Introduction
Subarachnoid hemorrhage (SAH) is a destructive type
of stroke, which contributes to ≥30% patients succumbing due to the
original hemorrhage and rehemorrhage despite the development of
novel treatment strategies (1–7). Although SAH is rare, it has a marked
impact, particularly on young individuals, and poor outcomes
(2,8,9). Therefore,
it is vital to investigate the risk factors of SAH and attempt to
prevent these with the aim of reducing the incidence of SAH.
Alcohol consumption is common throughout the world and numerous
observational studies have analyzed the role of alcoholic beverage
intake and SAH, with some demonstrating alcohol as a risk factor or
some presenting an unclear association (10–23).
In previous studies, alcohol intake and the risk of
hemorrhagic stroke were investigated in the two meta-analyses,
whereas intracerebral hemorrhage (ICH) and SAH were not classified
in either of the studies (24,25). Furthermore, their investigative
consequences primarily represented ICH, as the incidence of ICH was
double that of SAH (26). The impacts
of alcohol consumption on the incidence of SAH are inconclusive and
limited. Another two systematic reviews presented the association
between alcohol intake and the risk of SAH; however, the alcohol
consumption level was inexact and a further association was not
identified through dose-response analysis (27,28). Thus,
the current meta-analysis, which included a dose-response analysis,
was performed based on previous observational studies with the aim
of identifying the specific association between various levels of
alcohol consumption and the risk of SAH.
Materials and methods
Study selection
This meta-analysis was conducted on the basis of the
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
(PRISMA) statement issued in 2009 (29). A systematic literature search of the
Pubmed (http://www.ncbi.nlm.nih.gov/pubmed) and Embase
(http://www.embase.com) electronic databases for
pertinent studies published from their inception to 20 January 2016
was performed by two authors (Mr. Xiyang Yao and Dr Gang Chen)
independently. The search strategy used terms as follows:
‘Subarachnoid hemorrhage’ AND (‘alcohol’ OR ‘alcoholic beverages’
OR ‘ethanol’ OR ‘wine’ OR ‘liquor’ OR ‘spirits’ OR ‘beer’). No
language restriction was set. In addition, a manual search of the
probable missing publications of interest in our previous
literature search was based on the reference lists of relevant
studies.
Publications included in our meta-analysis complied
with the following criteria: i) A study of the association between
alcohol consumption and SAH; ii) case-control or cohort studies;
iii) the effect estimate [relative risk (RR), hazard ratio (HR),
odd ratio (OR)] and its corresponding 95% confidence intervals
(CIs) were supplied (or enough data to calclulate them); iv)
alcohol consumption was classified by quantity; v) the most
informative study remained in the current meta-analysis if two or
more published reports were based on the same research
population.
Data abstraction and quality
assessment
The following data were collected: First author's
last name, published year, country, study design, assessment of
alcohol consumption, sample size, female proportion, age at
baseline, follow up duration (years), effect estimate and its 95%
CI, and matched or adjusted factors. The Newcastle-Ottawa Scale
(NOS) (30) was selected to assess the
quality of the included studies. The NOS is composed of three
subscales as follows: Selection, comparability and outcome.
Furthermore, it includes a range from 0 to 9 stars, and a study was
considered to be of high quality when it obtained ≥7 stars. Two
authors (Mr. Xiyang Yao and Dr Gang Chen) gathered the data and
evaluated the study quality separately. An additional author
inspected and adjudicated the information according to the original
studies.
Statistical analysis
RR with 95% CI measured the association between
alcohol consumption and SAH. As the prevalence of SAH was
relatively low, ORs and HRs were directly considered to be RRs
(31). Adjusted RRs were employed in
the meta-analysis and the crude data was used when the study didn't
provide the adjusted data. The Q-statistic (P<0.1 was identified
as statistically significant) and I2 percentage
(I2: <50%, low heterogeneity; 50–75%, moderate
heterogeneity; >75%, high heterogeneity) were used to evaluate
the statistical heterogeneity between studies (32,33). The
random-effects model was used when heterogeneity existed between
studies and a fixed model was adopted otherwise (34). Subgroup analyses were performed
according to gender, study design, geographic area, matched or
adjusted status and study quality. Sensitivity analyses were
conducted by omitting one study each time to evaluate the influence
of the single study on the remaining studies. In addition,
publication bias for the association between alcohol consumption
and the risk of SAH was statistically assessed by Egger test
(35) and Begg's test (36). P<0.05 was considered to indicate a
statistically significant difference. When publication bias was
apparent the ‘trim and fill’ method was used to modify it (37).
One drink was defined as 12.5 g, with 1 ml as 0.8 g
and 1 oz as 28.35 g ethanol, and used grams of ethanol per day
(g/day) as a standard measurement of alcohol consumption (38). The midpoint of each classification was
taken as the quantity of alcohol consumption per day. When
referring to the open-ended upper dose classification, it was
counted as 1.2 times that of the lower bound for analyses (39). Non-drinkers were regarded as the
reference category. Alcohol consumption was placed in three
categories as follows: Light, <15 g/day; moderate, 15–30 g/day;
and heavy, >30 g/day (25). When
more than one category fell into one alcohol consumption level in
certain studies, the RRs within a single category were combined for
each study after which all studies were pooled (24). A dose-response analysis by generalized
least squares regression models (40)
was performed for further analysis and alcohol consumption (g/day)
was considered as the explanatory variable. The potential curve
linear correlation with alcohol consumption and SAH was evaluated
using restricted cubic splines with three knots at percentiles 25,
50 and 75% of the distribution (41).
The null hypothesis, where the coefficient of the second spline was
equal to 0, was used to assess linearity or non-linearity according
to a P-value (42). RRs with 95% CIs,
distribution of cases and controls or person-years for at least
three quantitative types of classifications have to be provided in
initial studies. STATA 12.0 (StataCorp LP, College Station, TX,
USA) was used to perform all statistical analyses.
