Introduction
Sub-clinical hypothyroidism occurs when
thyroid-stimulating hormone (TSH) exceeds the upper reference
limit, while free thyroxine and free tri-iodothyronine
(FT3) concentrations remain within the normal range.
Sub-clinical hypothyroidism occurs in 4–20% of the adult population
and is mostly caused by chronic lymphocytic thyroiditis (1). Other factors that cause sub-clinical
hypothyroidism include thyroid injury, such as radioactive iodine
treatment or external radiation therapy, drugs, such as
iodine-containing compounds, lithium carbonate or interferon, and a
period of sub-acute, post-partum or painless thyroiditis (2). The prevalence of sub-clinical
hypothyroidism increases with age and is higher in females than in
males. Sub-clinical hypothyroidism is a type of mild tissue
hypothyroidism; however, it is usually progressive, particularly
when serum TSH levels are >10 mU/l and when females are older
with the presence of anti-thyroid peroxidase antibodies (3). The symptoms of sub-clinical
hypothyroidism are not always obvious; however, they are associated
with disease severity, duration and individual sensitivity to
thyroid hormone deficiency. Anxiety and depression, as well as a
decline in cognitive and memory function are characteristic of
sub-clinical hypothyroidism.
The cardiovascular system is a major target of
thyroid hormone action. Thyroid hormone deficiency increases the
risk for cardiovascular disease by increasing systemic vascular
resistance and diastolic dysfunction, while reducing systolic
function and cardiac preload (3,4).
Hyperlipidemia is one of the risk factors for cardiovascular
disease. Thus, the reduction of serum lipid levels may decrease the
risk of cardiovascular mortality (5,6). The link
between hypothyroidism with dyslipidemia is well documented, while
data on this association in sub-clinical hypothyroidism remain
limited. Various cross-sectional studies have demonstrated the
positive association between TSH, and serum total cholesterol (TC)
and low-density lipoprotein-cholesterol (LDL-C) in patients with
sub-clinical hypothyroidism (7–9). Previous
studies have confirmed that L-thyroxine replacement treatment
decreases thyroid size, improves symptoms and signs of sub-clinical
hypothyroidism, and normalizes the hemodynamic alterations induced
by thyroid hormone deficiency (3).
However, only a small number of randomized controlled trials (RCTs)
have examined the effects of L-thyroxine on serum lipids in
patients with sub-clinical hypothyroidism, which has remained
controversial due to the inconsistency of the results (10–17).
Therefore, the present meta-analysis was performed to elucidate the
effect of L-thyroxine replacement treatment on serum lipid levels
in patients with sub-clinical hypothyroidism.
Materials and methods
Search strategy
A systematic search of the electronic Cochrane
Library (http://www.cochranelibrary.com/), PubMed (https://www.ncbi.nlm.nih.gov/pubmed), Medline
(https://www.medline.com/home.jsp),
Google Scholar (https://scholar.google.com) and Embase (http://www.embase.com) databases using the search
terms ‘thyroxine’, ‘levothyroxin’ and ‘levothyroxine’ was performed
(the last search was updated in July 2015). Furthermore, eligible
studies were retrieved from the reference lists of the studies
identified in order to identify any further studies. The search was
limited to studies on humans, but no language limitation was
set.
Study selection and data
extraction
Data were extracted by two independent
investigators, who reviewed all of the studies and cross-checked
each others' results to improve accuracy. Only studies that were
RCTs comparing the efficacy of L-thyroxine and a placebo treatment
on decreasing serum lipid levels in patients with sub-clinical
hypothyroidism were included. For each study, data collected
included the name of the first author, year of publication, number
of patients, the dose of L-thyroxine and placebo used, patient
history of thyroid disease, treatment period and serum TSH
levels.
Quality assessment
The quality and risk of bias were assessed for each
eligible study using Cochrane's risk of bias tool. Bias comprised
the following seven areas: Random sequence generation, allocation
concealment, blinding of participants and personnel, blinding of
outcome assessment, incomplete outcome data, selective reporting
and other biases (18,19). The risk of bias for each eligible study
was first assessed by two independent investigators and agreement
was then reached by consensus. If there was a disagreement, another
investigator would estimate the risk of bias for each eligible
study.
