Introduction
Previous studies indicated that periodontitis may be
associated with a higher risk of coronary heart disease (CAD)
(1–4),
independent of established cardiovascular risk factors.
Periodontitis is a chronic tissue-destruction inflammatory state
that is predominantly induced by Porphyromonus gingivalis
(P. gingivalis) in the gingival pockets of certain
individuals with advanced and severe periodontal disease.
P. gingivalis may promote transient
bacteremia during tooth brushing, chewing or dental procedures
(5–7).
Certain studies have identified that P. gingivalis was
detected frequently in atheromatous plaques of the aorta and
coronary artery, and it was reported to perpetuate systemic
inflammation (8–10). Additionally, P. gingivalis
induces macrophage foam cell formation (11) and stimulates oxidation of low-density
lipoprotein (12). Certain studies
show that P. gingivalis lipopolysaccharide (LPS) could
induce the expression of intercellular adhesion molecule 1 and
vascular cell adhesion molecule 1 in human umbilical vein
endothelial cells (HUVECs) (13,14), which
significantly enhances trans-endothelial migration of inflammatory
cells.
Furthermore, atherosclerosis can be triggered and
aggravated by the pathogen-driven antigenic peptide from P.
gingivalis heat-shock protein 60 (HSP60) (15–17). An
overall 55% homology exists between human and bacterial HSP60 that
can even reach 72% at certain domains of the 573-amino-acid-long
molecule (18). P. gingivalis
HSP60 is reported to accelerate the development of experimental
atherosclerosis by cross-reactivity of the immune response to
bacterial HSPs (19). However, Jeong
et al (20) found that P.
gingivalis HSP60 peptides have distinct roles in the
development of atherosclerosis; peptide 14 or 19 from P.
gingivalis HSP60 may have either an anti- or pro-atherogenic
role, respectively, in the ApoE(−/-) mouse model of
infection-triggered atherosclerosis through distinct mechanisms
operating in the polarization of T cells. Additionally, in a
clinical study, a strong positive correlation was found between
high levels of soluble HSP60 and the risk of CAD (21). Soluble HSP60 levels directly correlate
with the presence of classic risk factors of atherosclerosis, such
as elevated low-density lipid cholesterol levels, and with
particular proinflam¬matory markers, such as tumor necrosis
factor-α (22).
However, the potential pathways linking
periodontitis and cardiovascular disease remain to be elucidated
(23–25)
and the underlying molecular mechanisms from P. gingivalis
HSP60 regarding the association between periodontitis and
atherosclerosis require further investigation. In the present
study, the aim was to investigate whether P. gingivalis
HSP60 treatment leads to the dysfunction of HUVECs directly by
affecting the protein expression levels of endothelial nitric oxide
synthase (eNOS) and vascular endothelial (VE)-cadherin.
Materials and methods
Cell culture
HUVECs were kindly provided as a gift by Dr Yun Mu
(Tianjin Medical University, Tianjin, China). Cell culture media
and supplements were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Fetal bovine serum (FBS) was
purchased from Gibco (Thermo Fisher Scientific, Inc.). P.
gingivalis HSP60 was purchased from Hongling Longcheng
Technology Co., Ltd. (Beijing, China). The cells were cultured in
RPMI-1640 medium supplemented with 10% FBS at 37°C in a humidified
incubator with 5% CO2. The culture medium was exchanged
every 48 h. HUVECs up to passage 6 were used for the
experiments.
Cell viability
Cell viability was determined using the MTT assay.
HUVECs were seeded in 96-well culture plates at a density of
0.5×104 cells/well and incubated overnight at 37°C.
Following treatment with P. gingivalis HSP60 at different
concentrations (1, 10 and 100 ng/l), cells were incubated with 5
mg/ml MTT for 24 h. Subsequently, the MTT-containing growth medium
was replaced with 100 µl of dimethyl sulfoxide (DMSO) and mixed
thoroughly for 10 min. The optical density readings of each well
were determined at 570 nm using a microplate reader (ELx808; BioTek
Instruments, Inc., Winooski, VT, USA). The effect of P.
gingivalis HSP60 on cell viabilities was expressed as the
percentage of viable cells in the treated groups compared to the
DMSO control. Values [mean ± standard deviation (SD)] are from
three independent experiments.
Sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and western blot analysis
The cell layer was washed with 3 ml of
phosphate-buffered saline (PBS) twice. Following treatment (1, 10
and 100 ng/l P. gingivalis HSP60 for 2, 6, 12 or 24 h), the
cells were homogenized in an ice bath using sonification (3 times
for 15 sec, 50 Hz) with 1 ml of radioimmunoprecipitation assay
lysis buffer containing 400 µl of protease inhibitors of
phenylmethylsulfonyl fluoride (Thermo Fisher Scientific, Inc.). The
homogenate was collected and centrifuged at 12,000 × g for 15 min,
and the supernatant was used as a lysate for further
determinations. Protein concentration was determined by the BCA™
protein assay kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Western blot analysis was performed as
described previously (26). Equal
amounts of cellular protein (30 µg) underwent electrophoresis on a
gradient SDS-PAGE (4–10% gel) and the samples were
electrotransferred onto nitrocellulose membranes in a buffer
consisting of 25 mM Tris, 192 mM glycine and 20% methanol (pH 8.4)
for 2 h at a constant voltage (100 V) with cooling. The following
primary antibodies were used for blotting: β-actin (cat. no.
M20010; monoclonal mouse anti-human; 1:500; Abmart, Inc., Berkeley
Heights, NJ, USA), VE-cadherin (cat. no. SAB1306131; polyclonal
rabbit anti-human; 1:500; Sigma-Aldrich, St. Louis, MO, USA), eNOS
(cat. no. SAB4502013; polyclonal rabbit anti-human; 1:5,000;
Sigma-Aldrich), caspase-3 (cat. no. C9598; polyclonal rabbit
anti-human; 1:500; Sigma-Aldrich) and cleaved caspase-3 (cat. no.
SAB4503294; polyclonal rabbit anti-human; 1:500; Sigma-Aldrich).
The secondary antibodies include IRDye (1:3,000; Abmart, Inc.).
Immunocomplexes were detected using the ECL western blotting
detection kit (GenMed, Inc., Houston, TX, USA). All the other
reagents were purchased from Sigma-Aldrich. The specific proteins
were visualized by an Odyssey™ infrared imaging system (LI-COR,
Inc., Lincoln, NE, USA).
Flow cytometry analysis for apoptosis
quantification
Following the designated treatment (1, 10 and 100
ng/l P. gingivalis HSP60), annexin V-fluorescein
isothiocyanate-conjugated (FITC)/propidium iodide (PI) apoptosis
detection kit (Invitrogen, Thermo Fisher Scientific, Inc.) was used
according to the manufacturer's protocol. In brief, the cells were
centrifuged at 300 × g for 5 min, washed with cold PBS, and
resuspended in 100 µl of binding buffer. Annexin V-FITC (5 µl) and
PI (5 µl) were added to each sample, and the mixture was incubated
at 4°C in the dark for 15 min. The cells were immediately subjected
to fluorescence-activated cell sorting analysis (BD Accuri C6; BD
Biosciences, San Jose, CA, USA) within 1 h. For cells in the early
apoptotic stage, membrane phosphatidylserine was exposed and
combined with annexin V. The cells were stained with annexin V with
no PI fluorescence and recorded as annexin V (+)/PI (−). The
membranes of dead cells and cells in the late apoptotic stage were
permeable to PI. These cells were stained with annexin V and PI and
were recorded as annexin V (+)/PI (+). Finally, annexin V (+)/PI
(−) and annexin V (+)/PI (+) cells were detected under flow
cytometry, and the percentages of the total number of cells in each
group were compared.