Results
Literature search
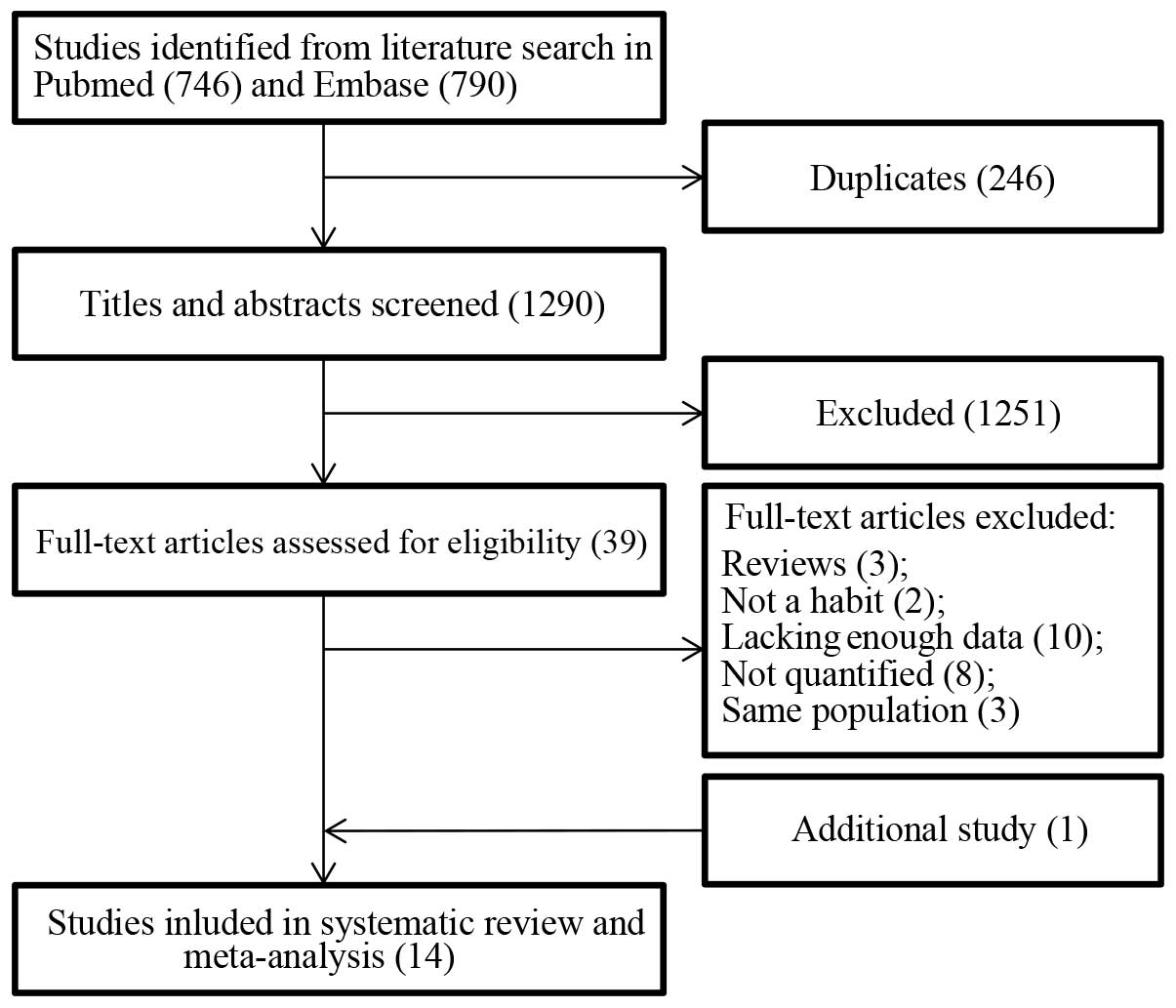
Fig. 1 demonstrates the
search flow. In total, 1,536 search records were yielded from the
PubMed and Embase databases. There were 246 duplicates that were
omitted, and 1,251 records were further removed after scanning the
titles and abstracts, as these did not meet the inclusion criteria.
The remaining 39 records were selected for full-text evaluation and
26 studies were excluded due to the following: Three studies were
excluded because they were reviews (43–45). Two
studies were excluded as they only investigated alcohol consumption
within 24 h and one week before SAH, which was not representative
of habitual alcohol consumption (46,47). Ten
studies (48–57) that lacked sufficient data calculating
RR estimates, and eight studies (58–65) where
alcohol consumption had not been quantified were excluded. The
remaining three studies reported on the same population (66–68).
Furthermore, only one additional study of interest was identified
during the manual search, which was then included (12). In total, 14 studies (10–23) were
included in the current meta-analysis of which nine were cohort
studies and five were case-control studies.
Study characteristics
The published period of the studies was from 1986 to
2013. Among the 14 studies, five were from the USA (10,11,14,20,22), one was from Britain (19), one was from Finland (18) and seven were from Japan (12,13,15–17,21,23). A total
of 480,014 individuals were reported in the cohort studies and
35.2% were male. The follow-up period of the cohorts ranged from
3.8 to 17.9 years and the number of participants ranged from 2,890
to 128,934 in every cohort study. In addition, there were 1,182
cases and 2,357 controls from the five case-control studies. Seven
of the studies achieved a rating of >6 stars and were,
therefore, considered to be high quality. Whereas, the remaining
seven studies were considered to be low quality as a result of the
star rating. Detailed characteristics are presented in Table I.
 | Table I.Key characteristics of the included
studies. |
Table I.
Key characteristics of the included
studies.
| Author, year
