Introduction
The prognosis of patients with renal failure has
improved due to the refinements and advances in hemodialysis
methods. Renal failure can lead to impairment of the immune system,
and therefore, patients undergoing hemodialysis can suffer from
various types of cancer. Consequently, the opportunity to undergo
chemotherapy for patients being treated by hemodialysis is
increasing.
A combination of paclitaxel and carboplatin (TC) is
first-line chemotherapy for patients with Müllerian cancer. There
are few studies on the TC pharmacokinetics in hemodialysis patients
with Müllerian cancer. Therefore, determination of the
administration method (dose and time interval between
administration of anticancer agent and hemodialysis initiation) is
difficult. The number of dialysis patients undergoing TC is
increasing, and therefore, the establishment of the optimal method
for administration of anticancer agents is important.
Certain previous studies have suggested that
paclitaxel pharmacokinetics are not affected by hemodialysis
(1–4). In
the present study, carboplatin concentrations were measured in a
patient with fallopian tube cancer (FTC) with chronic renal failure
who required hemodialysis.
Case report
A 73-year-old Japanese woman with chronic renal
failure associated with type-2 diabetes mellitus, who had undergone
hemodialysis three times a week for 3 years, presented with
abdominal distension. Magnetic resonance imaging revealed a tumor
in the left fallopian tube (diameter, 9 cm). Carcinoma of the left
adnexa was suspected, and the patient underwent abdominal total
hysterectomy, bilateral salpingo-oophorectomy, partial omentectomy
and pelvic lymphadenectomy. Histology revealed an endometrial
adenocarcinoma (grade 2) in the left fallopian tube. The patient
underwent optimal surgery and was diagnosed with stage-IC3
(International Federation of Gynecology and Obstetrics) FTC.
Postoperatively, the patient was administered TC (i.v.). The
chemotherapy regimen was 6 cycles, with 1 cycle every 3 weeks.
The paclitaxel regimen was determined to be 135
mg/m2 (total of 210 mg) over 3 h. After paclitaxel
administration, carboplatin was administered over 1 h. The
carboplatin dose was calculated according to the Calvert formula
[target area under the concentration-time curve (AUC): 5 mg•min/ml]
(5). The glomerular filtration rate
(GFR) was determined to be zero as the urinary volume per day of
the patient was 0 ml. Therefore, the carboplatin dose was
calculated as 125 mg.
Hemodialysis was initiated 1 h after completion of
carboplatin infusion and carried out for 4 h. Samples of
heparinized blood were collected at specific times. The blood
