Introduction
Coronary artery disease (CAD), traditionally
regarded as a disease of Western society, is currently the leading
cause of morbidity and mortality in China (1). A marked increase in the mortality rate of
coronary heart disease is the major reason for the long-term rise
of cardiovascular disease (CVD)-associated fatalities in the
Chinese population (1). CAD is a
slowly progressive disease that exhibits few symptoms at its early
stage when prevention is possible, thus, non-invasive approaches to
establish the severity of CAD is of great importance to patient
care.
MicroRNA (miRNA) is a class of small (19–25 nt)
single-stranded non-coding RNAs that post-transcriptionally
regulate gene expression and are crucial in a wide range of
biological processes, such as metabolism, differentiation,
proliferation and apoptosis (2–4). It has been
found that miRNA levels are tissue-specific and closely associated
with physiological/pathological changes in the cardiovascular
system (5). In addition, due to their
small size, miRNAs detected in body fluids are stable and resistant
to digestion of endogenous RNases (6).
These characteristics render miRNAs as ideal biomarkers for
CVD.
In recent years there have been numerous studies on
the potential role of miRNAs as diagnostic biomarkers in the field
of CVD. miR-499 and miR-208a/b belong to the miR-208 family, which
is almost specifically expressed in the heart. The expression
levels of these three miRNAs have been found to be promptly
increased to a significant extent in the circulation following
acute myocardial infarction (MI) and successfully discriminate MI,
more effectively than the traditional biomarker, cardiac troponin
(7–9). In
the early stages of CAD, although various miRNAs (for example,
miR-21, miR-145, miR-199a and miR-340) were found to be deregulated
(10–14), the findings were not conclusive. The
performance of certain miRNAs was inconsistent between different
studies. Thus, more cohort studies are required for further
validation.
In the present study, two plasma miRNAs (miR-208a
and miR-370) that are vital in the progression and development of
CAD were evaluated by reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) in a Chinese cohort of 95 CAD patients and
50 non-CAD control subjects. Their association with CAD was
investigated, and their ability to discriminated CAD was assessed
using receiver operating characteristic (ROC) analysis.
Materials and methods
Subjects
The ethics committee of the Affiliated Hospital of
Jining Medical University (Jining, China) approved the present
study, and written consent was obtained from all patients to
provide information and samples for research purposes.
Between July 21, 2005 and March 7, 2015, 95
consecutive patients (65 males and 30 females; age range, 44–78
years) who underwent diagnostic coronary angiography for chest pain
evaluation were recruited from inpatients admitted to the
Affiliated Hospital of Jining Medical University. Diagnosis of CAD
was confirmed by quantitative coronary angiography and was defined
as angiographic evidence of >50% luminal narrowing in at least
one segment of a main epicardial coronary artery. Two
cardiologists, blinded to which patients were in the study,
assessed the angiogram. Fifty individuals with neither detectable
coronary stenosis nor atherosclerotic vascular disease were
considered as non-CAD patients, who were matched for age, gender,
smoking habits, hypertension and diabetes. Patients with histories
of significant concomitant diseases, including cardiomyopathy,
congenital heart disease, hepatic failure, renal failure,
hepatitis, bleeding disorders, and malignant diseases were excluded
from the study.
Sample collection and plasma
isolation
A sample of fasting blood (~5 ml) was withdrawn in
ethylenediaminetetraacetic acid-containing tubes and immediately
centrifuged at 4°C for 10 min at 1,000 × g. Plasma was collected
and stored at −80°C in a freezer until RNA purification.
RNA purification and miRNA
quantification
RNA was extracted from 800 µl plasma using an
miRNeasy serum/plasma kit (Qiagen China, Co., Ltd., Shanghai,
China) according to the manufacturer's instructions. The RNA was
eluted in 50 µl nuclease free water and 40 ng RNA (in 5 µl) was
reverse transcribed with TaqMan® MicroRNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's instructions. cDNA was
quantitated using a TaqMan-based RT-qPCR assay specific for
miR-208a and miR-370 according to the manufacturer's recommended
protocol on an Applied Biosystems 7500 Sequence Detection System
(the miR-208a and miR-370 specific primers for RT and PCR were
enclosed in the kits). The cycling conditions were 95°C for 10 min
followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. All
reactions were performed in triplicate. The expression levels of
circulating miRNAs are presented as the quantitation cycle (Cq)
values. Exogenously added cel-miR-39 served as a control for
normalizing the data. The comparative Cq (ΔCq) method was used to
analyze the miRNA expression levels (15).