Statistical analysis
Statistical analyses were performed with SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Results are provided as
mean ± SD. One-way or two-way analysis of variance tests were
applied to compare the different groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
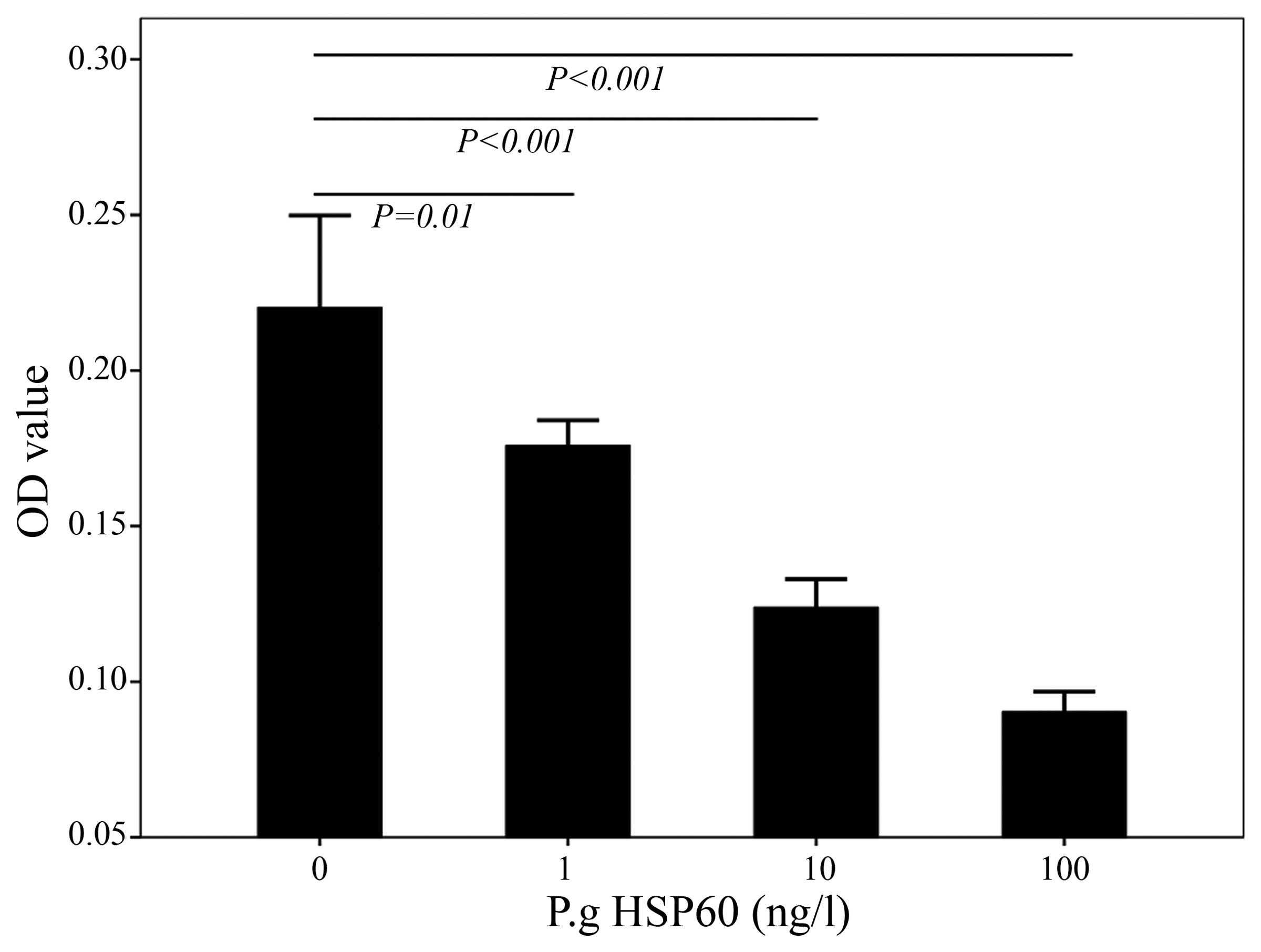
P. gingivalis HSP60 inhibits the
proliferation of HUVECs. To examine the effects of P.
gingivalis HSP60 on HUVECs, the HUVECs were first treated with
different concentrations of P. gingivalis HSP60, and the
cell viability was detected using the MTT assay. P.
gingivalis HSP60 at 1, 10 and 100 ng/l significantly altered
the viability of HUVECs (P<0.05) (Fig.
1).
