Introduction
During cerebral ischemia/reperfusion, the level of
the calcitonin gene-related peptide (CGRP) in the neurons of the
brain tissue shows a relative increase. CGRP has a role in the
dilation of the cerebral blood vessels and has an important
protective function in neuronal cells (1,2). The
exogenous supplementation of the CGRP can improve cerebral ischemia
(3). However, the specific mechanism
of action of the CGRP in the brain tissue remains to be elucidated.
Currently, studies have been published regarding the function of
the mitogen-activated protein kinase (MAPK) signal transduction
pathways in cerebral ischemia/reperfusion, including the
extracellular signal-regulated protein kinase (ERK), the c-Jun
N-terminal kinase (JNK) and the p38 pathways (4,5). However,
the association between the protective effect of CGRP on brain
tissues and these three signaling pathways remains to be
elucidated. Therefore, the present study used cerebral
ischemia/reperfusion rats as the study subjects to detect the
expression levels of proteins in the aforementioned pathways and
brain infarction areas to investigate the protective function of
CGRP in the brain tissue of cerebral ischemia/reperfusion rats and
the association of CGRP with the JNK, P38 and ERK signaling
pathways.
Materials and methods
Animals and reagents
All animal manipulations were approved by the Ethics
Committee of Jilin University (Changchun, China). Healthy male
Wistar rats (provided by the Experimental Animal Center of Jilin
University) with body weights of 250–300 g were selected. The
reagents, including CGRP, the CGRP inhibitor CGRP8–37, the ERK
inhibitor PD98059, and the p38 inhibitor SB203580, were purchased
from Sigma-Aldrich (St. Louis, MO, USA). The animals were randomly
divided into 6 groups: i) The sham-surgery group (sham group), ii)
the middle cerebral artery occlusion (MCAO) group, iii) the
MACO-CGRP group, iv) the MCAO+CGRP+CGRP8–37 group, v) the
MCAO+CGRP+PD98059 group, and vi) the MCAO+CGRP+SB203580 group. Each
group comprised of 24 animals. Drugs were administered according to
the design of each group. CGRP (3 g/kg) was intravenously injected;
CGRP8–37 (2.5 mg/kg) was intravenously injected; PD980549 (1 mg/kg)
was intraperitoneally injected; and SB203580 (5 mg/kg dissolved in
5 mg/ml dimethyl sulfoxide) was intraperitoneally injected.
Establishment of a cerebral
ischemia/reperfusion model in rats
Before the start of the experiments, the animals
were fasted for 12 h and water deprived for 4 h. Following chloral
hydrate anesthesia and disinfection, the carotid, internal carotid
and external carotid arteries were separated. One thread of nylon
monofilament line coated with poly-L-lysine was inserted along the
internal carotid artery; when resistance was sensed, the
monofilament was assumed to have reached the initial site of the
middle cerebral artery. Muscle and skin were temporarily full-layer
sutured. After 2 h of cerebral ischemia, the nylon line was removed
and the incision was sutured. Subsequently, reperfusion was
performed for 24 h. Following surgery, the rats were placed under
an illuminating lamp to maintain the body temperature of the rats
between 37–37.5°C (6).
Neurobehavioral scoring of the
rats
After the rats had awakened, the neurobehavioral
function of the rats was observed during the reperfusion period.
The Longa scoring standards were adopted, in which the neurological
findings were scored on a 5-point scale: A score of 0 indicated no
neurological deficit; a score of 1 (failure to extend left forepaw
fully), a mild focal neurological deficit; a score of 2 (circling
to the left), a moderate focal neurological deficit; a score of 3
(falling to the left), a severe focal deficit; and a score of 4,
inability to walk spontaneously and a depressed level of
consciousness (7). Animals with scores
between 1 and 3 points were selected as experimental subjects.
Animals with a score of 0 or 4 points indicated the model
establishment was not successful, and these animals were excluded.
Animals with scores that met the above requirements but experienced
a subarachnoid hemorrhage were excluded, and new animals were
supplemented.
2,3,5-triphenyltetrazolium chloride
(TTC) staining and measurement of the cerebral infarction
areas
For the TTC staining method, subsequent to the rats
being anesthetized, the brain was obtained by decapitation. The
olfactory bulb and lower brain stem were separated and carefully
removed. After being rapid-frozen in a −20°C freezer for ~20 min,
the brain tissues were sliced into five pieces with a thickness of
~2 mm. The tissues were stained in TTC buffer for ~30 min and were
fixed in 4% paraformaldehyde buffer. Normal tissues showed a pink
or red color, and ischemic tissues were white (8). Images were captured using a
high-resolution camera. The infarction area in the brain tissues
was calculated using ImageJ software (9).