Statistical analysis
Statistical analysis between numerical variables of
the two groups was performed using unpaired Student's t-test for
parametric data or Mann-Whitney rank sum test for nonparametric
data. P<0.05 was considered to indicate a statistically
significant difference. Logistic regression was conducted to
construct ROC curves using the expression level of miRNA adjusted
for the matching factors. The area under the curve (AUC) was
calculated with a 95% confidence interval (CI). The specificity and
sensitivity were identified by numerical integration of each ROC
curve. A prediction model was constructed by fitting miRNAs into a
logistic regression model, and the stepwise backward model was used
to determine miRNA combinations in discriminating CAD patients with
hypercholesterolemia A two-sided P<0.05 was considered to
indicate a statistically significant difference. Correlations
between miRNA expression levels were studied by Spearman analysis.
All analyses were performed using SPSS 15.0 software (SPSS, Inc.,
Chicago, IL, USA).
Results
Clinical characteristics of
patients
Clinicopathological characteristics of the patients
enrolled in the study are presented in Table I. The mean age was 65 years and the
population consisted of 68% males. The laboratory data of the
patients are presented in Table
II.
 | Table I.Clinical characteristics of study
subjects. |
Table I.
Clinical characteristics of study
subjects.
| Variables | CAD patients,
n=95 | Non-CAD subjects,
n=50 | P-value |
|---|
| Age (years) | 65 (44–78) | 65 (46–75) | 0.953 |
| Male/female | 65/30 | 34/16 | 0.613 |
| BMI