Statistical analysis
The present meta-analysis was performed using Review
Manager software (version 5.2; The Nordic Cochrane Centre,
Copenhagen, Denmark). All of the outcomes assessed were continuous
data. The majority of the studies included provided baseline, as
well as final measurements (6 or 12 month after thyroxine
treatment), enabling statistical analysis of differences between
data obtained at these two time-points (10,13,15–17). The
mean differences were obtained by subtracting the mean levels at
baseline from those at the end-point. The standard deviation (SD)
of the changes was calculated using the formula according to the
Cochrane Handbook for Systematic Reviews of Interventions (20). Statistical heterogeneity of each study
was assessed by visual inspection of forest plots and the extent of
inconsistency by I2 value. A fixed-effects model
was used for calculations unless significant heterogeneity existed
(I2>50%), in which case a random-effects model
was used (21). P<0.05 was
considered to indicate a statistically significant difference.
Results
Selection of studies
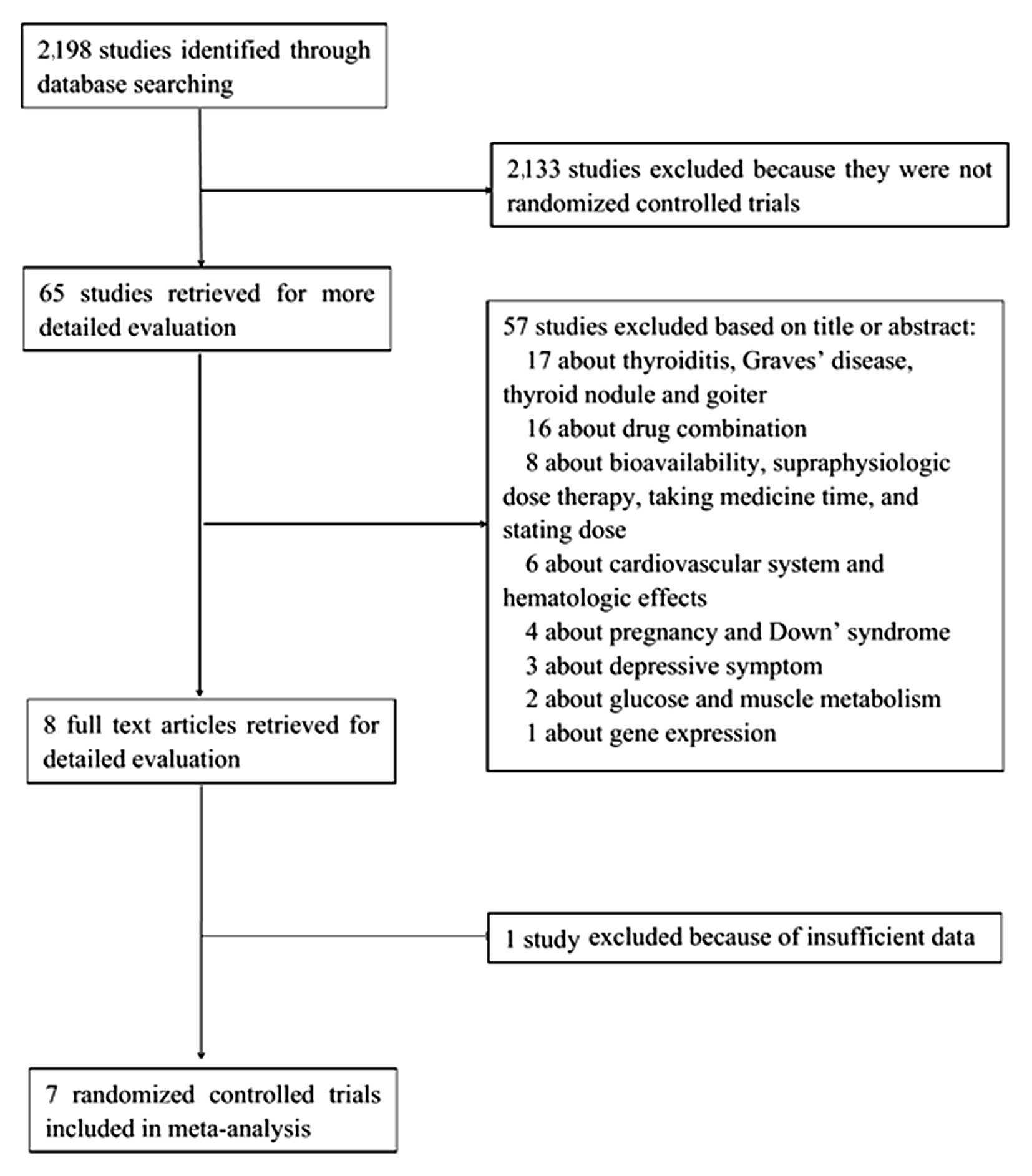