Detection of protein expression in the
brain tissues by western blotting
After reperfusion for 24 h, the brain tissues were
isolated from the ischemic penumbra cortices and homogenized in ice
cold buffer [1.5 mmol/l Tris based-HCl (pH 7.6), 1 mmol/l
dithiothreitol, 0.25 mol/l sucrose, 1 mmol/l MgCl2, 1.25
µg/ml pepstatin A, 10 µg/ml leupeptin, 2.5 µg/ml aprotinin, 0.5
mmol/l phenylmethane sulfonyl fluoride, 2.5 mmol/l
ethylenediaminetetraacetic acid, 1 mmol/l ethylene glycol
tetraacetic acid, 0.1 mol/l Na3VO4, 50 mmol/l
NaF and 2 mmol/l sodium pyrophosphate]. The homogenates were
centrifuged (1,000 × g for 20 min at 4°C) and the protein
concentration of the supernatants (containing cytoplasm) and
pellets (containing nucleus) was measured. Western blotting was
conducted with 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis. Following electrophoresis, the proteins were
transferred onto polyvinylidene fluoride membranes. The membranes
were probed with the following primary antibodies: Anti-JNK (cat.
no. sc-571; 1:500, polyclonal rabbit anti-rat), anti-p-JNK (cat.
no. sc-135642; 1:500, polyclonal rabbit anti-rat), anti-p38 (cat.
no. sc-535; 1:500, polyclonal rabbit anti-rat), anti-p-p38 (cat.
no. sc-17852-R; 1:500, polyclonal rabbit anti-rat), and
anti-β-actin (cat. no. sc-130657; 1:500, polyclonal rabbit
anti-rat) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
The horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (cat. no. 7074; 1:2,000, polyclonal goat anti-rabbit) was
obtained from Cell Signaling Technology (Danvers, MA, USA).
Following incubation with horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G, the blots were washed, and the
immunoreactive proteins were visualized on Kodak X-omat LS film
(Eastman Kodak Co., New Haven, CT, USA) with enhanced
chemiluminescence. The densitometry was performed with Kodak ID
image analysis software (Eastman Kodak Co.).
Statistical analysis
The statistical analyses were performed using SPSS
21.0 (IBM, Corp., Armonk, NY, USA). Measurement data are
represented as mean ± standard deviation. The data were analyzed by
analysis of variance, followed by SNK test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Neurobehavioral scores of the
rats
Compared to the MCAO group, the neurobehavioral
score of the MCAO+CGRP group was low. Compared to the MCAO+CGRP
group, the neurobehavioral scores of the MCAO+CGRP+CGRP8–37,
MCAO+CGRP+PD98059 and MCAO+CGRP+SB20358 groups increased
significantly (Table I).
 | Table I.Effects of CGRP on the neurobehavioral
scores of rats with focal cerebral ischemia. |
Table I.
Effects of CGRP on the neurobehavioral
scores of rats with focal cerebral ischemia.
| Group | Neurobehavioral
scores |
|---|
| Sham |
0.00±0.00 |
| MCAO |
3.75±0.36a |
| MCAO+CGRP |
2.13±0.29a,b |
|
MCAO+CGRP+CGRP8–37 |
3.50±0.33a,c |
|
MCAO+CGRP+SB203580 |
3.25±0.31a,c |
|
MCAO+CGRP+PD98059 |
3.63±0.38a,c |
TTC staining and infarction areas in
the brain tissues
Compared to the sham group, the area of white in the
TTC staining of the brain tissues in the MCAO group significantly
increased. Compared to the MCAO group, the area of white in the
MCAO+CGRP group significantly decreased. Compared to the MCAO+CGRP
group, the area of white in the 3 groups with the addition of the
inhibitor groups (MCAO+CGRP+CGRP8–37, MCAO+CGRP+PD9805 and
MCAO+CGRP+SB203580) significantly increased (Fig. 1 and Table
II).