(kg/m2) | 23 (20–26) | 22 (20–24) | 0.726 |
| Active smoker
(%) | 63.6 | 61.0 | 0.824 |
| Diabetes mellitus
(%) | 9.6 | 9.1 | 0.823 |
| Hypertension (%) | 52.3 | 50.8 | 0.208 |
| Systolic BP
(mmHg) | 131 (98–185) | 125 (112–145) | 0.349 |
| Diastolic BP
(mmHg) | 82 (56–97) | 81 (62–87) | 0.561 |
| Left ventricular
ejection fraction (%) | 58 (38–68) | 61 (50–65) | 0.145 |
 | Table II.Clinical laboratory data of the
subjects. |
Table II.
Clinical laboratory data of the
subjects.
| Variables | CAD patients,
n=95 | Non-CAD subjects,
n=50 | P-value |
|---|
| FBS (mmol/l) | 5.0 (4.1–6.9) | 5.0 (4.1–6.0) | 0.365 |
| TG (mmol/l) | 1.4 (0.3–6.1) | 1.2 (0.3–3.1) | 0.411 |
| TC (mmol/l) | 4.6 (1.8–7.5) | 4.2 (1.6–6.1) | 0.214 |
| HDL-C (mmol/l) | 1.1 (0.9–1.5) | 1.1 (1.0–1.5) | 0.632 |
| LDL-C (mmol/l) | 2.6 (1.5–5.2) | 2.4 (1.4–4.0) | 0.519 |
| AST (U/l) | 23.5
(15.3–41.3) | 17.6 (11.2–34.7) | 0.313 |
| ALT (U/l) | 26.0
(11.0–54.2) | 20.1 (11.2–32.7) | 0.289 |
| Cr (µmol/l) | 67.2
(46.9–98.3) | 67.3 (47.1–91.4) | 0.768 |
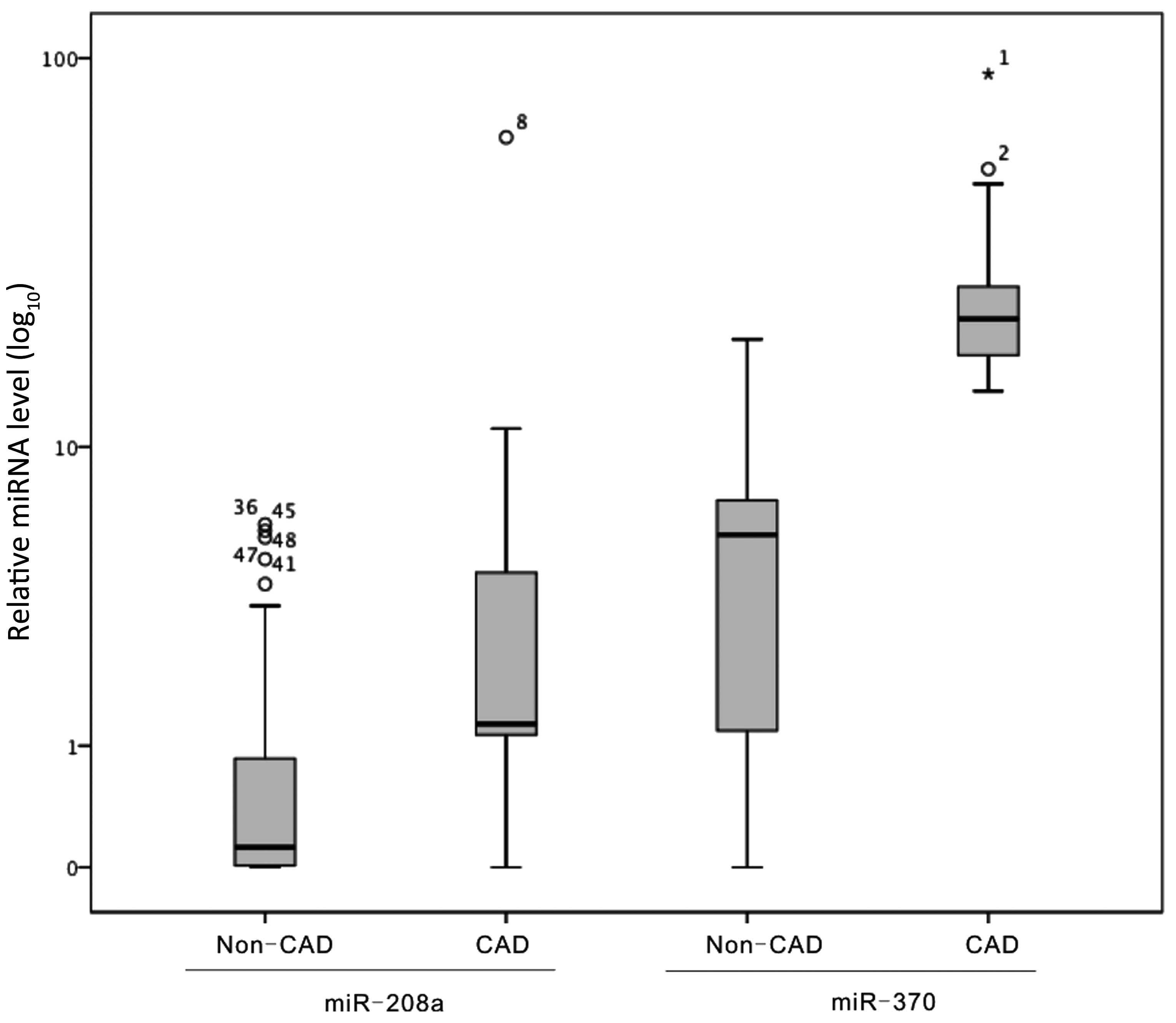
Relative expression levels of plasma
miR-208a and miR-370 in CAD patients and non-CAD subjects
The relative plasma levels of miR-208a and miR-370
in CAD patients and non-CAD patients were determined. The results
are presented in Fig. 1. The median
values of miR-208a and miR-370 were 1.57 and 15.21 in the CAD
patients, respectively; whereas, in the non-CAD patients, those of
miR-208a and miR-370 were 0.12 and 5.67, respectively. Plasma
expression levels of miR-208a (P=0.006) and miR-370 (P=0.003) were
significantly higher in the CAD group than in the non-CAD
group.