(Refs.) | Country | Subjects, n | Gender | Cases, n | Years of duration
or study period | Exposure
assessment | Adjusted or matched
variables | NOS score |
|---|
| Cohort studies |
|
|
Donahue, 1986 (10) | USA | 8006 | M | 32 | 12 | IPI | a,b,f,g,i,j,k | 6 |
|
Stampfer, 1988 (11) | USA | 87526 | F | 28 | 3.8 | SAQ | a | 5 |
| Iso,
1995 (12) | Japan | 2890 | M | 18 | 10.5 | IPI | a | 6 |
| Sankai,
2000 (13) | Japan | 12372 | M+F | 71 | 9.4 | IPI | a,b,d,f-i | 8 |
|
Klatsky, 2002 (14) | USA | 128934 | M+F | 133 | 7 | SAQ | a-d,g,s | 7 |
| Yamada,
2003 (15) | Japan | 109293 | M+F | 244 | 9.9 | SAQ | a | 7 |
| Iso,
2004 (16) | Japan | 19544 | M | 73 | 11 | SAQ | None | 7 |
|
Ikehara, 2013 (17) | Japan | 47100 | F | 338 | 16.7 | SAQ | a,d-f-i,m-o | 7 |
| Korja,
2013 (18) | Finland | 64349 | M+F | 437 | Median 17.9 | SAQ | a,b | 6 |
| Case-control
studies |
|
| Gill,
1991 (19) | Britain | 766 | M+F | 193 | NR | SAQ | a-d,f,p-q,r | 6 |
|
Longstreth, 1992 (20) | USA | 447 | M+F | 149 | 1987–1989 | IPI | a,b | 7 |
| Kubota,
2001 (21) | Japan | 254 | M+F | 127 | NR | SAQ | a,b | 7 |
|
Qureshi, 2001 (22) | USA | 1292 | M+F | 323 | 1990–1997 | MR | a-c | 4 |
| Ohkuma,
2003 (23) | Japan | 780 | M+F | 390 | 2000–2001 | SAQ | None | 5 |
Alcohol consumption and risk of
SAH
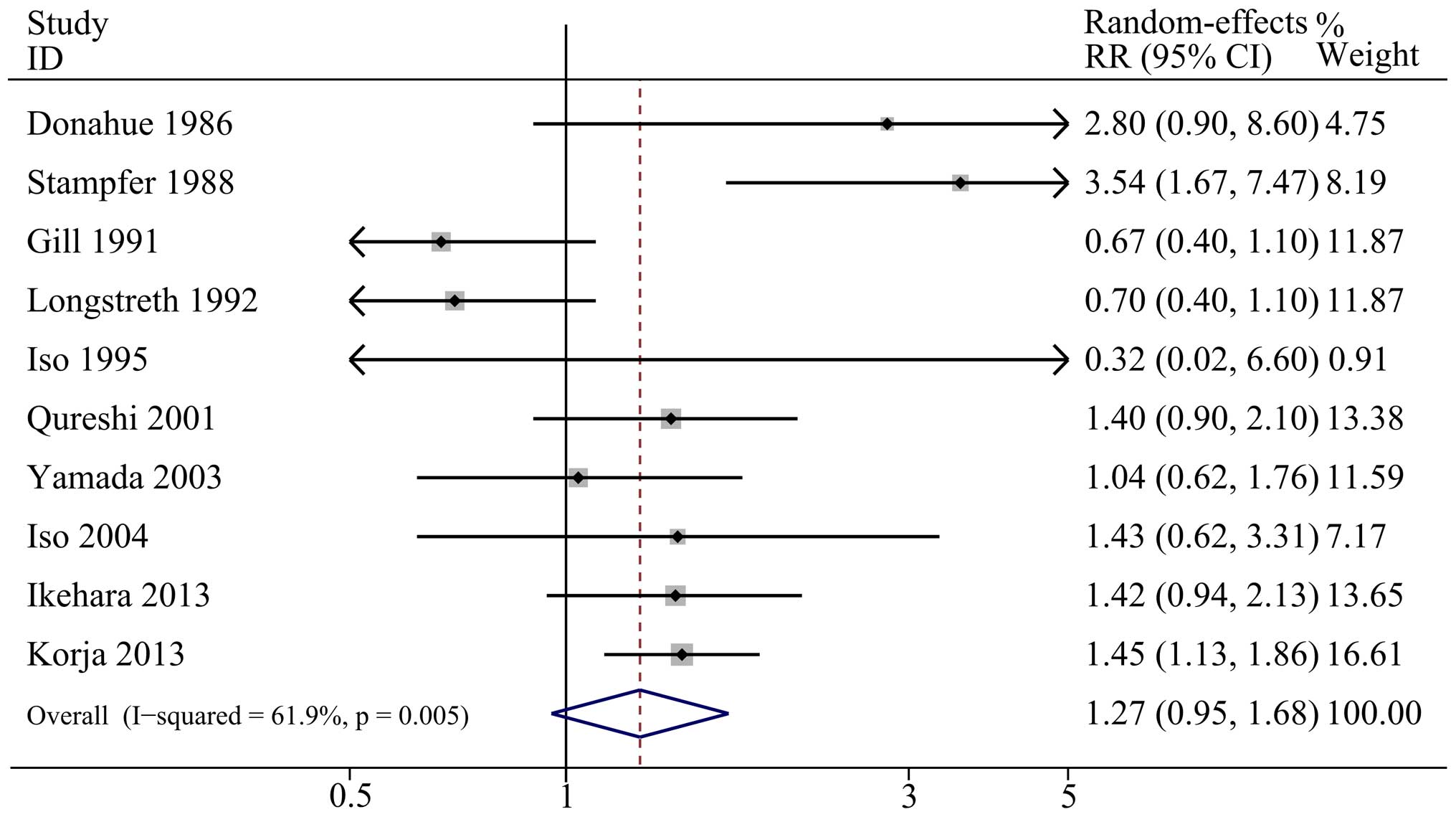
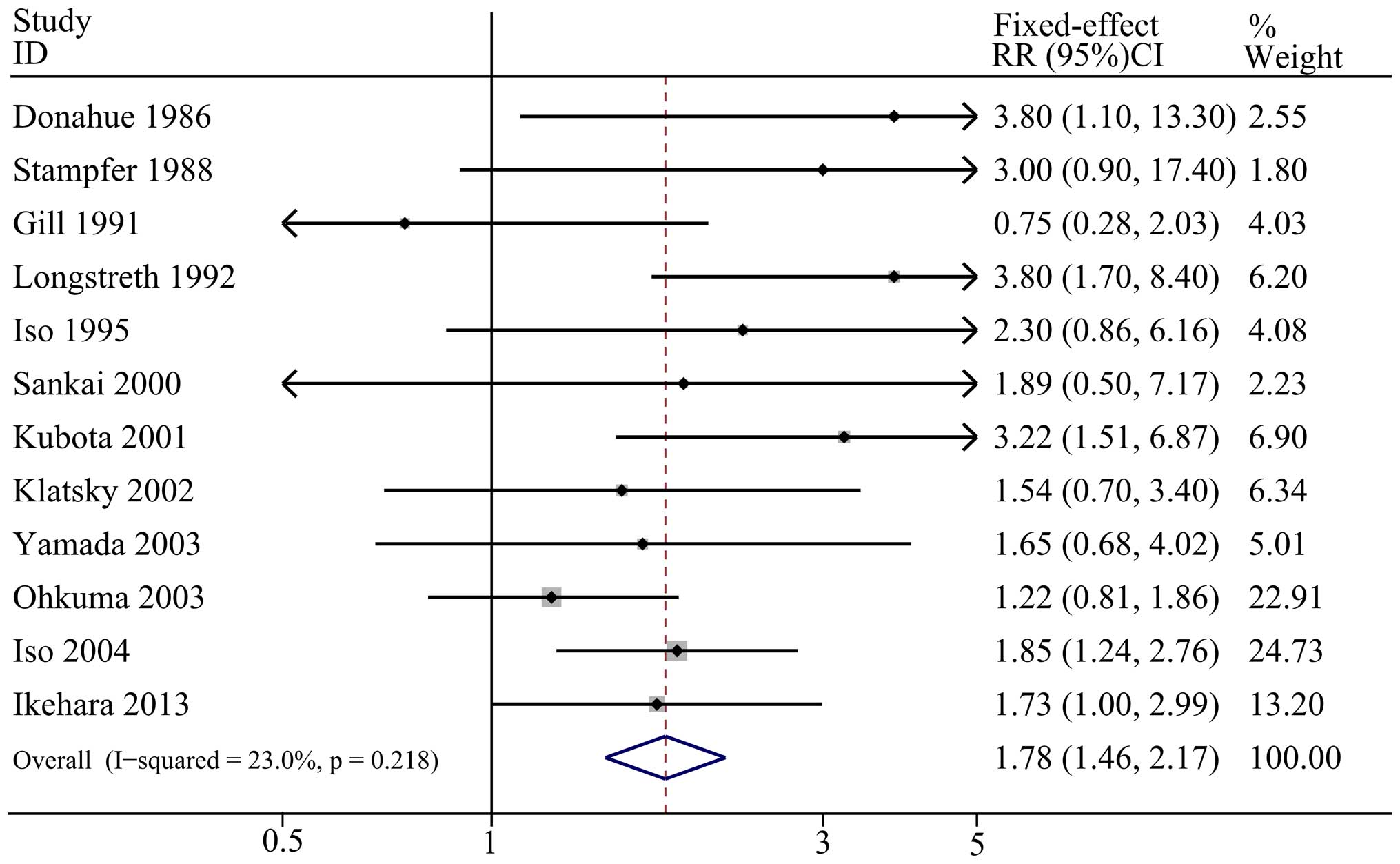
Figs. 2–4 demonstrate the outcomes from the
random-effects model (light and moderate alcohol consumption) or
fixed-effect model (heavy alcohol consumption) of pooled RRs for
SAH. The combined RR of light alcohol consumption (<15 g/day)
and moderate alcohol consumption (15–30 g/day) compared with no
alcohol consumption were 1.27 (95% CI: 0.95, 1.68;
I2=61.9%; P=0.005 for heterogeneity) and 1.33 (95% CI:
0.84, 2.09; I2=59.6%; P=0.022 for heterogeneity),
respectively, which indicated no significant association between
light or moderate alcohol consumption and SAH. Furthermore, the
summary RR showed an increased risk of SAH when heavy alcohol
consumption (>30 g/day) was compared with no alcohol
consumption, with a pooled result of 1.78 (95% CI: 1.46, 2.17;
I2=23.0%; P=0.218 for heterogeneity).