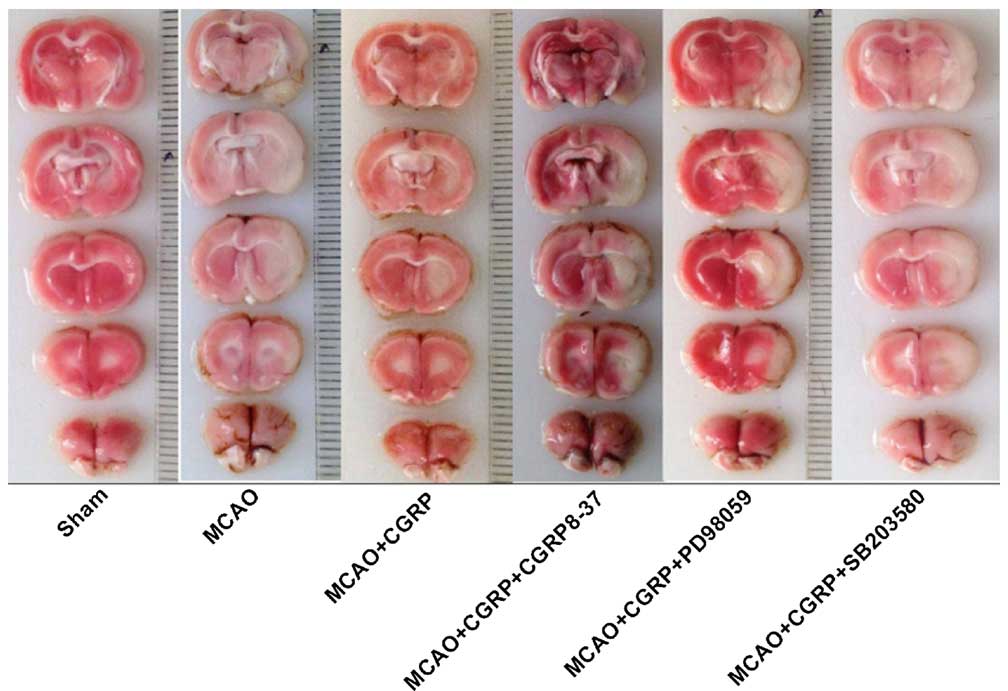 | Figure 1.TTC staining in all the treatment
groups. Normal tissues were a pink or red color, whereas the
ischemic tissues were white. Compared to the sham group, the white
area of TTC staining in the brain tissues in the MCAO group
significantly increased. Compared to the MCAO group, the white area
in the MCAO+CGRP group significantly decreased. Compared to the
MCAO+CGRP group, the white area in the MCAO+CGRP+CGRP8–37,
MCAO+CGRP+PD9805 and MCAO+CGRP+SB203580 groups significantly
increased. TTC, 2,3,5-triphenyltetrazolium chloride; CGRP,
calcitonin gene-related peptide; MCAO, middle cerebral artery
occlusion. |
 | Table II.Effects of CGRP on the cerebral
infarction area of rats with focal cerebral ischemia. |
Table II.
Effects of CGRP on the cerebral
infarction area of rats with focal cerebral ischemia.
| Group | Infarction area,
% |
|---|
| Sham |
0.0±0.0 |
| MCAO |
40.1±2.5a |
| MCAO+CGRP |
22.7±1.3a,b |
|
MCAO+CGRP+CGRP8–37 |
35.2±2.8a,c |
|
MCAO+CGRP+SB203580 |
34.2±2.6a,c |
|
MCAO+CGRP+PD98059 |
35.3±3.4a,c |