samples were centrifuged at 1,350 × g for 5 min at 4°C. The plasma
aliquots were centrifuged at 1,350 × g for 15 min at 4°C in an
Amicon® system (Millipore, Bedford, MA, USA) for
evaluation of the free platinum based on the method of LeRoy et
al (6).
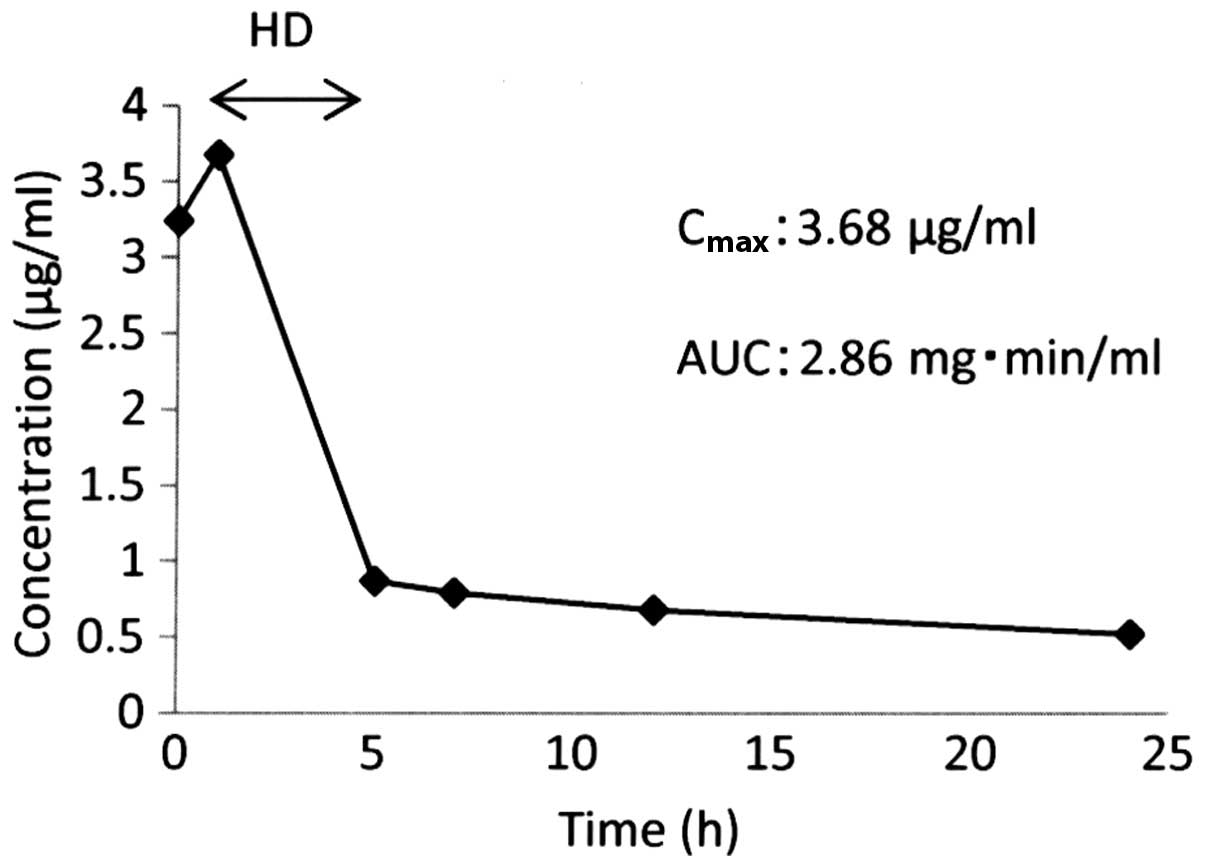
The maximum plasma concentration (Cmax)
of the free platinum was 3.68 µg/ml and the AUC of the free
platinum was 2.86 mg•min/ml (Fig. 1).
Adverse events were evaluated based on the Common Terminology
Criteria for Adverse Events set by the National Cancer Institute
(version 4) (7). Adverse events of
grade 3 or 4 were not observed.
Due to the low AUC of free platinum in the first
cycle of chemotherapy, the time interval between the end of
carboplatin infusion and the start of hemodialysis was extended in
the next cycle. The time interval was prolonged to 16 h at the
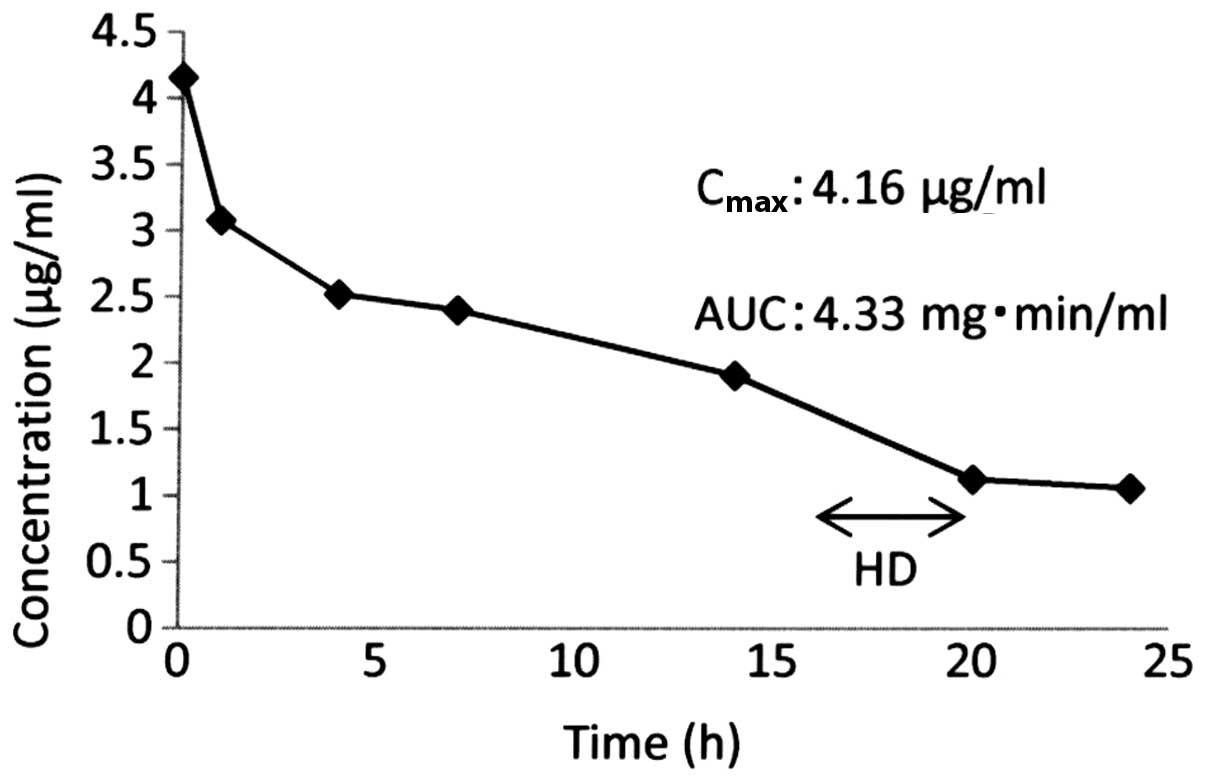
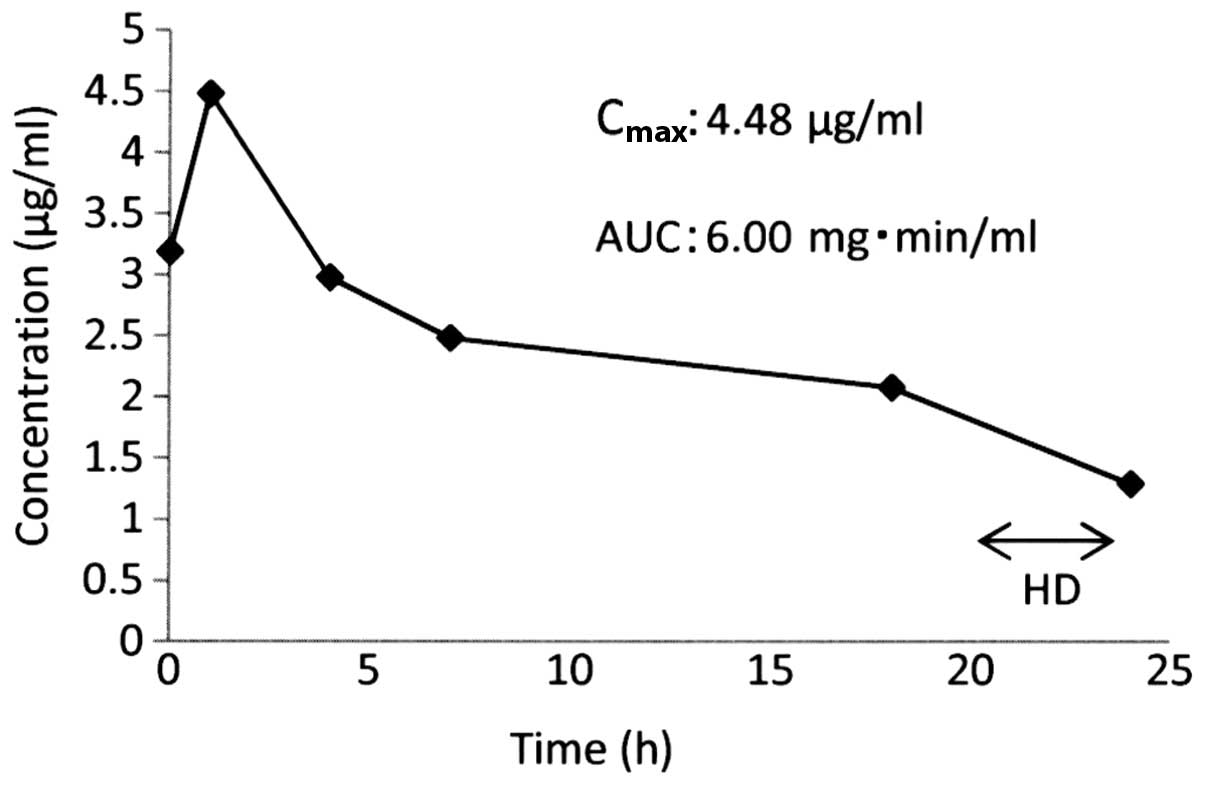
second cycle and to 20 h at the third cycle. The Cmax of
the drug in the serum at the second and third cycles were 4.16 and
4.48 µg/ml, and the AUCs were 4.33 and 6.00 mg•min/ml, respectively
(Figs. 2 and 3). Grade-3 leukocytopenia and neutropenia
were observed; however, chemotherapy was (in general)
well-tolerated.
The desired AUC of free platinum was demonstrated
and only mild side effects were observed at the third cycle.