Correlations of plasma miR-208a and
miR-370 expression levels with blood lipid profiles
The correlation between plasma miR-208a and miR-370
expression levels and blood lipid profiles was analyzed. miR-370
was found to be positively correlated with total cholesterol (TC),
triglycerides (TG), low-density lipoprotein cholesterol (LDL-C),
but not high-density lipoprotein cholesterol (HDL-C); while
miR-208a was not correlated with any of these parameters (Table III).
 | Table III.Spearman analysis of the correlations
of miR-208a and miR-370 with blood lipid profiles. |
Table III.
Spearman analysis of the correlations
of miR-208a and miR-370 with blood lipid profiles.
| Groups | Total
cholesterol | Triglycerides | Low-density
lipoprotein cholesterol | High-density
lipoprotein cholesterol |
|---|
| CAD patients
(n=95) |
|
|
|
|
|
miR-208a | 0.143 | 0.107 | 0.122 |
0.025 |
|
miR-370 | 0.466a | 0.421b | 0.457a | −0.041 |
| Non-CAD patients
(n=95) |
|
|
|
|
|
miR-208a | 0.137 | 0.126 | 0.143 | −0.019 |
|
miR-370 | 0.453a | 0.397b | 0.461a | −0.036 |
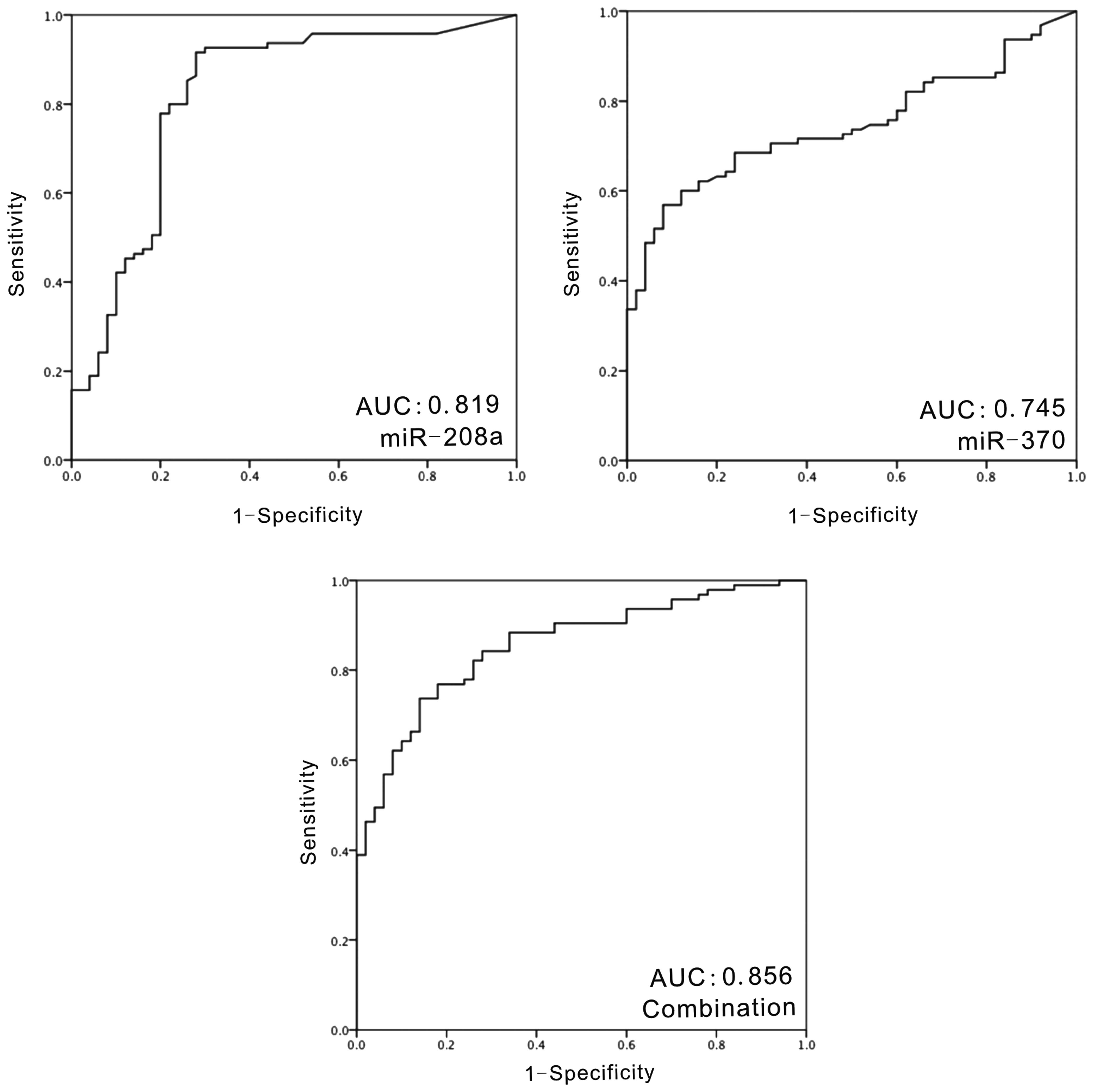
Diagnostic potential of miR-208a and
miR-370 in CAD patients
ROC analysis was performed to examine the potential
of miR-208a and miR-370 as biomarkers for CAD patients (Fig. 2). The AUC of miR-208a was 0.819 (95%
CI, 0.739–0.898; P=2.97×10−7). For miR-370, the AUC was
0.745 (95% CI, 0.667–0.823; P=1×10−6). At the optimal
cut-off value of the relative quantification values, the
sensitivity of miR-208a was 91.6% and the specificity was 72.0%;
the sensitivity of miR-370 was 55.8% and the specificity was
92.0%.
Combination of miR-208a and miR-370
exhibited improved AUC values than each individual miRNA
The distribution of plasma levels of miR-208 and
miR-370 were different among all of the subjects. Spearman analysis
was used to analyze the association between miR-208a and miR-370
expression. There was only a weak, however statistically
significant, correlation between miR-208a and miR-370
(rs=0.229; P=0.006). Combination of these two miRNAs was
subjected to ROC analysis. The AUC was 0.856 (95% CI, 0.796–0.917;