Western blotting results
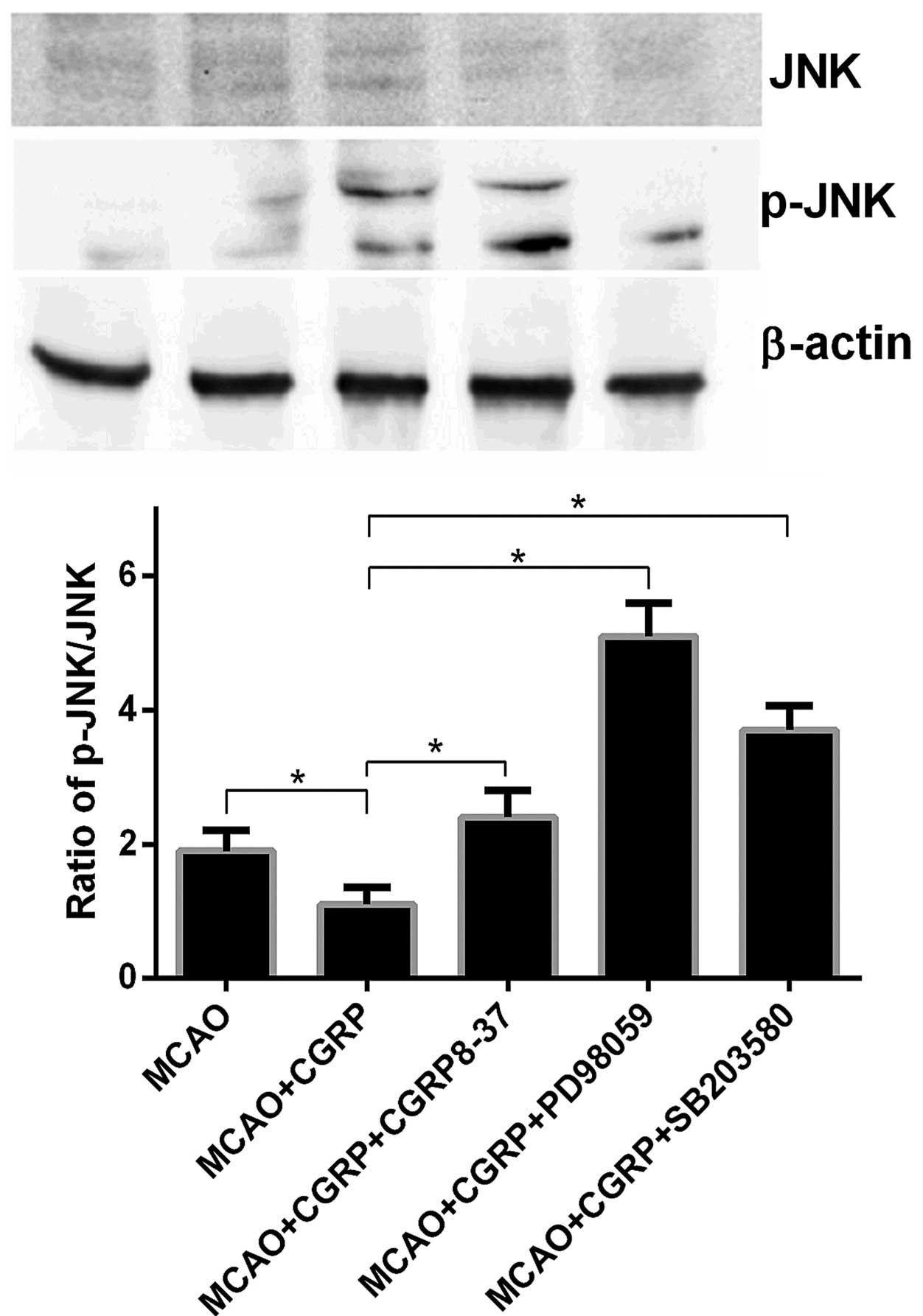
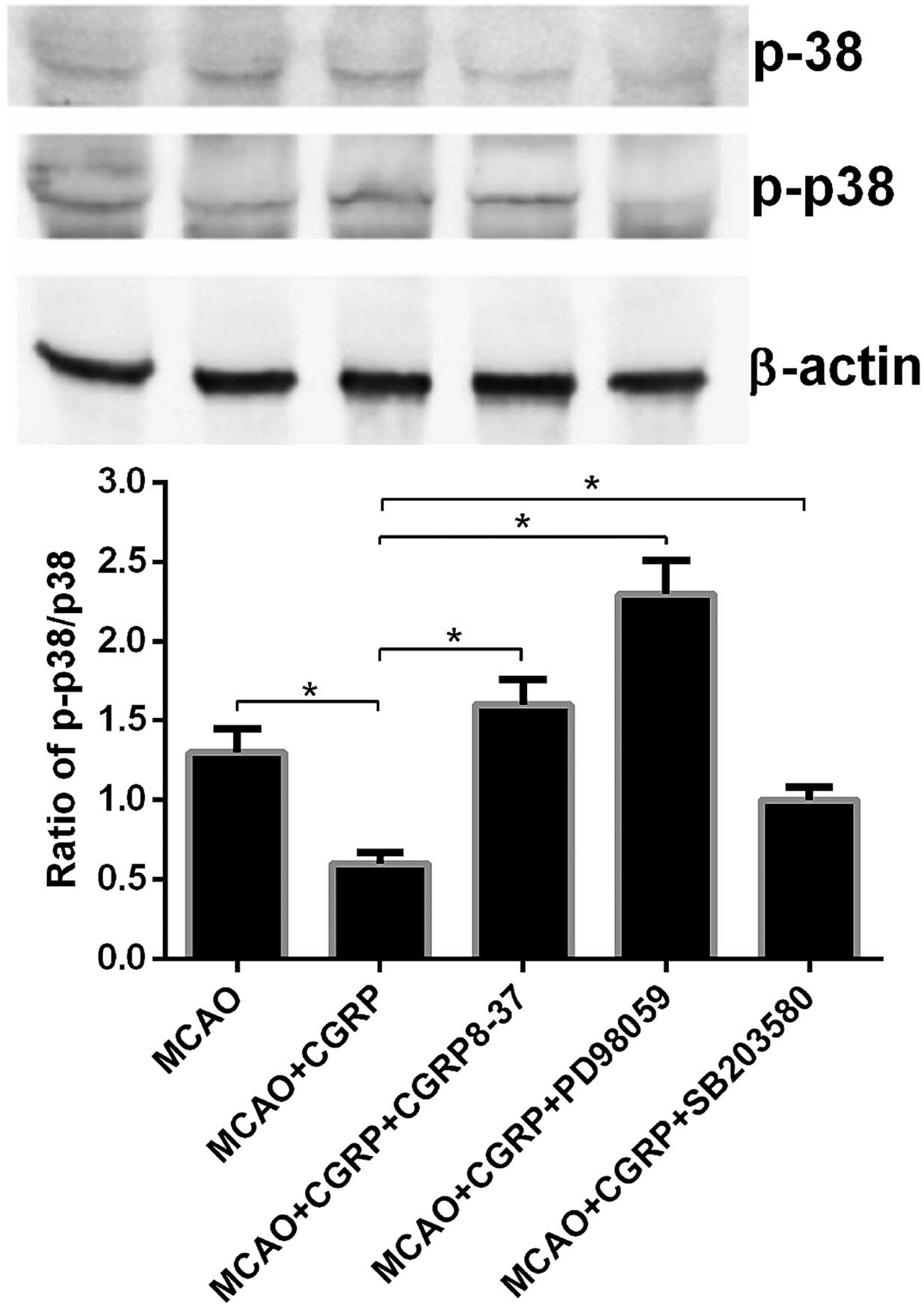
p-JNK, JNK, p-p38, p38, p-ERK and ERK were detected.
Compared to the sham group, the expression levels of p-JNK and
p-p38 in the brain tissues in the MCAO group significantly
increased (P<0.05), whereas no significant change was observed
for the expression levels of JNK and p38. Compared to the MCAO
group, the expression levels of p-JNK and p-p38 in the MCAO+CGRP
group significantly decreased (P<0.05); in addition, no
significant changes in the expression levels of JNK and p38 were
identified. Compared to the MCAO+CGRP group, the expression levels
of p-JNK and p-p38 significantly increased in the
MCAO+CGRP+CGRP8–37, MCAO+CGRP+PD98059 and MCAO+CGRP+SB203580 groups
(P<0.05), whereas no significant change in the protein
expression levels of JNK and p38 was identified (Figs. 2 and 3).
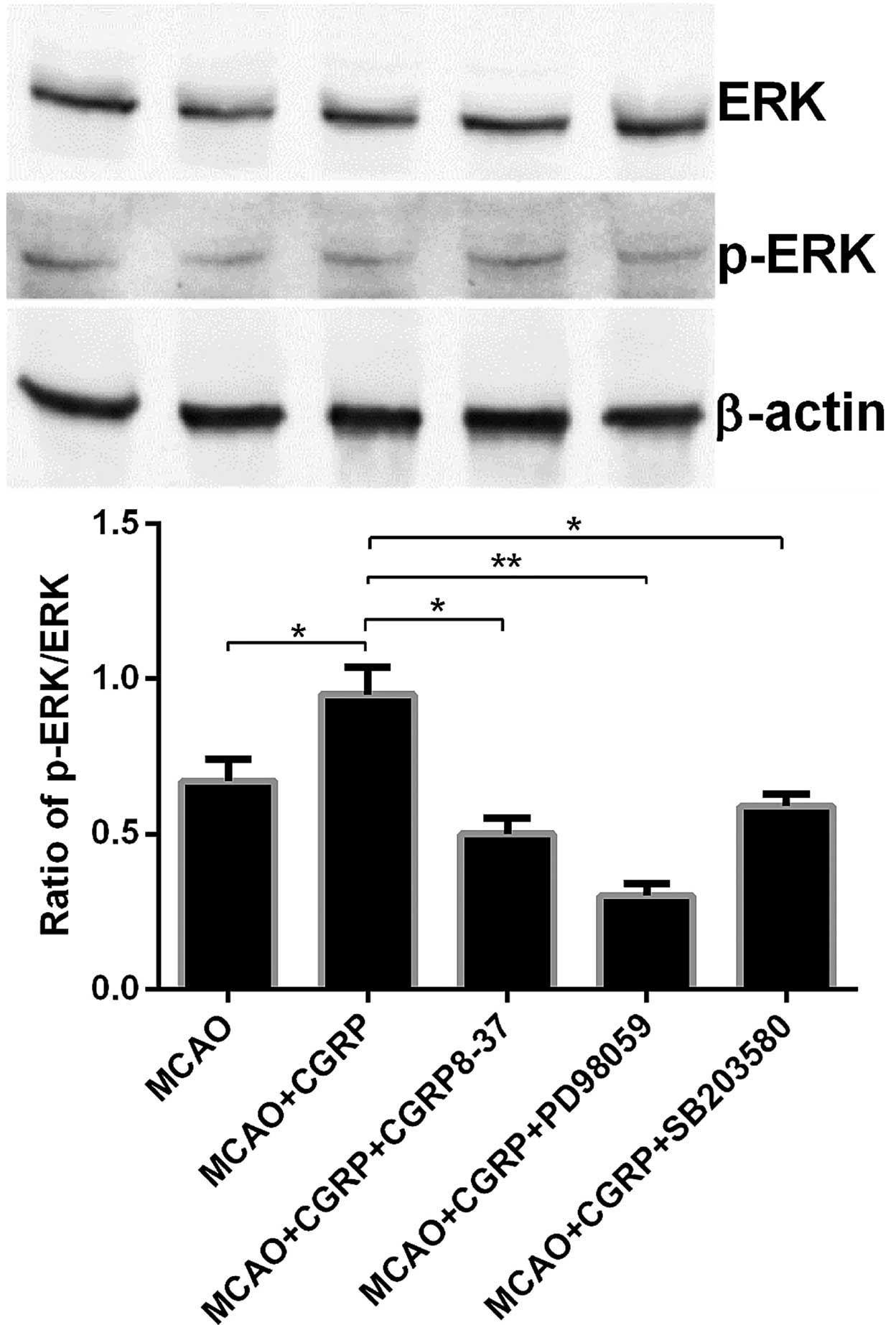
Compared to the sham group, the expression level of
p-ERK in the brain tissues in the MCAO groups significantly
decreased (P<0.05), whereas no significant change in the ERK