Therefore, hemodialysis was initiated 20 h after completion of
carboplatin infusion at cycles 4–6. The total chemotherapy planned
was completed without severe adverse events. Recurrence was not
observed at the 22-month follow-up.
Discussion
The prognosis of patients with renal failure has
improved due to the advances in hemodialysis methods. In addition,
TC is being administered increasingly often in patients with
Müllerian cancer who require hemodialysis. However, the optimal
drug dose and timing of hemodialysis remain to be elucidated. In
the present study, TC was administered safely to a patient with FTC
requiring hemodialysis, and the carboplatin pharmacokinetics were
examined.
Paclitaxel is metabolized extensively by the hepatic
cytochrome P450 and secreted into bile, with <10% being
extracted by the kidneys (8).
Therefore, it is assumed that paclitaxel pharmacokinetics are
slightly affected by renal function. It has been reported that the
Cmax, AUC and attenuation pattern of paclitaxel in
patients with renal failure requiring hemodialysis are comparable
with the pharmacokinetics of patients with normal renal function,
and that paclitaxel can be administered safely for patients with
renal failure without hepatic dysfunction prior or subsequent to
hemodialysis at the same dose as patients with normal renal
function (1–4,9). Therefore,
in this instance, paclitaxel was administered in a patient with
renal failure instead of measuring the paclitaxel concentration in
blood.
Carboplatin is metabolized almost completely by the
kidneys, and renal function affects its pharmacokinetics. In
general, the carboplatin dose administered is calculated based on
the Calvert formula (1). In practice,
the GFR in hemodialysis patients is approximately zero, so the
carboplatin dose should be the target AUC ×25. Carboplatin can be
dialyzed readily during hemodialysis because of its low binding
capacity to proteins and intermediate molecular weight (10). Therefore, carboplatin is excreted
mainly during hemodialysis in patients with renal failure, and its
AUC is dependent upon the carboplatin dose and timing of
hemodialysis (11,12).
In the present study, hemodialysis was initiated 1 h
after completion of the administration of carboplatin (125 mg) at
the first chemotherapy cycle, and the target AUC was not achieved.
Therefore, the time interval between the end of carboplatin
administration and beginning of hemodialysis was prolonged. An AUC
of 4.33 mg/ml•min was obtained at the second chemotherapy cycle
when hemodialysis was started 16 h after carboplatin
administration. An adequate AUC of 6.00 mg/ml•min was achieved at
the third chemotherapy cycle at a time interval of 20 h. Therefore,
hemodialysis was initiated 20 h after completion of carboplatin
infusion at cycles 4–6. The total chemotherapy planned was
completed without severe adverse events.
Several studies have described the carboplatin dose,
AUC of free platinum and interval between completion of
administration and initiation of hemodialysis in hemodialysis
patients (1,3,4,12–15)
(Table I). In this way, the optimal
dose of carboplatin and timing of hemodialysis remains
controversial. Therefore, guidelines for the optimal method to
administer carboplatin in hemodialysis patients are required.
Measurement of the concentration of free platinum following
administration may be useful for determination of the optimal dose
of carboplatin and interval following administration to obtain an
adequate AUC. The present study suggests that carboplatin can be
administered to a patient undergoing hemodialysis, and that an
adequate interval between the end of carboplatin administration and
hemodialysis initiation could be ~20 h.
 | Table I.CBDCA dose, AUC of free platinum and
interval between completion of CBDCA administration and the
initiation of hemodialysis. |
Table I.
CBDCA dose, AUC of free platinum and
interval between completion of CBDCA administration and the
initiation of hemodialysis.
