mature
granulation tissues of chronic suppurative otitis media.
| T-cell
subtypes | Fresh granulation
tissues, no. (n=6) | Mature granulation
tissues, no. (n=9) |
|---|
|
CD4+ | 6.3±4.5 | 7.7±6.6 |
|
CD8+ |
48±30a | 10.3±16.8 |
| Granzyme B
positive | 1.5±0.8 | 4.2±8.6 |
| Forkhead box
P3+ |
41.3±22.8a | 8.8±6.4 |
|
OX40+ | 2.5±2.0 | 1.9±2.0 |
Discussion
CSOM is an inflammatory-related chronic disease in
the middle ear mastoid cavity with different granulation tissue
phases. The present study revealed that the maturity of granulation
tissues had no correlation with the disease course of CSOM. There
was no evidence showing that the longer the duration is, the more
mature the granulation tissues are. In a previous histopathological
study of the temporal bone slice, granulation tissues formed in the
inflammatory drainage in two different ways; and subsequently,
effusion was gradually covered (2).
The purpose of granulation tissue formation is to cover and
organize effusion that could not be completely absorbed. In the
local inflammatory response of CSOM, it is a persistent
pathological course and acts as an important pathological sign.
Scar tissues contain a small amount of fibroblasts and an abundant
amount of collagen, which forms following apoptosis and absorption
of granulation tissues. In this way, each CSOM occurrence leaves a
mark in the middle ear cavity. A mixture of granulation tissues and
scars in the middle ear cavity would form following several acute
attacks of the disease.
Macrophages, T cells and B cells were presented
time-dependently in the acute inflammatory response in acute otitis
media (AOM) (5). Different subtypes of
T lymphocyte were identified in various lesions in patients with
CSOM and cholesteatoma (6).
Macrophages were considered to be the firsT cells to invade the
tissue following AOM induction (5),
and these macrophages in the middle ear may have a profound
influence in regulating immune response in the middle ear in
patients who have otitis media with effusion (7). CD4+ T cells are known as
mature T-helper cells that help regulate immune response and
activate other immune cells by releasing T-cell cytokines. These
cells are essential in B-cell antibody class switching, activation
and growth of cytotoxic T cells, and enhancement of the
bactericidal activity of phagocytes such as macrophages.
CD4+ T cells could be observed in granulation tissues in
all 15 cases in the present study. No significant difference was
observed in the number of CD4+ T cells in different
granulation tissue phases (fresh or mature), or in lesions of CSOM
patients with or without recent ear discharge (acute or stationary
phase).
However, the population of CD4+ T cells
expressing OX40 increased in granulation tissues in the acute phase
of CSOM. OX40, also known as CD134, is a member of the superfamily
of TNF receptors; which belongs to the type I transmembrane
protein. OX40 was initially found on activated CD4+ T
cells (8,9), but subsequent studies reported that OX40
is also expressed on activated CD8+ T cells and
neutrophils (8,10). OX40 expression levels on naive CD4
cells peaked 2–3 days after T-cell receptor (TCR) stimulation, and
were downregulated 4–5 days after the initial TCR stimulation
(11). OX40 expression on activated
CD4+ T cells was known to enable proliferating T cells
to accumulate as differentiated effector cells. However, no
significant difference was identified in the number of
OX40-positive cells in fresh and mature granulation tissues.
FOXP3, also known as scurfin, is a protein involved
in the immune system responses (12).
This protein appears to function as a master regulator in the
development and function of Treg cells, which generally turn down
immune responses (9,13,14).
FOXP3+ is an important surface marker of activated
CD4+CD25+ Treg. Activated FOXP3+
Treg may inhibit T-cell activation and proliferation in immune
responses (12). In the present study,
FOXP3+ Treg expression levels were relatively higher in
granulation tissues, particularly in specimens from patients with
ear discharge within the last 6 months and patients with fresh
granulation tissues. Similar results were reported by Germanidis
et al (15) wherein
FOXP3+ Treg was significantly downregulated in the
remission of chronic hepatitis B virus and FOXP3 transcripts were
positively correlated to the intensity of inflammation. Thus,
higher expression levels of FOXP3+ Treg in acute
inflammation and fresh granulation tissues could avoid excessive
damage during an immune response. It has previously been reported
that although there were no significant differences in
immunoglobulin levels, patients treated with allergen immunotherapy
had lower numbers of FOXP3+ lymphocytes than patients
that were not treated (16). The
percentage of T lymphocyte with IL-7R (CD127 and CD132) increased
in hypertrophic adenoid, which appeared to influence the number and
function of lymphocytes in tonsils; and further influenced the
course of chronic otitis media with effusion (10). The decline of memory T cells was
considered a risk factor of chronic otitis media with effusion in
adolescents (11). A previous study by
Wang et al (17) revealed that
CD4+CD25+FOXP3+ Treg cells were
involved in chronic otitis media with effusion as immune cells for
regulation.
Another critical component of cellular immune
response is CD8+ T lymphocytes, which are effector cells
that could kill infected and cancer cells. In the present study,
CD8+ T cells could be observed in granulation tissues
from all 15 cases, suggesting that T cell-mediated cellular immune
response is involved in the inflammatory immune process of the
disease. Known as cytotoxic T cells, CD8+ T cells could
recognize targeT cells with surface antigen of major
histocompatibility complex class I molecules; and subsequently,
destroy these cells. Once activated, CD8+ T cells would
release perforin and granzymes, which could trigger the caspase
cascade and eventually lead to apoptosis. Granzymes are serine
proteases released by cytoplasmic granules within cytotoxic T
cells, and are natural killer cells. They can induce apoptosis of
virus-infected cells (18). Granzyme B
is one of the most important types of granzyme (19), which is secreted along with the
pore-forming protein, perforin, to mediate apoptosis in targeT
cells (20). A second way to induce
apoptosis is via the binding of the Fas ligand (FasL) expressed on
the surface of activated T cells and Fas molecules expressed on the
surface of targeT cells. The present experimental results revealed
that CD8+ T-cell expression levels were significantly
high, while granzyme B-positive cell expression levels were
relatively low. Therefore, CD8+ T-cell accumulation in
lesions of CSOM may induce apoptosis mainly via the Fas/FasL
pathway.