expression level was identified. Compared to the MCAO group, p-ERK
expression in brain tissues in the MCAO+CGRP group significantly
increased (P<0.05); whereas no significant change in ERK
expression was identified. Compared to the MCAO+CGRP group, the
expression level of p-ERK in the MCAO+CGRP+CGRP8–37,
MCAO+CGRP+PD98059 and MCAO+CGRP+SB203580 groups significantly
decreased (P<0.05); the reduction in the MCAO+CGRP+PD98059 group
was evident; and no significant change in the ERK expression level
was identified (Fig. 4).
Discussion
CGRP is a polypeptide composed of 37 amino acids,
and it is extensively expressed in the central and peripheral
nervous systems. Currently, CGRP is considered the most potent
vasodilator (10,11). CGRP serves a protective function in
brain tissues. When brain tissues suffer ischemia, they are induced
to produce numerous self-protective substances; of these, CGRP is
one of the most important (3). Studies
have shown that during cerebral ischemia/reperfusion, the CGRP
level in the neuronal cells in the brain tissue exhibits a relative
increase. This increase is intended to cause dilation of the
cerebral blood vessels to provide blood supply to the ischemic
brain tissue as quickly as possible. However, when the cerebral
ischemia was relatively severe, the CGRP produced by the brain
tissues could not completely defend this injury; therefore, severe
cerebral ischemia occurred (12).
Thus, the concept that an increase in exogenous CGRP could relieve
ischemia/reperfusion brain injury was a possibility. The results
from the present study show that, in an ischemia/reperfusion rat
model, the intravenous injection of exogenous CGRP can effectively
improve the neurobehavioral function of rats and increase their
quality of life. Pathological examination of the brain tissue
showed that CGRP effectively decreased the infarction area in the
rat brain tissue induced by the suture. These results could be
blocked by the CGRP inhibitor CGRP8–37. Although CGRP could
effectively relieve nerve injury caused by ischemia/reperfusion,
the specific mechanism of the CGRP remains to be elucidated.