The process for the selection of studies for
meta-analysis is shown in Fig. 1. The
initial literature search yielded 2,198 studies, of which 2,133
were excluded, as they were not RCTs and 57 were excluded based on
the title or abstract (were not associated with serum lipids).
After full-text scrutiny, one study was excluded due to
insufficient data (11), and the seven
remaining RCTs that met the inclusion criteria were analyzed
(10,12–17).
Baseline characteristics of the patients included in the RCTs
selected are presented in Table I.
 | Table I.Characteristics of trials included in
the present meta-analysis. |
Table I.
Characteristics of trials included in
the present meta-analysis.
| First author, year
(Refs.) | Patient no. | Thyroid disease
history | Serum TSH levels in
L-T4 group (mIU/l) | Serum TSH levels in
placebo group (mIU/l) | Initial dose of
L-T4 (µg) | Treatment period
(weeks) |
|---|
| Cooper et
al, 1984 (10) | 33 | Previously treated
hyperthyroidism | 10.8±2.2 | 11.1±3.2 | 50 | 48 |
| Jaeschke et
al, 1996 (12) | 31 | Not mentioned | 12.1±6.8 | 9.4±3.1 | 25 | 24 |
| Meier et al,
2001 (13) | 63 | Autoimmune
thyroiditis, previously treated Graves's disease and goiter,
idiopathic subclinical hypothyroid | 14.4±1.7 | 11.3±1.0 | 25 | 48 |
| Kong et al,
2002 (14) | 27 | No history of
thyroid disease |
8.0±1.5 |
7.3±1.6 | 50 | 24 |
| Razvi et al,
2007 (15)a | 71 | Not mentioned | 5.4 (3.8–15.8) | 5.3 (3.7–13.9) | 100 | 24 |
| Monzani et
al, 2004 (16)a | 45 | Hashimoto's
thyroiditis, previously treated toxic adenoma or multinodular toxic
goiter | 6.03
(3.65–15.00) | 5.68
(3.66–12.60) | 25 | 24 |
| Caraccio et
al, 2002 (17)a | 49 | Autoimmune
thyroiditis, previously treated hyperthyroidism | 6.0
(3.70–15.00) | 4.90
(3.65–9.00) | 25 | 24 |
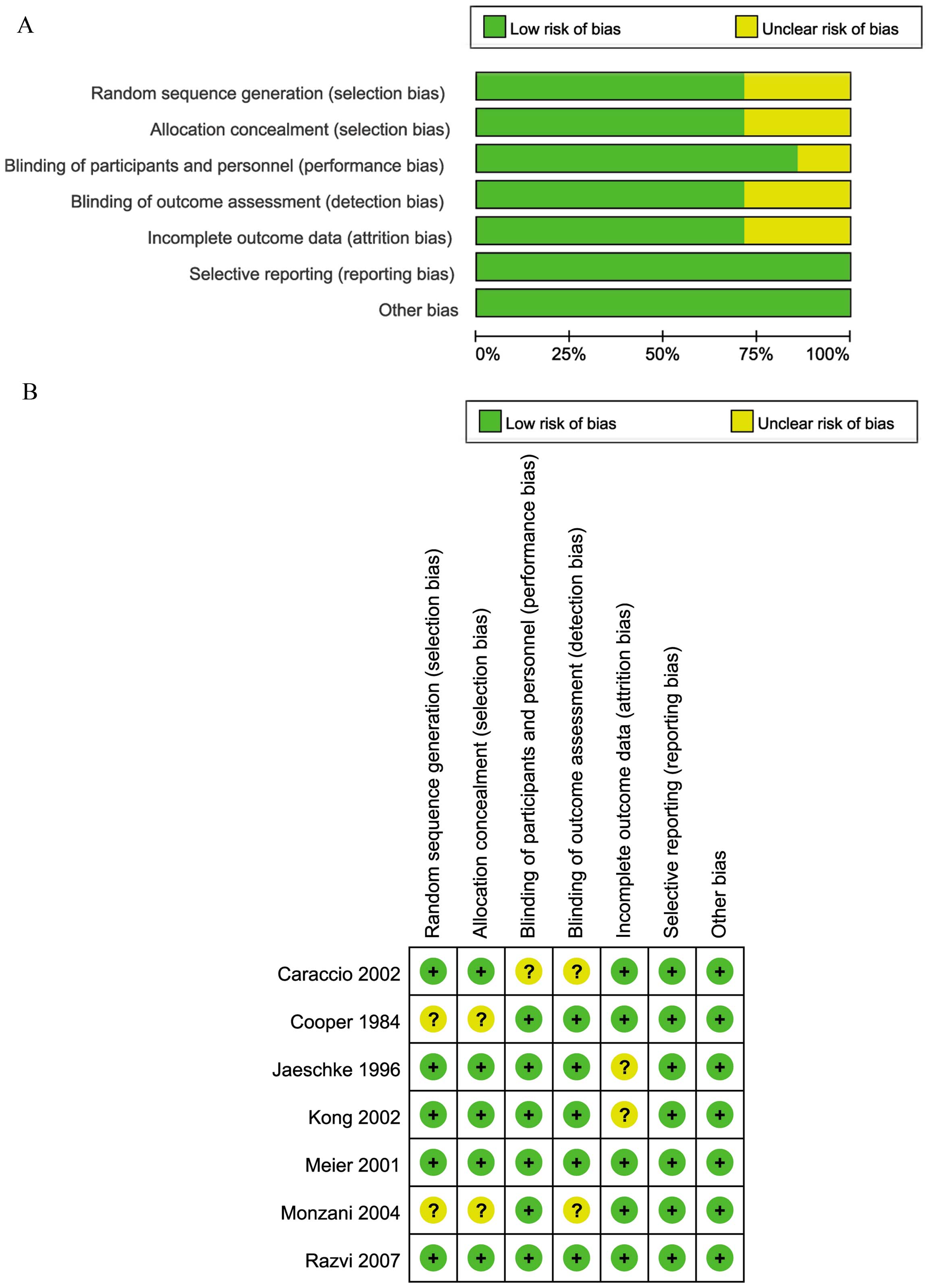
Quality assessment
The quality of the studies was assessed by
constructing a risk of bias graph and risk of bias summary
(Fig. 2). Each risk of bias item was
presented as percentages across all studies included. Fig. 2A shows that the overall methodological
quality of the studies was good. High risk of bias was not detected
in any of the studies included (Fig.
2B). In five of the studies, the risk of bias was unclear in
1–3 categories, while the two remaining studies met the
high-quality criteria.