Stratified analyses
To minimize heterogeneity, stratified analyses were
conducted between the included studies. The subgroups were
generated in terms of pivotal study characteristics: Study design,
gender, geographic area, type of SAH, study quality and adjustment
status (smoking and blood pressure). The intact stratified results
are presented in Table II.
 | Table II.Summary RRs for alcohol consumption
and SAH. |
Table II.
Summary RRs for alcohol consumption
and SAH.
|
| Low alcohol
consumption | Moderate alcohol
consumption | Heavy low alcohol
consumption |
|---|
|
|
|
|
|
|---|
|
| RR (95% CI) |
P-valuea | RR (95% CI) |
P-valuea | RR (95% CI) |
P-valuea |
|---|
| Total outcomes | 1.27
(0.95,1.68) |
0.10 | 1.33
(0.84,2.09) |
0.23 | 1.78
(1.46,2.17) | <0.01 |
| Study design |
|
|
Cohort | 1.53
(1.16,2.02) | <0.01 | 2.08
(0.90,4.81) |
0.09 | 1.88
(1.46,2.43) | <0.01 |
|
Case-control | 0.88
(0.54,1.68) |
0.63 | 1.33
(0.84,2.09) |
0.68 | 1.64
(1.20,2.24) | <0.01 |
| Gender |
|
|
Men | 1.46
(1.11,1.93) | <0.01 | 3.5 (1.5,20.7) |
0.04 | 1.77
(1.36,2.30) | <0.01 |
|
Women | 1.33
(0.82,2.18) |
0.25 | 2.41
(0.66,8.78) |
0.18 | 1.27
(0.90,1.79) |
0.18 |
| Geographic
area |
|
| North
America | 1.63
(0.81,3.27) |
0.17 | 2.16
(1.29,3.59) | <0.01 | 2.64
(1.63,4.28) | <0.01 |
|
Asia | 1.26
(0.94,1.70) |
0.13 | 0.82
(0.62,1.09) |
0.17 | 1.71
(1.37,2.14) | <0.01 |
|
Europe | 1.02
(0.48,2.16) |
0.96 | / | / | 0.75
(0.28,2.02) |
0.57 |
| Type of SAH |
|
| Total
SAH | 1.27
(0.95,1.68) |
0.10 | 1.61
(1.07,2.44) |
0.02 | 1.90
(1.50,2.41) | <0.01 |
|
aSAH | – | – | 0.80
(0.58,1.09) |
0.16 | 1.53
(1.06,2.20) |
0.02 |
| Study quality |
|
|
Low | 1.49
(0.75,2.98) |
0.26 | 2.19
(0.54,8.85) |
0.27 | 1.41
(1.01,1.97) |
0.04 |
|
High | 1.20
(0.91,1.57) |
0.20 | 1.12
(0.77,1.63) |
0.55 | 2.02
(1.58,2.59) |
0.02 |
| Adjusted
smoking |
|
|
Yes | 1.23
(0.62,2.45) |
0.56 | 1.54
(0.71,3.36) |
0.27 | 1.62
(1.09,2.39) |
0.04 |
| No | 1.31
(0.94,1.83) |
0.12 | 1.25
(0.68,2.30) |
0.47 | 2.02
(1.45,2.81) | <0.01 |
| Adjusted blood
pressure |
|
|
Yes | 1.23
(0.62,2.45) |
0.57 | 1.65
(0.46,5.92) |
0.44 | 1.64
(0.94,2.87) |
0.01 |
| No | 1.31
(0.94,1.83) |
0.12 | 1.27
(0.74,2.18) |
0.38 | 1.93
(1.44,2.58) | <0.01 |
For light alcohol consumption, total RRs of light
alcohol consumption were not identified to be associated with the
risk of SAH and had a moderate heterogeneity. However, if the study
design was cohort, light alcohol consumption was observed to have
an increased association with the risk of SAH and low
heterogeneity, with RR of 1.53 (95% CI: 1.16, 2.02;
I2=35.7%; P=0.156 for heterogeneity). Furthermore, the
pooled RR of 1.46 (95% CI: 1.11, 1.93; I2=0%; P=0.468
for heterogeneity) was statistically significant and there was no
evidence of heterogeneity with regard to men and light alcohol
consumption. No further statistically significant data was found in
the geographic area, types of SAH, adjustment for smoking,
adjustment for blood pressure and study quality.
For moderate alcohol consumption, no statistical
significance was identified with the overall RRs of moderate
alcohol consumption compared with the risk of SAH, and moderate
heterogeneity was indicated. Notably, low heterogeneity was
observed and an increased risk between moderate alcohol consumption
and SAH was identified when the geographic area was North America
and the type of SAH was total SAH. Furthermore, only one study
mentioned the data regarding association with moderate alcohol
consumption and the risk of SAH in a male population. No
statistically significant information was found according to the
results of the groups, such as study design, study quality and
adjustments.
For heavy alcohol consumption, the RRs of women and
adjustment for blood pressure along with heavy alcohol consumption
did not show statistical significance with the risk of SAH, while
other items indicated a statistically significant increased risk
(Table II).