P=1.89×10−9). At the optimal cut-off, the sensitivity of
the combination of the two miRNAs was 73.7% and the specificity was
86.0%.
Discussion
CAD is the most common lethal factor in
industrialized countries and poses a marked burden on the public
health system (1). The well-determined
risk factors of CAD include current smoking habit, hypertension,
obesity, diabetes, stress and high blood lipids. miRNAs that are
important in these biological processes may have diagnostic
potential to discriminate CAD. The miR-208 family (consisting of
miR-208a, miR-208b and miR-499) exert critical functions in muscle
performance (16). Transgenic
overexpression of miR-208a in the heart was identified to be
sufficient to induce hypertrophic growth in rodents via inhibiting
thyroid hormone-associated protein 1 and myostatin (17). Anti-miR-208a treatment prevented
pathological cardiac remodeling and improved cardiac function and
survival during hypertension-induced heart failure in Dahl
hypertensive rats (18). miRNA
distribution analysis revealed that miR-208a was exclusively
expressed in the heart, which is important for diagnostic purposes
(19,20). The miR-208a expression level (as well
as miR-208b and miR-499) was elevated markedly following MI and the
miR-208 family has been recommended as a biomarker for MI replacing
traditional circulating cardiac troponin I in a number of studies
(19,21–23). As for
CAD, there are fewer reports regarding miR-208a, and the results
are inconsistent. Fichtlscherer et al (11) reported that the expression level of
miR-208a tends to be higher in patients with CAD, while Nabiałek
et al (24) reported that the
miR-208a level was not different between stable CAD patients and
healthy control subjects. In the present study, the miR-208a
expression level was identified to be significantly higher in CAD
patients when compared with non-CAD control subjects. The
discrepancy between the current results and those of Nabiałek et
al (24) may result from the
smaller number of subjects in the previous study (four stable CAD
patients and five control subjects).