L-thyroxine improves LDL-C levels in
patients with sub-clinical hypothyroidism
In the present study, four separate meta-analyses
were performed to assess the effects of L-thyroxine vs. those of a
placebo on the levels of TC, LDL-C, triglyceride (TG) and
high-density lipoprotein-cholesterol (HDL-C) in patients with
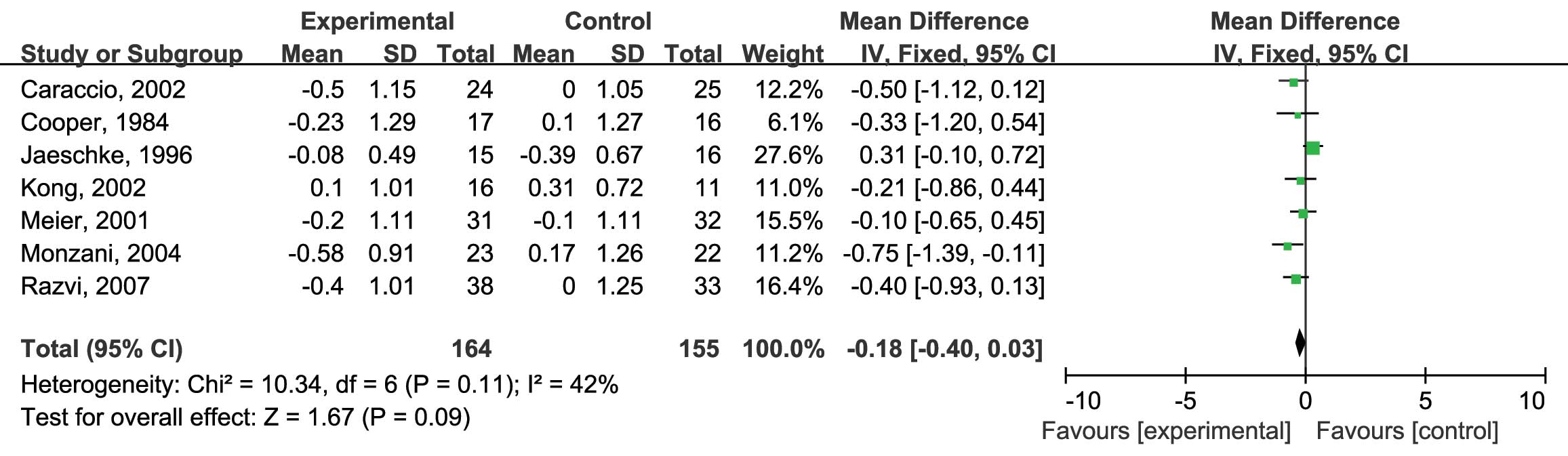
sub-clinical hypothyroidism. To assess the effects of L-thyroxine
vs. placebo on serum TC levels (mmol/l), all of the seven RCTs were
included (10,12–17). No
heterogeneity was identified (I2=42%; P=0.11) and
the fixed-effects model was therefore used. The analysis showed no
significant differences in the reduction of serum TC levels by