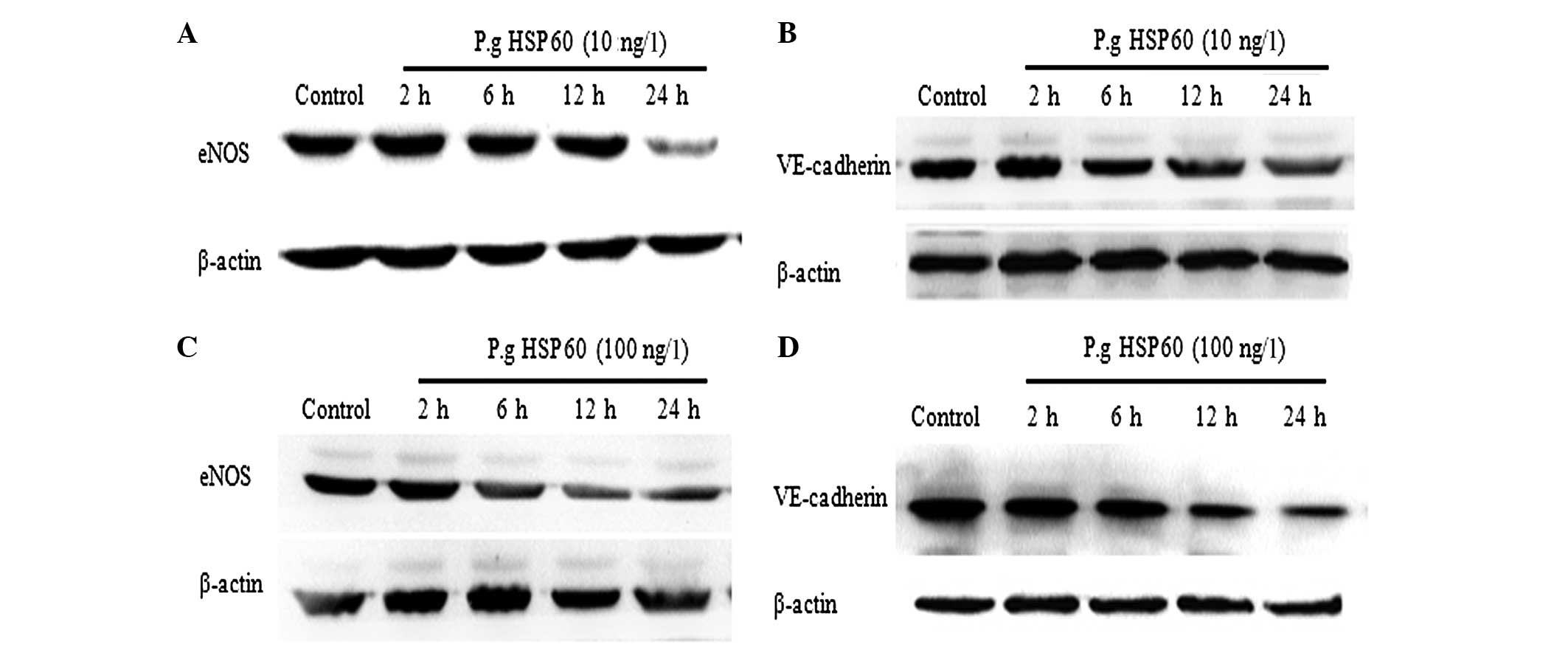
P. gingivalis HSP60 downregulates the
expression levels of eNOS and V-cadherin in HUVECs. HUVECs were
incubated with P. gingivalis HSP60 (1, 10 and 100 ng/l) at
different time-points (2, 6, 12 and 24 h) and the levels of
VE-cadherin and eNOS were detected by western blot analysis. The
expression levels of VE-cadherin and eNOS proteins were comparable
in HUVECs treated with 1 ng/l of P. gingivalis HSP60. The
protein expression levels of eNOS at 24 h following treatment with
P. gingivalis HSP60 (10 ng/l) were significantly decreased
as compared with those at 2, 6 and 12 h (Fig. 2A), and the protein expression level of
VE-cadherin had an opposing effect (Fig.
2B). Additionally, HUVECs treated with P. gingivalis
HSP60 (100 ng/l) exhibited a significantly lower eNOS protein
expression level following 12 h of treatment in contrast to the
levels at 2 and 6 h (Fig. 2C), and the
protein expression of VE-cadherin was significantly decreased after
12 h (Fig. 2D). Taken together, these
results provide evidence that P. gingivalis HSP60 may lead
to endothelial dysfunction by the regulation of VE-cadherin and
eNOS protein expression levels.