Sensitivity analyses and publication
bias
The outcomes of sensitivity analyses were not
identified to be significantly varied (data not shown). The Egger
test (P=0.906) and Begg's test (P=0.719) indicated no evidence of
publication bias for the association with light alcohol consumption
and the risk of SAH. For heavy alcohol consumption, similar results
were observed with the Egger test (P=0.235) and Begg's test
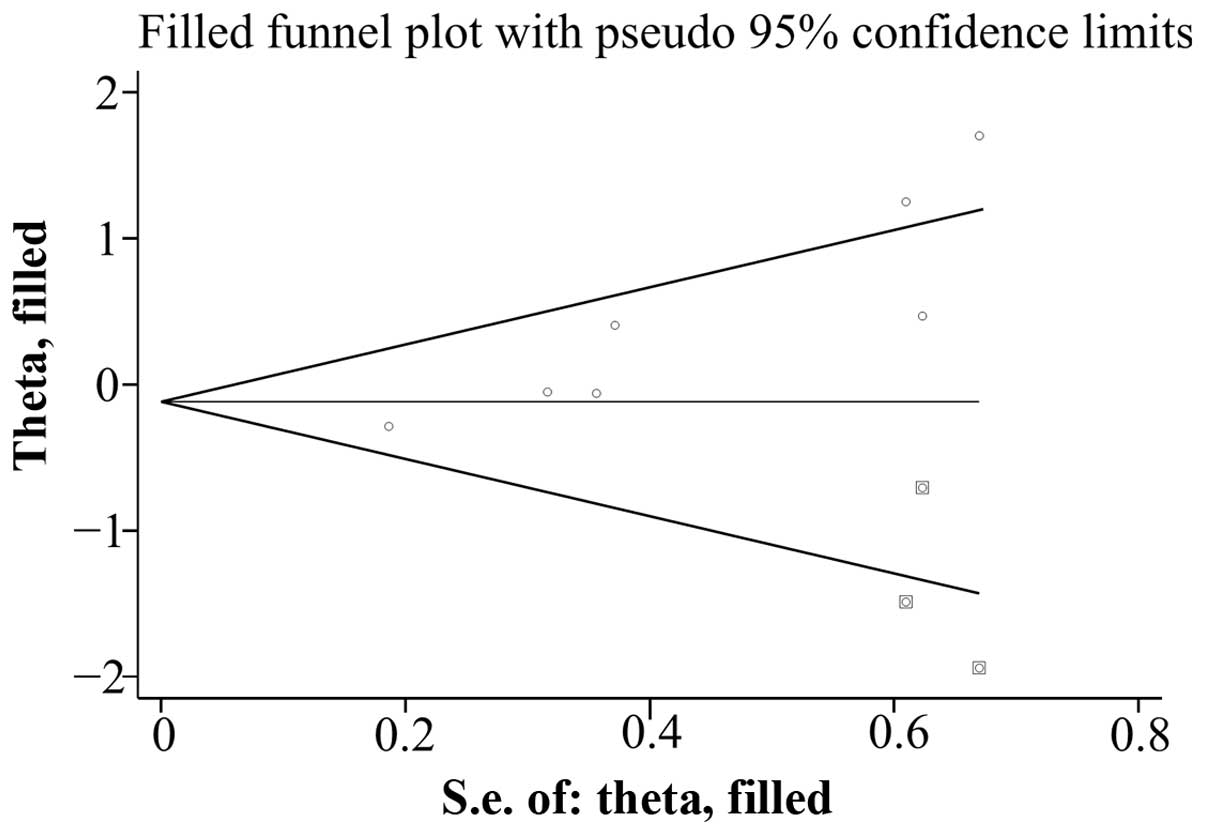
(P=0.493). However, potential evidence of publication bias was
found for SAH risk with moderate alcohol consumption according to
the Egger test (P=0.004) and Begg's test (P=0.011). The adjusted
result from the ‘trim and fill’ method for publication bias was
0.94 (95% CI: 0.57, 1.53), which did not change the former
conclusions for SAH risk and moderate alcohol consumption. Fig. 5 demonstrates the funnel plot after
applying the ‘trim and fill’ method.
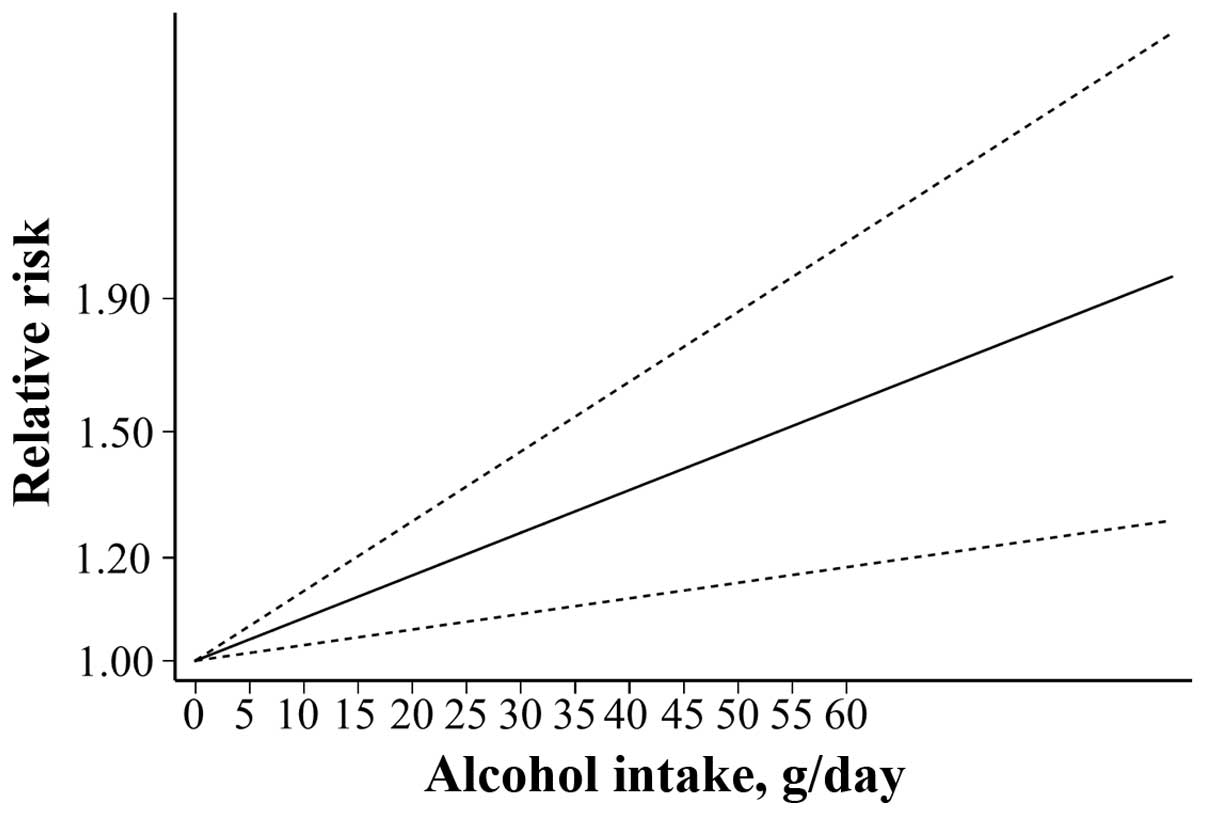
Dose-response association
Seven studies were included in the dose-response
analysis of alcohol consumption with SAH risk (10,11,14,16,17,19,20). A linear increase in SAH with increasing
alcohol consumption (P=0.013) was observed after accessing
dose-response patterns for alcohol consumption (g/day) for alcohol
consumers vs. teetotal individuals (Fig.
6). Furthermore, the risk increased by 12.1% for each
additional 10 g/day of alcohol consumption.
Discussion
In the past several decades, the role of alcohol
intake in the development of SAH has been increasingly recognized.
Teunissen et al (27) conducted
a systematic review about the risk factors for SAH, which five
studies referring to alcohol intake and the risk of SAH. Alcohol
abuse was identified to be a significant risk factor for SAH, with
the results of drinking ≥21.4 g/day alcohol being RR=4.7 (95% CI:
2.1, 10.5) according to two longitudinal studies, and odds ratio
(OR)=1.5 (95% CI: 1.1, 1.9) according to three case control
studies. Subsequently, an updated systematic review of
epidemiological studies was conducted by the same group, which
demonstrated no different conclusions with the inclusion of extra
studies (28). The current
meta-analysis was based on observational studies that had
quantified alcohol consumption with the aim of identifying probable
correlations between alcohol consumption and the risks of SAH. The
analysis included 483,553 individuals and 2,556 patients from nine
cohort and five case-control studies. The results indicated that
there was no correlation between light or moderate alcohol
consumption and the risk of SAH, while an increased risk of SAH was
found to be associated with heavy alcohol consumption. The
dose-response analysis evidenced a distinct linear association
between alcohol consumption and SAH, which was consistent with the
findings of Leppala et al (69). In addition, the dose-response analysis
indicated an increased risk of 12.1% for every increase of 10 g/day
alcohol when comparing drinkers to teetotal individuals.