The main cause of CAD is atherosclerosis, which is a
chronic lipid-driven inflammatory disease, with accumulation of
lipids on the arterial wall. Hyperlipidemia is a major risk factor
for CAD. miR-370 is a key miRNA in lipid metabolism and
downregulates the expression of the carnitine palmitoyl transferase
1α gene controlling fatty acid oxidation (25). Motawae et al (26) reported that miR-370 was positively
associated with body mass index, LDL-C and LDL-C/HDL-C ratio, and
was significantly higher in CAD patients and type 2 diabetes
patients with CAD (26). In the
present study, miR-370 was found to be positively correlated with
TC, TG, LDL-C, and significantly higher in the CAD patients than in
the control subjects.
ROC analysis was conducted to investigate the
performance of miR-208a and miR-370. miR-208a may be a more
efficacious biomarker (AUC=0.819) than miR370 (AUC=0.745). miR-208a
exhibits a higher sensitivity (91.6%) than miR-370 (55.8%); while
the specificity of miR-370 was higher (92.0%) than that of miR-208a
(72.0%). As the two miRNAs are involved in different biological
pathways, it may be better to use the two miRNAs in combination
rather than individually. The present study found that the AUC of
the combination of miR-208a and miR-380 was largest at 0.856.
In conclusion, expression levels of miR-208a
(exclusively expressed in the heart) and miR-370 (vital to lipid
metabolism) were significantly higher in CAD patients, rendering
them as potential diagnostic tools for discriminating CAD to
facilitate with the management of patient care. However, to date no
single miRNA could be used as potential biomarker for CAD due to
unsatisfactory sensitivity and specificity values. The present
study indicates that the combination of miRNAs in different
pathways concerning CAD may act more effectively than individual
ones for the diagnosis of CAD. However, in the current study the
sensitivity of the combination (73.7%) at the optimal cut-off is
not satisfactory, as it was lower than that of miR-208a alone.
Therefore, further investigations are required to establish
suitable miRNAs and their combinations for the early diagnosis of
CAD.
Acknowledgements
The present study was supported by the Medical and
Health Science and Technology Development Plan of Shandong Province
of China (grant no. 2014WS0516) and Natural Science Foundation of
Liaoning Province of China (grant no. 2013023044).
References
|
1
|
Chen WW, Gao RL, Liu LS, Zhu ML, Wang W,
Wang YJ, Wu ZS, Li HJ, Zheng Z, Jiang LX, et al: Outline of the
report on cardiovascular disease in China, 2014. Chinese
Circulation Journal. 30:617–622. 2015.(In Chinese).
|
|
2
|
Goedeke L, Aranda JF and
Fernández-Hernando C: MicroRNA regulation of lipoprotein
metabolism. Curr Opin Lipidol. 25:282–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dangwal S and Thum T: MicroRNA
therapeutics in cardiovascular disease models. Annu Rev Pharmacol
Toxicol. 54:185–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Creemers EE, Tijsen AJ and Pinto YM:
Circulating microRNAs: novel biomarkers and extracellular
communicators in cardiovascular disease? Circ Res. 110:483–495.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gilad S, Meiri E, Yogev Y, Benjamin S,
Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed
N, et al: Serum microRNAs are promising novel biomarkers. PLoS One.
3:e31482008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gidlöf O, Andersson P, van der Pals J,
Götberg M and Erlinge D: Cardiospecific microRNA plasma levels
correlate with troponin and cardiac function in patients with ST
elevation myocardial infarction, are selectively dependent on renal
elimination, and can be detected in urine samples. Cardiology.