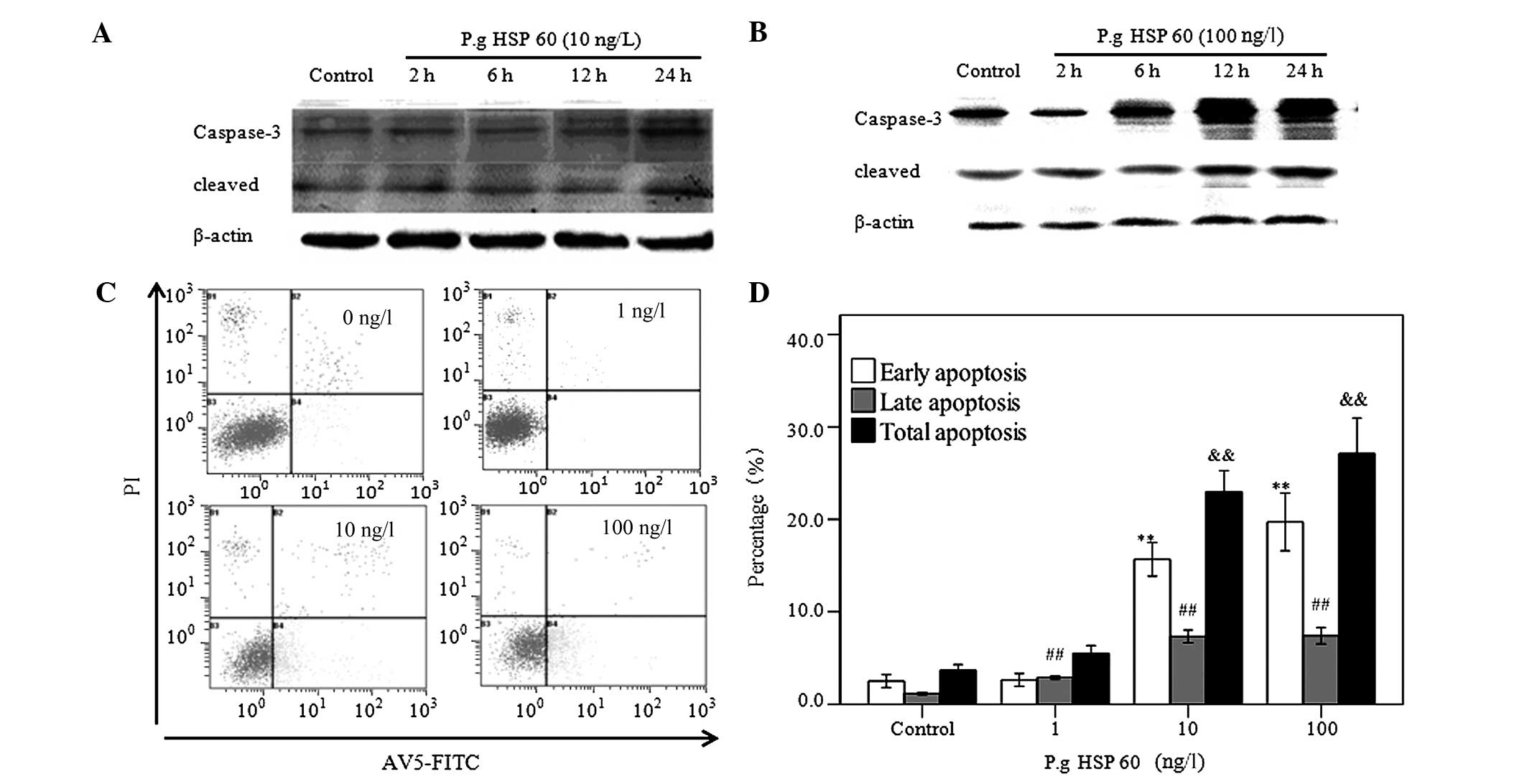
P. gingivalis HSP60 upregulates the
expression of caspase-3 and induces apoptosis of HUVECs in a
concentration-dependent manner
Caspase-3 was detected by western blot analysis. The
expression of the cleavage of caspase-3 at 24 h was significantly
increased as compared with that of 2, 6 and 12 h treatment with
P. gingivalis HSP60 (10 ng/l) (Fig.
3A), and when HUVECs were treated with P. gingivalis
HSP60 at 100 ng/l, the expression of the cleavage of caspase-3 at
12 h was significantly increased as compared with that at 2 and 6 h
(Fig. 3B). The apoptosis of HUVECs was
analyzed by annexin V/FITC staining, as shown in Fig. 3C and D. The percentage of total
apoptotic cells was 3.86±0.60, 5.52±0.82, 22.99±2.28 and
27.11±3.87% in cells untreated and cells treated with 1, 10 and 100
ng/l of P. gingivalis HSP60, respectively. These results
showed that P. gingivalis HSP60 (1, 10 and 100 ng/l)
stimulation for 24 h significantly increased the apoptotic rate and
induced significant apoptosis on HUVECs in a
concentration-dependent manner.