The current findings confirmed the results from
previous systematic reviews (27,28).
Furthermore, the accuracy of the risk estimates was augmented with
the accumulative data and stratified analyses were executed to
evaluate the origin of heterogeneity (70), thereby advancing the clinical
association of the present findings. Subgroup analysis indicated
that light alcohol consumption was associated with an increase in
SAH risk in cohort studies and men. Smoking was confirmed to be an
independent risk factor for SAH (20,21,52,71,72) and men smoked more than women, which may
be the explanation for the association between light alcohol
consumption and the increased risk of SAH in men. Moderate alcohol
consumption was associated with an increased risk of SAH in North
American individuals; however, the explanation of this finding was
unclear and requires additional investigation to be confirmed.
Hypertension (73), reducing platelet
aggregation (74) and enhancing
fibrinolysis from endothelial cells (75) may be the reasons for the association
between an increased risk of SAH and heavy alcohol consumption;
although heavy alcohol consumption was not significantly associated
with SAH in women. The potential reasons are as follows: i) Heavy
drinkers were likely to be inclined to take part in the surveys as
participants and less willing to complete repeat questionnaires in
detail (11); ii) all volunteers in
the study by Stampfer et al (11) were female nurses who may have had
knowledge of the negative outcomes of drinking excessively, and
therefore avoided heavy drinking (11); and iii) small population number
(13,19). Notably, although hypertension has been
consistently considered as the strongest predictor of SAH in
previous studies (76–80), no correlation was found between heavy
alcohol consumption and SAH risk in individuals from the included
studies, which adjusted for blood pressure (10,13,17,19).
However, the association between alcohol and stroke diminished
markedly when adjusting for varying hypertension (3).
A notable strength of the present study is that it
contains a broader individual population and has a longer follow-up
when compared with previous studies. However, when interpreting the
outcomes of the current meta-analysis, certain limitations should
also be considered. First, the impact of potential confounding
factors is a well-known issue resulting from the observational
design of all of the included studies. In consideration of an
osculating association between alcohol consumption and demographic,
family history and lifestyle factors, the residual confounding
factor should be categorized distinctly between these variables in
future research. Second, information, select and recall bias
usually lead to overestimating and underestimating the real
associations in the observational studies. Furthermore, evidence of
publication bias existed when pooling the results of correlation
with moderate alcohol consumption and the risk of SAH, although the
conclusion remained unchanged following the ‘trim and fill’ method.
Third, the strength of the correlation may have been weakened by
misclassification bias. As the alcohol consumption assessment was
based on self-reports and self-administered questionnaires,
misclassification bias was inevitable. Finally, stratified analyses
to examine the influence of different types of alcohol consumption
on these correlations were conducted on account of the limited
number of studies.
The present findings reveal various implications
that are significant to public health. Individuals who reduce their
alcohol consumption may also reduce their risk of SAH. Therefore,
subjects who are alcohol drinkers should be encouraged to modify
their habits to diminish the possibility of heavy alcohol
consumption. Although small quantities of alcohol consumption are
thought to reduce the risk of ischemic stroke (24,25), alcohol
indeed promotes certain types of cancer, such as colorectal and
gastric cancer (81,82) and, according to the present
meta-analysis, increases the risk of SAH.
In conclusion, heavy alcohol consumption (>30
g/day) is associated with increased risk of SAH. The association
between alcohol consumption and SAH has clinical implications for
primary and secondary prevention of SAH. The implications of the
findings require careful evaluation, as any suggestion regarding
alcohol consumption must be tailored to the risks of each
individual patient.
Acknowledgements
The present study was supported by Suzhou Key
Medical Center (grant no. Szzx201501), grants from the National
Natural Science Foundation of China (grant nos. 81571115, 81422013
and 81471196), the Scientific Department of Jiangsu Province (grant
no. BL2014045), Suzhou Government (grant nos. LCZX201301, SZS201413
and SYS201332) and A Project Funded by the Priority Academic
Program Development of Jiangsu Higher Education Institutions.
References
|
1
|
Hanel RA, Xavier AR, Mohammad Y, Kirmani
JF, Yahia AM and Qureshi AI: Outcome following intracerebral
hemorrhage and subarachnoid hemorrhage. Neurol Res. 24:(Suppl 1).
S58–S62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnston SC, Selvin S and Gress DR: The
burden, trends, and demographics of mortality from subarachnoid
hemorrhage. Neurology. 50:1413–1418. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hasegawa Y, Suzuki H, Uekawa K, Kawano T
and Kim-Mitsuyama S: Characteristics of cerebrovascular injury in
the hyperacute phase after induced severe subarachnoid hemorrhage.
Transl Stroke Res. 6:458–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marbacher S, Nevzati E, Croci D, Erhardt
S, Muroi C, Jakob SM and Fandino J: The rabbit shunt model of
subarachnoid haemorrhage. Transl Stroke Res. 5:669–680. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pluta RM, Bacher J, Skopets B and Hoffmann
V: A non-human primate model of aneurismal subarachnoid hemorrhage
(SAH). Transl Stroke Res. 5:681–691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turan N, Heider RA, Zaharieva D, Ahmad FU,
Barrow DL and Pradilla G: Sex differences in the formation of
intracranial aneurysms and incidence and outcome of subarachnoid
hemorrhage: Review of experimental and human studies. Transl Stroke
Res. 7:12–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng C, Jiang L, Yang Y, Wu H, Huang Z
and Sun X: Effect of APOE gene polymorphism on early cerebral
perfusion after aneurysmal subarachnoid hemorrhage. Transl Stroke
Res. 6:446–450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Linn FH, Rinkel GJ, Algra A and van Gijn
J: Incidence of subarachnoid hemorrhage: Role of region, year, and
rate of computed tomography: A meta-analysis. Stroke. 27:625–629.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Etminan N: Aneurysmal subarachnoid
hemorrhage-status quo and perspective. Transl Stroke Res.
6:167–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Donahue RP, Abbott RD, Reed DM and Yano K:
Alcohol and hemorrhagic stroke. The honolulu heart program. JAMA.
255:2311–2314. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stampfer MJ, Colditz GA, Willett WC,
Speizer FE and Hennekens CH: A prospective study of moderate
alcohol consumption and the risk of coronary disease and stroke in
women. N Engl J Med. 319:267–273. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iso H, Kitamura A, Shimamoto T, Sankai T,
Naito Y, Sato S, Kiyama M, Iida M and Komachi Y: Alcohol intake and
the risk of cardiovascular disease in middle-aged Japanese men.
Stroke. 26:767–773. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sankai T, Iso H, Shimamoto T, Kitamura A,
Naito Y, Sato S, Okamura T, Imano H, Iida M and Komachi Y:
Prospective study on alcohol intake and risk of subarachnoid
hemorrhage among Japanese men and women. Alcohol Clin Exp Res.