118:217–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olivieri F, Antonicelli R, Lorenzi M,
D'Alessandra Y, Lazzarini R, Santini G, Spazzafumo L, Lisa R, La
Sala L, Galeazzi R, et al: Diagnostic potential of circulating
miR-499-5p in elderly patients with acute non ST-elevation
myocardial infarction. Int J Cardiol. 167:531–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He
J, Qin YW and Jing Q: Circulating microRNA: a novel potential
biomarker for early diagnosis of acute myocardial infarction in
humans. Eur Heart J. 31:659–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren J, Zhang J, Xu N, Han G, Geng Q, Song
J, Li S, Zhao J and Chen H: Signature of circulating microRNAs as
potential biomarkers in vulnerable coronary artery disease. PLoS
One. 8:e807382013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fichtlscherer S, De Rosa S, Fox H,
Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T,
Müller-Ardogan M, et al: Circulating microRNAs in patients with
coronary artery disease. Circ Res. 107:677–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jansen F, Yang X, Proebsting S, Hoelscher
M, Przybilla D, Baumann K, Schmitz T, Dolf A, Endl E, Franklin BS,
et al: MicroRNA expression in circulating microvesicles predicts
cardiovascular events in patients with coronary artery disease. J
Am Heart Assoc. 3:e0012492014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sondermeijer BM, Bakker A, Halliani A, de
Ronde MW, Marquart AA, Tijsen AJ, Mulders TA, Kok MG, Battjes S,
Maiwald S, et al: Platelets in patients with premature coronary
artery disease exhibit upregulation of miRNA340* and miRNA624*.
PLoS One. 6:e259462011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schulte C and Zeller T: microRNA-based
diagnostics and therapy in cardiovascular disease-summing up the
facts. Cardiovasc Diagn Ther. 5:17–36. 2015.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Rooij E, Quiat D, Johnson BA,
Sutherland LB, Qi X, Richardson JA, Kelm RJ Jr and Olson EN: A
family of microRNAs encoded by myosin genes governs myosin
expression and muscle performance. Dev Cell. 17:662–673. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Callis TE, Pandya K, Seok HY, Tang RH,
Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, et al:
MicroRNA-208a is a regulator of cardiac hypertrophy and conduction
in mice. J Clin Invest. 119:2772–2786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Montgomery RL, Hullinger TG, Semus HM,
Dickinson BA, Seto AG, Lynch JM, Stack C, Latimer PA, Olson EN and
van Rooij E: Therapeutic inhibition of miR-208a improves cardiac
function and survival during heart failure. Circulation.
124:1537–1547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji X, Takahashi R, Hiura Y, Hirokawa G,
Fukushima Y and Iwai N: Plasma miR-208 as a biomarker of myocardial
injury. Clin Chem. 55:1944–1949. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Rooij E, Sutherland LB, Qi X,
Richardson JA, Hill J and Olson EN: Control of stress-dependent
cardiac growth and gene expression by a microRNA. Science.
316:575–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Fan Z, Zhao T, Cao W, Zhang L, Li
H, Xie Q, Tian Y and Wang B: Plasma miR-1, miR-208, miR-499 as
potential predictive biomarkers for acute myocardial infarction: an
independent study of Han population. Exp Gerontol. 72:230–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y and Li J: MicroRNA208 family in
cardiovascular diseases: therapeutic implication and potential
biomarker. J Physiol Biochem. 71:479–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiao J, Shen B, Li J, Lv D, Zhao Y, Wang F
and Xu J: Serum microRNA-499 and microRNA-208a as biomarkers of
acute myocardial infarction. Int J Clin Exp Med. 7:136–141.
2014.PubMed/NCBI
|
|
24
|
Nabiałek E, Wańha W, Kula D, Jadczyk T,
Krajewska M, Kowalówka A, Dworowy S, Hrycek E, Włudarczyk W, Parma
Z, et al: Circulating microRNAs (miR-423-5p, miR-208a and miR-1) in
acute myocardial infarction and stable coronary heart disease.
Minerva Cardioangiol. 61:627–637. 2013.PubMed/NCBI
|
|
25
|
Iliopoulos D, Drosatos K, Hiyama Y,
Goldberg IJ and Zannis VI: MicroRNA-370 controls the expression of
microRNA-122 and Cpt1alpha and affects lipid metabolism. J Lipid
Res. 51:1513–1523. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Motawae TM, Ismail MF, Shabayek MI and
Seleem MM: MicroRNAs 9 and 370 association with biochemical markers
in T2D and CAD Complication of T2D. PLoS One. 10:e01269572015.
View Article : Google Scholar : PubMed/NCBI
|