Discussion
There is increasing evidence that P.
gingivalis has a key role in contributing to the progression of
atherosclerosis (8–11). According to a recent study, LPS of
P. gingivalis may induce arterial endothelial cell apoptosis
and the expression of adhesion molecules in endothelial cells in
vitro, which may promote atherogenesis (27). Although the P.
gingivalis-induced dysfunction of HUVECs, including LPS and the
immunological mechanism, are well established, little is known
regarding the mechanisms involved in P. gingivalis HSP60.
The association between P. gingivalis HSP60 stimulation and
HUVEC dysfunction and associated mechanisms are insufficient and
require further analysis. The present study assessed the impact of
P. gingivalis HSP60 on HUVECs. The results proved that
co-culture of HUVECs with P. gingivalis HSP60 led to
decreased viability of HUVECs, as determined by the MTT assay.
As endothelial dysfunction and apoptosis are vital
factors in the progression of atherosclerosis, the associated
mechanisms of P. gingivalis HSP60 on the induction of HUVECs
dysfunction and apoptosis were investigated in the present study.
The data showed that P. gingivalis HSP60 downregulated the
expression level of the eNOS protein, which is able to modulate the
biologically active gas production of NO. Endothelium-derived NO
has an important physiological role in the regulation of vascular
tone, and endothelial cell survival and migration (28,29).
Additionally, similar influences of VE-cadherin
expression were observed in P. gingivalis HSP60-treated
HUVECs. VE-cadherin is localized to the adherens junctions,
associating with α-catenin, β-catenin, p120-catenin and plakoglobin
via its cytoplasmic domains. It has previously been reported that
VE-cadherin, as a major regulator of adherens junctions, in
particular has an essential role in the regulation of endothelial
cell permeability (30–32), migration and assembly of new blood
vessels. Loss of entire VE-cadherin or a lack of its cytoplasmic
domain induced endothelial apoptosis and prevented normal vascular
development in vivo (33).
VE-cadherin is an endothelial cell-specific adhesive molecule for
the integrity of endothelial cell contacts (31). VE-cadherin has significant functions in
the processes of atherosclerosis (33). Thus far, there have been limited
studies regarding VE-cadherin expression when HUVECs were treated
with P. gingivalis HSP60. Information is limited regarding
the mechanisms of P. gingivalis HSP60-induced
atherosclerosis involving the expression of VE-cadherin. The
present data identified that VE-cadherin may be one of the factors
leading to endothelial dysfunction.
Additionally, the present results showed that P.
gingivalis HSP60 induced significant apoptosis of HUVECs in a
concentration-dependent manner, as shown by the annexin V-FITC/PI
assay. Apoptosis is primarily mediated by the activity of caspases.
The extrinsic apoptotic pathway involves binding of specific
ligands to membrane-bound death receptors, such as Fas/cluster of
differentiation 95, which in turn activates caspase-8, facilitating
the subsequent activation of terminal effector caspases, such as
caspase-3, −6 and −7 (34,35). Thus, caspase-3 is pivotal to the death
process. Furthermore, P. gingivalis HSP60 was also found to
a certain extent to induce HUVECs apoptosis through a mechanism
that involved caspase-3 activation. It was proved that apoptosis of
VE cells resulted in the loss of endothelial integrity, and was a
risk factor of atherosclerosis. Therefore, the present study
verified the mechanism of P. gingivalis HSP60, which led to
atherosclerosis by further accelerating apoptosis in HUVECs.
In conclusion, taken together with the results of
other studies, we hypothesize that P. gingivalis HSP60 has
an essential role in the dysfunction of HUVECs via the mechanism of
regulating eNOS and VE-cadherin expression levels, as well as
apoptosis by activating caspase-3 in a unique manner. These
findings provide new mechanistic insights into the effect of P.
gingivalis HSP60 on HUVECs and the associated pathogenesis of
cardiovascular disease and periodontitis. Further study is required
to determine the pathway of the P. gingivalis HSP60-induced
decreased expression of VE-cadherin and eNOS.