24:386–389. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Klatsky AL, Armstrong MA, Friedman GD and
Sidney S: Alcohol drinking and risk of hemorrhagic stroke.
Neuroepidemiology. 21:115–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamada S, Koizumi A, Iso H, Wada Y,
Watanabe Y, Date C, Yamamoto A, Kikuchi S, Inaba Y, Toyoshima H, et
al: Risk factors for fatal subarachnoid hemorrhage: The Japan
collaborative cohort study. Stroke. 34:2781–2787. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iso H, Baba S, Mannami T, Sasaki S, Okada
K, Konishi M and Tsugane S: JPHC Study Group: Alcohol consumption
and risk of stroke among middle-aged men: The JPHC study cohort I.
Stroke. 35:1124–1129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ikehara S, Iso H, Yamagishi K, Kokubo Y,
Saito I, Yatsuya H, Inoue M and Tsugane S: JPHC Study group:
Alcohol consumption and risk of stroke and coronary heart disease
among Japanese women: The Japan public health center-based
prospective study. Prev Med. 57:505–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Korja M, Silventoinen K, Laatikainen T,
Jousilahti P, Salomaa V, Hernesniemi J and Kaprio J: Risk factors
and their combined effects on the incidence rate of subarachnoid
hemorrhage-a population-based cohort study. PLoS One. 8:e737602013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gill JS, Shipley MJ, Tsementzis SA, Hornby
RS, Gill SK, Hitchcock ER and Beevers DG: Alcohol consumption-a
risk factor for hemorrhagic and non-hemorrhagic stroke. Am J Med.
90:489–497. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Longstreth WT Jr, Nelson LM, Koepsell TD
and van Belle G: Cigarette smoking, alcohol use, and subarachnoid
hemorrhage. Stroke. 23:1242–1249. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kubota M, Yamaura A and Ono J: Prevalence
of risk factors for aneurysmal subarachnoid haemorrhage: Results of
a Japanese multicentre case control study for stroke. Br J
Neurosurg. 15:474–478. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qureshi AI, Suri MF, Yahia AM, Suarez JI,
Guterman LR, Hopkins LN and Tamargo RJ: Risk factors for
subarachnoid hemorrhage. Neurosurgery. 49:607–613. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohkuma H, Tabata H, Suzuki S and Islam MS:
Risk factors for aneurysmal subarachnoid hemorrhage in Aomori,
Japan. Stroke. 34:96–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reynolds K, Lewis B, Nolen JD, Kinney GL,
Sathya B and He J: Alcohol consumption and risk of stroke: A
meta-analysis. JAMA. 289:579–588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Qin YY, Chen Q, Jiang H, Chen XZ,
Xu CL, Mao PJ, He J and Zhou YH: Alcohol intake and risk of stroke:
A dose-response meta-analysis of prospective studies. Int J
Cardiol. 174:669–677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Broderick JP, Brott T, Tomsick T, Miller R
and Huster G: Intracerebral hemorrhage more than twice as common as
subarachnoid hemorrhage. J Neurosurg. 78:188–191. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teunissen LL, Rinkel GJ, Algra A and van
Gijn J: Risk factors for subarachnoid hemorrhage: A systematic
review. Stroke. 27:544–549. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feigin VL, Rinkel GJ, Lawes CM, Algra A,
Bennett DA, van Gijn J and Anderson CS: Risk factors for
subarachnoid hemorrhage: An updated systematic review of
epidemiological studies. Stroke. 36:2773–2780. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med. 6:e10000972009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V and Tugwell P: The Newcastle-Ottawa Scale (NOS) for
Assessing the Quality of Nonrandomised Studies in Meta-Analyses.
Available online. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htmAccessed.
February 25–2016.
|
|
31
|
Greenland S: Quantitative methods in the
review of epidemiologic literature. Epidemiol Rev. 9:1–30.
1987.PubMed/NCBI
|
|
32
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ali-Hassan-Sayegh S, Mirhosseini SJ,
Tahernejad M, Mahdavi P, Haddad F, Shahidzadeh A, Lotfaliani MR,
Sedaghat-Hamedani F, Kayvanpour E, Weymann A, et al: Administration
of erythropoietin in patients with myocardial infarction: Does it
make sense? An updated and comprehensive meta-analysis and
systematic review. Cardiovasc Revasc Med. 16:179–189. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Egger M, Smith G Davey, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Duval S and Tweedie R: Trim and fill: A
simple funnel-plot-based method of testing and adjusting for
publication bias in meta-analysis. Biometrics. 56:455–463. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Galeone C, Malerba S, Rota M, Bagnardi V,
Negri E, Scotti L, Bellocco R, Corrao G, Boffetta P, La Vecchia C
and Pelucchi C: A meta-analysis of alcohol consumption and the risk
of brain tumours. Ann Oncol. 24:514–523. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Berlin JA, Longnecker MP and Greenland S:
Meta-analysis of epidemiologic dose-response data. Epidemiology.
4:218–228. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Greenland S and Longnecker MP: Methods for
trend estimation from summarized dose-response data, with
applications to meta-analysis. Am J Epidemiol. 135:1301–1309.
1992.PubMed/NCBI
|
|
41
|
Ma Y, Zhang P, Wang F, Yang J, Liu Z and
Qin H: Association between vitamin D and risk of colorectal cancer:
A systematic review of prospective studies. J Clin Oncol.
29:3775–3782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Harrell FE Jr, Lee KL and Pollock BG:
Regression models in clinical studies: Determining relationships
between predictors and response. J Natl Cancer Inst. 80:1198–1202.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Daniel S and Bereczki D: Alcohol as a risk
factor for hemorrhagic stroke. Ideggyogy Sz. 57:247–256.
2004.PubMed/NCBI
|
|
44
|
Hillbom M, Saloheimo P and Juvela S:
Alcohol consumption, blood pressure, and the risk of stroke. Curr
Hypertens Rep. 13:208–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Andreasen TH, Bartek J Jr, Andresen M,
Springborg JB and Romner B: Modifiable risk factors for aneurysmal
subarachnoid hemorrhage. Stroke. 44:3607–3612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Juvela S, Hillbom M, Numminen H and
Koskinen P: Cigarette smoking and alcohol consumption as risk
factors for aneurysmal subarachnoid hemorrhage. Stroke. 24:639–646.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Anderson C, Ni Mhurchu C, Scott D, Bennett
D, Jamrozik K and Hankey G: Australasian Cooperative Research on
Subarachnoid Hemorrhage Study Group: Triggers of subarachnoid
hemorrhage: Role of physical exertion, smoking, and alcohol in the
Australasian cooperative research on subarachnoid hemorrhage study
(ACROSS). Stroke. 34:1771–1776. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Acuña MY and Cifuentes AL: Aneurismal
subarachnoid hemorrhage in a Chilean population, with emphasis on
risk factors. BMC Res Notes. 4:4642011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Broderick JP, Viscoli CM, Brott T, Kernan
WN, Brass LM, Feldmann E, Morgenstern LB, Wilterdink JL and Horwitz
RI: Hemorrhagic Stroke Project Investigators: Major risk factors
for aneurysmal subarachnoid hemorrhage in the young are modifiable.