Studies have shown that in the cerebral
ischemia/reperfusion injury model, the MAPK signal transduction
pathways have important roles in cell apoptosis (13–15). The
MAPK signal transduction pathways mainly include the ERK, JNK and
p38 pathways. The functions of these three signaling pathways in
cerebral ischemia/reperfusion are different (16–18). Studies
have shown that the JNK signaling pathway has an important role in
the programmed cell death process following cerebral
ischemia/reperfusion injury and can promote cell apoptosis
(19). The p38 signaling pathway is
activated at the early stage of cerebral ischemia to allow
participation in free radical injury, cell apoptosis, cytotoxicity,
and the inflammatory reaction (20).
The p38 inhibitor has an important protective function in neurons
(16). The classic ERK signaling
pathway has important roles in numerous diseases such as myocardial
infarction, tumor and diabetes mellitus (21–23).
Following cerebral ischemia/reperfusion, the ERK signaling pathway
was activated to reduce the NMDA receptor activity in cerebral
ischemia/reperfusion injury, inhibit the intracellular Ca2+ influx,
and has an important protective role in neuronal function (4). These pathways also should experience
similar changes in the cerebral ischemia/reperfusion models in
rats. However, the mechanism underlying the protection of brain
tissues by CGRP and the association between this protective
function and the three signaling pathways of the MAPK family remain
to be elucidated. The present study exogenously supplemented CGRP
and used the CGRP inhibitor CGRP8–37, the ERK inhibitor PD98059 and
the p38 inhibitor SB203580 to investigate this association.
The results of the study showed that in the cerebral
ischemia/reperfusion model in rats, an exogenous increase in the
CGRP significantly increased the phosphorylation level of ERK as
shown by western blotting. In addition, TTC staining showed that
the infarction area significantly decreased. When the ERK inhibitor
PD98059 and CGRP were administered together, the western blotting
results showed that the phosphorylation level of ERK significantly
decreased. In addition, TTC staining showed that the infarction
area in the brain tissues significantly increased. These results
indicate that an exogenous increase in CGRP could exert a
protective function in nerve cells; however, following the
inhibition of the ERK signaling pathway, the protective function of
CGRP was no longer evident. Therefore, the CGRP function may be
achieved through the activation of the ERK pathway and the
promotion of its phosphorylation. Analysis of the JNK and p38
signaling pathways showed that following the administration of
CGRP, the phosphorylation levels of the JNK and p38 significantly
decreased according to western blotting. In addition, the TTC
staining results showed that the infarction area significantly
decreased. The p38 inhibitor SB203580 could reverse the protective
function of CGRP; thus, the p38 inhibitor could influence the
phosphorylation of JNK and p38 simultaneously. Therefore, the
present study demonstrated that in the cerebral
ischemia/reperfusion model, the JNK and p38 signaling pathways were
significantly activated, whereas the ERK signaling pathway
experienced certain inhibitions. When the CGRP level in the brain
tissues was exogenously increased, the expression levels of p-JNK
and p-p38 decreased, and the protein expression level of p-ERK
increased. In addition, CGRP could significantly improve the
neuronal function in the brain tissues and decrease the occurrence
of cell apoptosis. Therefore, CGRP may achieve the protective
function on neurons in the brain tissues via its influence on the
JNK, p38 and ERK signaling pathways. With the addition of the
inhibitors of these three MAPK pathways, the protective function of
the exogenously increased CGRP attenuated or disappeared,
indicating that these three pathways are interdependent. The
protective function of CGRP could not be achieved when any one of
these pathways was inhibited.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81200888).
References
|
1
|
Zhang ZH, Fang XB, Xi GM, Li WC, Ling HY
and Qu P: Calcitonin gene-related peptide enhances CREB
phosphorylation and attenuates tau protein phosphorylation in rat
brain during focal cerebral ischemia/reperfusion. Biomed
Pharmacother. 64:430–436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Z, Liu Q, Cai H, Xu C, Liu G and Li Z:
Calcitonin gene-related peptide prevents blood-brain barrier injury
and brain edema induced by focal cerebral ischemia reperfusion.
Regul Pept. 171:19–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shao B, Zhou YL, Wang H and Lin YS: The
role of calcitonin gene-related peptide in post-stroke depression
in chronic mild stress-treated ischemic rats. Physiol Behav.
139:224–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou J, Du T, Li B, Rong Y, Verkhratsky A
and Peng L: Crosstalk between MAPK/ERK and PI3K/AKT signal pathways
during brain ischemia/reperfusion. ASN Neuro. 7:72015. View Article : Google Scholar
|
|
5
|
Wei SG, Yu Y, Weiss RM and Felder RB:
Inhibition of brain mitogen-activated protein kinase signaling
reduces central endoplasmic reticulum stress and inflammation and
sympathetic nerve activity in heart failure rats. Hypertension.