Acknowledgements
The study was completed in the Department of Urology
Institute in Tianjin. The authors thank Dr Yuanjie Niu and Dr Ning
Jiang for their skilled technical assistance.
References
|
1
|
Dietrich T, Jimenez M, Krall Kaye EA,
Vokonas PS and Garcia RI: Age-dependent associations between
chronic periodontitis/edentulism and risk of coronary heart
disease. Circulation. 117:1668–1674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bahekar AA, Singh S, Saha S, Molnar J and
Arora R: The prevalence and incidence of coronary heart disease is
significantly increased in periodontitis: A meta-analysis. Am Heart
J. 154:830–837. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dietrich T, Sharma P, Walter C, Weston P
and Beck J: The epidemiological evidence behind the association
between periodontitis and incident atherosclerotic cardiovascular
disease. J Periodontol. 84(Suppl 4): S70–S84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tonetti MS and Van Dyke TE: working group
1 of the joint EFP/AAP workshop: Periodontitis and atherosclerotic
cardiovascular disease: Consensus report of the Joint EFP/AAP
Workshop on Periodontitis and Systemic Diseases. J Periodontol.
84(Suppl 4): S24–S29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Forner L, Larsen T, Kilian M and Holmstrup
P: Incidence of bacteremia after chewing, tooth brushing and
scaling in individuals with periodontal inflammation. J Clin
Periodontol. 33:401–407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kinane DF, Riggio MP, Walker KF, MacKenzie
D and Shearer B: Bacteraemia following periodontal procedures. J
Clin Periodontol. 32:708–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guntheroth WG: How important are dental
procedures as a cause of infective endocarditis? Am J Cardiol.
54:797–801. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yumoto H, Chou HH, Takahashi Y, Davey M,
Gibson FC III and Genco CA: Sensitization of human aortic
endothelial cells to lipopolysaccharide via regulation of Toll-like
receptor 4 by bacterial fimbria-dependent invasion. Infect Immun.
73:8050–8059. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kozarov EV, Dorn BR, Shelburne CE, Dunn WA
Jr and Progulske-Fox A: Human atherosclerotic plaque contains
viable invasive Actinobacillus actinomycetemcomitans and
Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol.
25:e17–e18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Darveau RP: Periodontitis: A polymicrobial
disruption of host homeostasis. Nat Rev Microbiol. 8:481–490. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyakawa H, Honma K, Qi M and Kuramitsu
HK: Interaction of Porphyromonas gingivalis with low-density
lipoproteins: Implications for a role for periodontitis in
atherosclerosis. J Periodontal Res. 39:1–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giacona MB, Papapanou PN, Lamster IB, Rong
LL, D'Agati VD, Schmidt AM and Lalla E: Porphyromonas
gingivalis induces its uptake by human macrophages and promotes
foam cell formation in vitro. FEMS Microbiol Lett. 241:95–101.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khlgatian M, Nassar H, Chou HH, Gibson FC
III and Genco CA: Fimbria-dependent activation of cell adhesion
molecule expression in Porphyromonas gingivalis-infected
endothelial cells. Infect Immun. 70:257–267. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakamura N, Yoshida M, Umeda M, Huang Y,
Kitajima S, Inoue Y, Ishikawa I and Iwai T: Extended exposure of
lipopolysaccharide fraction from Porphyromonas gingivalis
facilitates mononuclear cell adhesion to vascular endothelium via
Toll-like receptor-2 dependent mechanism. Atherosclerosis.
196:59–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeong E, Lee JY, Kim SJ and Choi J:
Predominant immunoreactivity of Porphyromonas gingivalis
heat shock protein in autoimmune diseases. J Periodontal Res.
47:811–816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rajaiah R and Moudgil KD: Heat-shock
proteins can promote as well as regulate autoimmunity. Autoimmun
Rev. 8:388–393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Puijvelde GH, van Es T, van Wanrooij
EJ, Habets KL, de Vos P, van der Zee R, van Eden W, van Berkel TJ
and Kuiper J: Induction of oral tolerance to HSP60 or an
HSP60-peptide activates T cell regulation and reduces
atherosclerosis. Arterioscler Thromb Vasc Biol. 27:2677–2683. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Craig EA, Gambill BD and Nelson RJ: Heat
shock proteins: Molecular chaperones of protein biogenesis.