|
|
|
| Hemodialysis |
|
|
|---|
|
|
|
|
|
|
|
|---|
| First author,
year | Type of cancer | CBDCA, mg/body | Interval after CDBCA
administration, h | Duration, h | AUC, mg•min/ml | Refs. |
|---|
| Watanabe, 2002 | Ovarian | 125 | 16 | 4 | 4.4 | (1) |
| Yoshida, 2009 | Ovarian | 125 | 1 | 4 | 1.0 | (3) |
| Kodama, 2010 | Ovarian | 150 | 24 | 3 | 4.6 | (4) |
| Suzuki, 1997 | Merkel cell | 150 | 2 | 4 | 4.8 | (12) |
| Chatelut, 1994 | Ovarian | 150 | 24 | 4 | 6.7 | (13) |
| Yokoyama, 2006 | Ovarian | 200 | 16 | 4 | 5.7 | (14) |
| Fong, 2014 | Neurocrine | 200 |
1.5 | 3.5 | 2.9 | (15) |
| Wada, 2016 | FT | 125 | 1 | 4 | 2.9 | (Present study) |
|
|
| 125 | 16 | 4 | 4.2 |
|
|
|
| 125 | 20 | 4 | 6.0 |
|
References
|
1
|
Watanabe M, Aoki Y, Tomita M, Sato T,
Takaki Y, Kato N, Kikuchi M, Kase H and Tanaka K: Paclitaxel and
carboplatin combination chemotherapy in a hemodialysis patient with
advanced ovarian cancer. Gynecol Oncol. 84:335–338. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomita M, Kurata H, Aoki Y, Tanaka K and
Kazama JJ: Pharmacokinetics of paclitaxel and cisplatin in a
hemodialysis patient with recurrent ovarian cancer. Anticancer
Drugs. 12:485–487. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshida H, Sumi T, Abe K and Ishiko O:
Pharmacokinetics of paclitaxel and carboplatin in a hemodialysis
patient with advanced ovarian cancer. Eur J Gynaecol Oncol.
30:583–585. 2009.PubMed/NCBI
|
|
4
|
Kodama J, Sasaki A, Masahiro S, Seki N,
Kusumoto T, Nakamura K, Hongo A and Hiramatsu Y: Pharmacokinetics
of combination chemotherapy with paclitaxel and carboplatin in a
patient with advanced epithelial ovarian cancer undergoing
hemodialysis. Oncol Lett. 1:511–513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calvert AH, Newell DR, Gumbrell LA,
O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME and
Wiltshaw E: Carboplatin dosage: Prospective evaluation of a simple
formula based on renal function. J Clin Oncol. 7:1748–1756.
1989.PubMed/NCBI
|
|
6
|
LeRoy AF, Wehling ML, Sponseller HL,
Friauf WS, Solomon RE, Dedrick RL, Litterst CL, Gram TE, Guarino AM
and Becker DA: Analysis of platinum in biological materials by
flameless atomic absorption spectrophotometry. Biochem Med.
18:184–191. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Cancer Institute. National
Institutes of Health, US Department of Health and Human Services:
Common terminology criteria for adverse events (CTCAE) version 4.0.
NIH Publication no. 09–5410. Bethesda, MD: 2009.
|
|
8
|
Longnecker SM, Donehower RC, Cates AE,
Chen TL, Brundrett RB, Grochow LB, Ettinger DS and Colvin M:
High-performance liquid chromatographic assay for taxol in human
plasma and urine and pharmacokinetics in a phase I trial. Cancer
Treat Rep. 71:53–59. 1987.PubMed/NCBI
|
|
9
|
Woo MH, Gregornik D, Shearer PD, Meyer WH
and Relling MV: Pharmacokinetics of paclitaxel in an anephric
patient. Cancer Chemother Pharmacol. 43:92–96. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hiraike M, Hiraki Y, Misumi N, Hanada K,
Tsuji Y, Kamimura H, Karube Y and Kashiwabara K: Pharmacokinetics
of carboplatin in a hemodialysis patient with small-cell lung
cancer. Cancer Chemother Pharmacol. 69:845–848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koeller JM, Trump DL, Tutsch KD, Earhart
RH, Davis TE and Tormey DC: Phase I clinical trial and
pharmacokinetics of carboplatin (NSC 241240) by single monthly
30-minute infusion. Cancer. 57:222–225. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki S, Koide M, Sakamoto S and Matsuo
T: Pharmacokinetics of carboplatin and etoposide in a haemodialysis
patient with Merkel-cell carcinoma. Nephrol Dial Transplant.
12:137–140. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chatelut E, Rostaing L, Gualano V, Vissac
T, De Forni M, Ton-That H, Suc JM, Houin G and Canal P:
Pharmacokinetics of carboplatin in a patient suffering from
advanced ovarian carcinoma with hemodialysis-dependent renal
insufficiency. Nephron. 66:157–161. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokoyama Y, Futagami M, Higuchi T and
Mizunuma H: Pharmacokinetic analysis of paclitaxel and carboplatin
in a patient with advanced ovarian cancer during hemodialysis -
case report. Eur J Gynaecol Oncol. 27:437–439. 2006.PubMed/NCBI
|
|
15
|
Fong MK, Fetterly GJ Jr, McDougald LJ and
Iyer RV: Carboplatin pharmacokinetics in a patient receiving
hemodialysis. Pharmacotherapy. 34:e9–e13. 2014. View Article : Google Scholar : PubMed/NCBI
|