67:229–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagasawa H and Kogure K: Correlation
between cerebral blood flow and histologic changes in a new rat
model of middle cerebral artery occlusion. Stroke. 20:1037–1043.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bederson JB, Pitts LH, Germano SM,
Nishimura MC, Davis RL and Bartkowski HM: Evaluation of
2,3,5-triphenyltetrazolium chloride as a stain for detection and
quantification of experimental cerebral infarction in rats. Stroke.
17:1304–1308. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mistrova E, Wiegand S, Sviglerova J, Pfeil
U, Kuncova J, Slavikova J, Kummer W and Chottova Dvorakova M:
Adrenomedullin and the calcitonin receptor-like receptor system
mRNA expressions in the rat heart and sensory ganglia in
experimentally-induced long-term diabetes. Gen Physiol Biophys.
33:215–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hagner S, Stahl U, Knoblauch B, McGregor
GP and Lang RE: Calcitonin receptor-like receptor: Identification
and distribution in human peripheral tissues. Cell Tissue Res.
310:41–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai H, Xu X, Liu Z, Wang Q, Feng G, Li Y,
Xu C, Liu G and Li Z: The effects of calcitonin gene-related
peptide on bFGF and AQP4 expression after focal cerebral ischemia
reperfusion in rats. Pharmazie. 65:274–278. 2010.PubMed/NCBI
|
|
13
|
Zhang CX, Liu JX, Li D, Li L, Fu JH, Hou
JC, Du XM and Zhang FC: Effect of guanmaitong tablet on ERK and p38
protein of TLR2 pathway expression in cerebral ischemia/reperfusion
rats: An experimental study. Zhongguo Zhong Xi Yi Jie He Za Zhi.
35:712–716. 2015.(In Chinese). PubMed/NCBI
|
|
14
|
Nozaki K, Nishimura M and Hashimoto N:
Mitogen-activated protein kinases and cerebral ischemia. Mol
Neurobiol. 23:1–19. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun X, Liu C, Qian M, Zhao Z and Guo J:
Ceramide from sphingomyelin hydrolysis differentially mediates
mitogen-activated protein kinases (MAPKs) activation following
cerebral ischemia in rat hippocampal CA1 subregion. J Biomed Res.
24:132–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piao CS, Kim JB, Han PL and Lee JK:
Administration of the p38 MAPK inhibitor SB203580 affords brain
protection with a wide therapeutic window against focal ischemic
insult. J Neurosci Res. 73:537–544. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonny C, Borsello T and Zine A: Targeting
the JNK pathway as a therapeutic protective strategy for nervous
system diseases. Rev Neurosci. 16:57–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haddad JJ: The role of Bax/Bcl-2 and
pro-caspase peptides in hypoxia/reperfusion-dependent regulation of
MAPK(ERK): Discordant proteomic effect of MAPK(p38). Protein Pept
Lett. 14:361–371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen CP, Tsimberg Y, Salvadore C and
Meller E: Activation of Erk and JNK MAPK pathways by acute swim
stress in rat brain regions. BMC Neurosci. 5:362004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piao CS, Che Y, Han PL and Lee JK: Delayed
and differential induction of p38 MAPK isoforms in microglia and
astrocytes in the brain after transient global ischemia. Brain Res
Mol Brain Res. 107:137–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang M, Wang L and Jiang HH: Role of
spinal MAPK-ERK signal pathway in myocardial ischemia-reperfusion
injury. Zhongguo Dang Dai Er Ke Za Zhi. 15:387–391. 2013.(In
Chinese). PubMed/NCBI
|
|
22
|
Anfuso CD, Motta C, Giurdanella G, Arena
V, Alberghina M and Lupo G: Endothelial PKCα-MAPK/ERK-phospholipase
A2 pathway activation as a response of glioma in a triple culture
model. A new role for pericytes? Biochimie. 99:77–87. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim TK, Lee JS, Jung HS, Ha TK, Kim SM,
Han N, Lee EJ, Kim TN, Kwon MJ, Lee SH, et al: Triiodothyronine
induces proliferation of pancreatic β-cells through the MAPK/ERK
pathway. Exp Clin Endocrinol Diabetes. 122:240–245. 2014.
View Article : Google Scholar : PubMed/NCBI
|