Microbiol Rev. 57:402–414. 1993.PubMed/NCBI
|
|
19
|
Ford PJ, Gemmell E, Hamlet SM, Hasan A,
Walker PJ, West MJ, Cullinan MP and Seymour GJ: Cross-reactivity of
GroEL antibodies with human heat shock protein 60 and
quantification of pathogens in atherosclerosis. Oral Microbiol
Immunol. 20:296–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeong E, Kim K, Kim JH, Cha GS, Kim SJ,
Kang HS and Choi J: Porphyromonas gingivalis HSP60 peptides
have distinct roles in the development of atherosclerosis. Mol
Immunol. 63:489–496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, He M, Cheng L, Chen Y, Zhou L,
Zeng H, Pockley AG, Hu FB and Wu T: Elevated heat shock protein 60
levels are associated with higher risk of coronary heart disease in
Chinese. Circulation. 118:2687–2693. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lewthwaite J, Owen N, Coates A, Henderson
B and Steptoe A: Circulating human heat shock protein 60 in the
plasma of British civil servants: Relationship to physiological and
psychosocial stress. Circulation. 106:196–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Dyke TE and van Winkelhoff AJ:
Infection and inflammatory mechanisms. J Clin Periodontol. 40(Suppl
14): S1–S7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schenkein HA and Loos BG: Inflammatory
mechanisms linking periodontal diseases to cardiovascular diseases.
J Periodontol. 84(Suppl 4): S51–S69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kebschull M, Demmer RT and Papapanou PN:
‘Gum bug, leave my heart alone!’ - epidemiologic and mechanistic
evidence linking periodontal infections and atherosclerosis. J Dent
Res. 89:879–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kajiyama H, Kikkawa F, Khin E, Shibata K,
Ino K and Mizutani S: Dipeptidyl peptidase IV overexpression
induces up-regulation of E-cadherin and tissue inhibitors of matrix
metalloproteinases, resulting in decreased invasive potential in
ovarian carcinoma cells. Cancer Res. 63:2278–2283. 2003.PubMed/NCBI
|
|
27
|
Andrukhov O, Steiner I, Liu S, Bantleon
HP, Moritz A and Rausch-Fan X: Different effects of
Porphyromonas gingivalis lipopolysaccharide and TLR2 agonist
Pam3CSK4 on the adhesion molecules expression in endothelial cells.
Odontology. 103:19–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hobbs AJ, Higgs A and Moncada S:
Inhibition of nitric oxide synthase as a potential therapeutic
target. Annu Rev Pharmacol Toxicol. 39:191–220. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Busse R and Mülsch A: Calcium-dependent
nitric oxide synthesis in endothelial cytosol is mediated by
calmodulin. FEBS Lett. 265:133–136. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smadja DM, Bièche I, Helley D, Laurendeau
I, Simonin G, Muller L, Aiach M and Gaussem P: Increased VEGFR2
expression during human late endothelial progenitor cells expansion
enhances in vitro angiogenesis with up-regulation of integrin
alpha(6). J Cell Mol Med. 11:1149–1161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Franco CA, Mericskay M, Parlakian A,
Gary-Bobo G, Gao-Li J, Paulin D, Gustafsson E and Li Z: Serum
response factor is required for sprouting angiogenesis and vascular
integrity. Dev Cell. 15:448–461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baluk P, Fuxe J, Hashizume H, Romano T,
Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E,
et al: Functionally specialized junctions between endothelial cells
of lymphatic vessels. J Exp Med. 204:2349–2362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Potter MD, Barbero S and Cheresh DA:
Tyrosine phosphorylation of VE-cadherin prevents binding of p120-
and beta-catenin and maintains the cellular mesenchymal state. J
Biol Chem. 280:31906–31912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marsden VS, O'Connor L, O'Reilly LA, Silke
J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ,
et al: Apoptosis initiated by Bcl-2-regulated caspase activation
independently of the cytochrome c/Apaf-1/caspase-9 apoptosome.
Nature. 419:634–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003. View Article : Google Scholar : PubMed/NCBI
|