Stroke. 34:1375–1381. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jung SY, Bae HJ, Park BJ and Yoon BW:
Acute Brain Bleeding Analysis Study Group: Parity and risk of
hemorrhagic strokes. Neurology. 74:1424–1429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bateman BT, Olbrecht VA, Berman MF,
Minehart RD, Schwamm LH and Leffert LR: Peripartum subarachnoid
hemorrhage: Nationwide data and institutional experience.
Anesthesiology. 116:324–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Juvela S: Prevalence of risk factors in
spontaneous intracerebral hemorrhage and aneurysmal subarachnoid
hemorrhage. Arch Neurol. 53:734–740. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiménez-Yepes CM and Londoño-Fernández JL:
Risk of aneurysmal subarachnoid hemorrhage: The role of confirmed
hypertension. Stroke. 39:1344–1346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hamdan A, Barnes J and Mitchell P:
Subarachnoid hemorrhage and the female sex: Analysis of risk
factors, aneurysm characteristics, and outcomes. J Neurosurg.
121:1367–1373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ben-Shlomo Y, Markowe H, Shipley M and
Marmot MG: Stroke risk from alcohol consumption using different
control groups. Stroke. 23:1093–1098. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jee SH, Park JW, Lee SY, Nam BH, Ryu HG,
Kim SY, Kim YN, Lee JK, Choi SM and Yun JE: Stroke risk prediction
model: A risk profile from the Korean study. Atherosclerosis.
197:318–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Singh JK, Ranjan P, Kumari A, Dahale AS,
Jha R and Das R: Types, outcome and risk factors of stroke in
Tribal Patients. Int J Stroke. 8:675–680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang J, Liu G, Arima H, Li Y, Cheng G,
Shiue I, Lv L, Wang H, Zhang C, Zhao J, et al: Incidence and risks
of subarachnoid hemorrhage in China. Stroke. 44:2891–2893. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lindekleiv H, Sandvei MS, Romundstad PR,
Wilsgaard T, Njølstad I, Ingebrigtsen T, Vik A and Mathiesen EB:
Joint effect of modifiable risk factors on the risk of aneurysmal
subarachnoid hemorrhage: A cohort study. Stroke. 43:1885–1889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Vlak MH, Rinkel GJ, Greebe P, Greving JP
and Algra A: Lifetime risks for aneurysmal subarachnoid
haemorrhage: Multivariable risk stratification. J Neurol Neurosurg
Psychiatry. 84:619–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shiue I, Arima H, Hankey GJ and Anderson
CS: ACROSS Group: Modifiable lifestyle behaviours account for most
cases of subarachnoid haemorrhage: A population-based case-control
study in Australasia. J Neurol Sci. 313:92–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Koshy L, Easwer HV, Premkumar S, Alapatt
JP, Pillai AM, Nair S, Bhattacharya RN and Banerjee M: Risk factors
for aneurysmal subarachnoid hemorrhage in an Indian population.
Cerebrovasc Dis. 29:268–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Inagawa T: Risk factors for aneurysmal
subarachnoid hemorrhage in patients in Izumo City, Japan. J
Neurosurg. 102:60–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Harmsen P, Rosengren A, Tsipogianni A and
Wilhelmsen L: Risk factors for stroke in middle-aged men in
Goteborg, Sweden. Stroke. 21:223–229. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Inagawa T: Risk factors for the formation
and rupture of intracranial saccular aneurysms in Shimane, Japan.
World Neurosurg. 73:155–164, e23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sandvei MS, Romundstad PR, Müller TB,
Vatten L and Vik A: Risk factors for aneurysmal subarachnoid
hemorrhage in a prospective population study: The HUNT study in
Norway. Stroke. 40:1958–1962. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lindekleiv H, Sandvei MS, Njølstad I,
Løchen ML, Romundstad PR, Vatten L, Ingebrigtsen T, Vik A and
Mathiesen EB: Sex differences in risk factors for aneurysmal
subarachnoid hemorrhage: A cohort study. Neurology. 76:637–643.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sankai T, Iso H, Shimamoto T, Kitamura A,
Naito Y, Sato S, Okamura T, Imano H, Iida M and Komachi Y: Cohort
study on risk factors for subarachnoid hemorrhage among Japanese
men and women. Nihon Eiseigaku Zasshi. 53:587–595. 1999.(In
Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Leppälä JM, Paunio M, Virtamo J, Fogelholm
R, Albanes D, Taylor PR and Heinonen OP: Alcohol consumption and
stroke incidence in male smokers. Circulation. 100:1209–1214. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Thompson SG: Why sources of heterogeneity
in meta-analysis should be investigated. BMJ. 309:1351–1355. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Feigin V, Parag V, Lawes CM, Rodgers A,
Suh I, Woodward M, Jamrozik K and Ueshima H: Asia Pacific Cohort
Studies Collaboration: Smoking and elevated blood pressure are the
most important risk factors for subarachnoid hemorrhage in the
Asia-Pacific region: An overview of 26 cohorts involving 306,620
participants. Stroke. 36:1360–1365. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Canhão P, Pinto AN, Ferro H and Ferro JM:
Smoking and aneurysmal subarachnoid haemorrhage: A case-control
study. J Cardiovasc Risk. 1:155–158. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Stokes GS: Hypertension and alcohol: Is
ther a link? J Chronic Dis. 35:759–762. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Moncada S and Radomski MW: The problems
and the promise of prostaglandin influences in atherogenesis. Ann N
Y Acad Sci. 454:121–130. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Laug WE: Ethyl alcohol enhances
plasminogen activator secretion by endothelial cells. JAMA.
250:772–776. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Knekt P, Reunanen A, Aho K, Heliövaara M,
Rissanen A, Aromaa A and Impivaara O: Risk factors for subarachnoid
hemorrhage in a longitudinal population study. J Clin Epidemiol.
44:933–939. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bonita R: Cigarette smoking, hypertension
and the risk of subarachnoid hemorrhage: A population-based
case-control study. Stroke. 17:831–835. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Petitti DB and Wingerd J: Use of oral
contraceptives, cigarette smoking, and risk of subarachnoid
haemorrhage. Lancet. 2:234–235. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Longstreth WT, Nelson LM, Koepsell TD and
van Belle G: Subarachnoid hemorrhage and hormonal factors in women.
A population-based case-control study. Ann Intern Med. 121:168–173.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Inman WH: Oral contraceptives and fatal
subarachnoid haemorrhage. Br Med J. 2:1468–1470. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fedirko V, Tramacere I, Bagnardi V, Rota
M, Scotti L, Islami F, Negri E, Straif K, Romieu I, La Vecchia C,
et al: Alcohol drinking and colorectal cancer risk: An overall and
dose-response meta-analysis of published studies. Ann Oncol.
22:1958–1972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tramacere I, Negri E, Pelucchi C, Bagnardi
V, Rota M, Scotti L, Islami F, Corrao G, La Vecchia C and Boffetta
P: A meta-analysis on alcohol drinking and gastric cancer risk. Ann
Oncol. 23:28–36. 2012. View Article : Google Scholar : PubMed/NCBI
|