In conclusion, there was no evident association
between the course of the disease and granulation tissue formation.
T cell-mediated cellular immunity was involved in granulation
tissue formation in CSOM.
Acknowledgements
The present study was financially supported by the
Cancer Research Institute of the Medical School of Xi'an Jiaotong
University.
References
|
1
|
Afolabi OA, Salaudeen AG, Ologe FE,
Nwabuisi C and Nwawolo CC: Pattern of bacterial isolates in the
middle ear discharge of patients with chronic suppurative otitis
media in a tertiary hospital in North central Nigeria. Afr Health
Sci. 12:362–367. 2012.PubMed/NCBI
|
|
2
|
Paparella MM, Schachern PA, Yoon TH,
Abdelhammid MM, Sahni R and da Costa SS: Otopathologic correlates
of the continuum of otitis media. Ann Otol Rhinol Laryngol Suppl.
148:17–22. 1990.PubMed/NCBI
|
|
3
|
da Costa SS, Paparella MM, Schachern PA,
Yoon TH and Kimberley BP: Temporal bone histopathology in
chronically infected ears with intact and perforated tympanic
membranes. Laryngoscope. 102:1229–1236. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaya E, Dag I, Incesulu A, Gurbuz MK, Acar
M and Birdane L: Investigation of the presence of biofilms in
chronic suppurative otitis media, nonsuppurative otitis media, and
chronic otitis media with cholesteatoma by scanning electron
microscopy. ScientificWorldJournal. 2013:6387152013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forséni M, Hansson GK, Bagger-Sjöbäck D
and Hultcrantz M: Infiltration of immunocompetenT cells in the
middle ear during acute otitis media: A temporal study. Am J Otol.
20:152–157. 1999.PubMed/NCBI
|
|
6
|
Kähönen K, Palva T, Bergroth V, Konttinen
YT and Reitamo S: Immunohistochemical identification of
inflammatory cells in secretory and chronic otitis media and
cholesteatoma using monoclonal antibodies. Acta Otolaryngol.
97:431–436. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamanaka T, Bernstein JB, Cumella J,
Parker C and Ogra PL: Immunologic aspects of otitis media with
effusion: Characteristics of lymphocyte and macrophage reactivity.
J Infect Dis. 145:804–810. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baumann R, Yousefi S, Simon D, Russmann S,
Mueller C and Simon HU: Functional expression of CD134 by
neutrophils. Eur J Immunol. 34:2268–2275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gramaglia I, Weinberg AD, Lemon M and
Croft M: Ox-40 ligand: A potent costimulatory molecule for
sustaining primary CD4 T cell responses. J Immunol. 161:6510–6517.
1998.PubMed/NCBI
|
|
10
|
Żelazowska-Rutkowska B, Wysocka J,
Ratomski K, Kasprzycka E and Skotnicka B: Increased percentage of T
cells with the expression of CD127 and CD132 in hypertrophic
adenoid in children with otitis media with effusion. Eur Arch
Otorhinolaryngol. 269:1821–1825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharma SK, Casey JR and Pichichero ME:
Reduced memory CD4+ T-cell generation in the circulation
of young children may contribute to the otitis-prone condition. J
Infect Dis. 204:645–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakaguchi S, Miyara M, Costantino CM and
Hafler DA: FOXP3+ regulatory T cells in the human immune
system. Nat Rev Immunol. 10:490–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Redmond WL, Ruby CE and Weinberg AD: The
role of OX40-mediated co-stimulation in T-cell activation and
survival. Crit Rev Immunol. 29:187–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Devaud C, Darcy PK and Kershaw MH: Foxp3
expression in T regulatory cells and other cell lineages. Cancer
Immunol Immunother. 63:869–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Germanidis G, Argentou N, Hytiroglou P,
Vassiliadis T, Patsiaoura K, Germenis AE and Speletas M: Liver
FOXP3 and PD1/PDL1 expression is down-regulated in chronic HBV
hepatitis on maintained remission related to the degree of
inflammation. Front Immunol. 4:2072013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coria-Ramírez É, Vargas-Camaño ME,
Guido-Bayardo RL, García-Castillo G, Zepeda-García C, Mèndez-Medina
J and Castrejón-Vázquez MI: Levels of CD4+ and
FOXP3+ lymphocytes in patients with allergic rhinitis.
Rev Alerg Mex. 60:5–10. 2013.(In Spanish). PubMed/NCBI
|
|
17
|
Wang DN, Li J, Zhao SQ, Wang Y and Yang L:
Role of FOXP3 CD4 CD25 T cells in otitis media with effusion.
Chinese Archives of Otolaryngology-Head and Neck Surgery.
20:126–128. 2013.
|
|
18
|
Mallett S, Fossum S and Barclay AN:
Characterization of the MRC OX40 antigen of activated CD4 positive
T lymphocytes -a molecule related to nerve growth factor receptor.
EMBO J. 9:1063–1068. 1990.PubMed/NCBI
|
|
19
|
Ewen CL, Kane KP and Bleackley RC: A
quarter century of granzymes. Cell Death Differ. 19:28–35. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chowdhury D and Lieberman J: Death by a
thousand cuts: Granzyme pathways of programmed cell death. Annu Rev
Immunol. 26:389–420. 2008. View Article : Google Scholar : PubMed/NCBI
|