either L-thyroxine or the placebo [mean difference (MD): −0.18; 95%
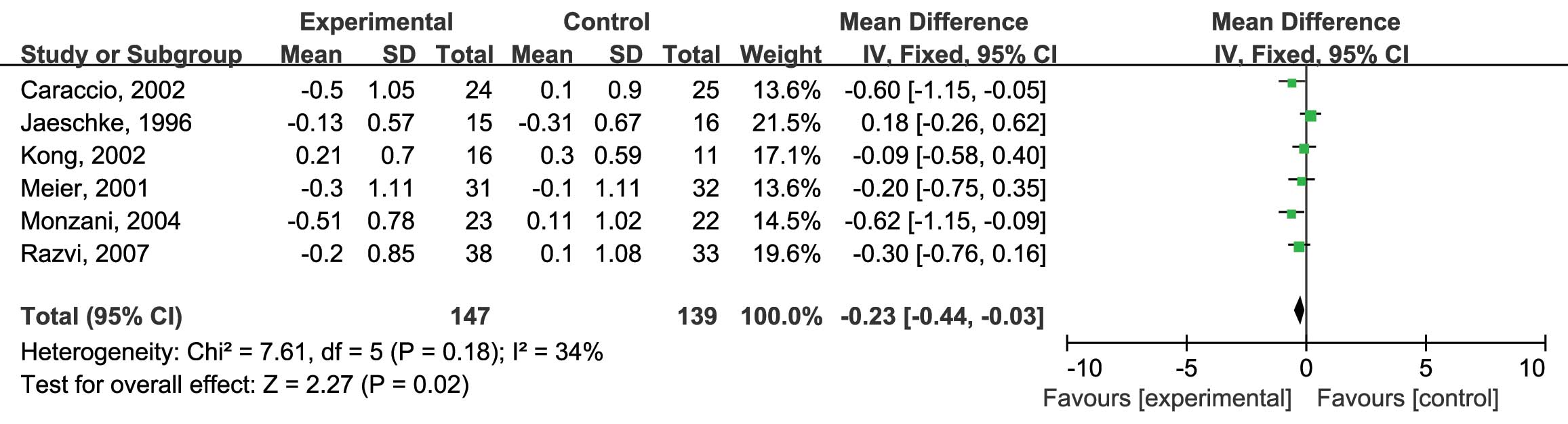
confidence interval (CI): −0.40, 0.03; P=0.09] (Fig. 3). Next, the effects of L-thyroxine vs.
placebo on serum LDL-C levels were assessed. Six RCTs were included
in this analysis and no heterogeneity was found
(I2=34%; P=0.18) (12–17);
therefore, the fixed-effects model was used. The analysis showed a
significantly higher reduction of serum LDL-C in the patients
treated with L-thyroxine compared with that in the placebo group
(MD: −0.23; 95% CI: −0.44, −0.03; P=0.02) (Fig. 4). Furthermore, the effects of
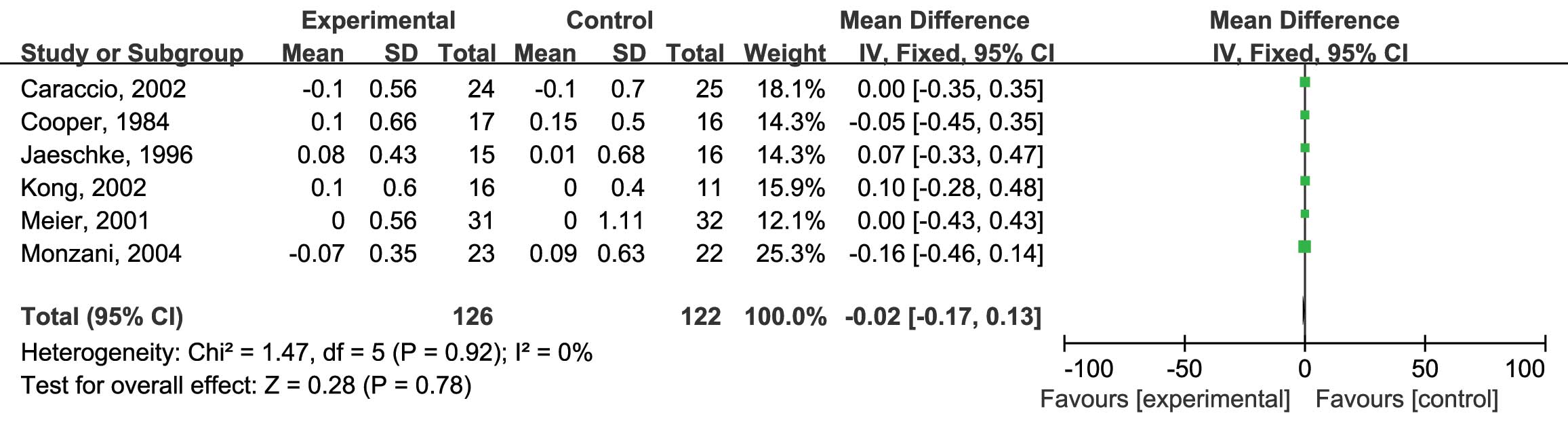
L-thyroxine vs. placebo on serum TG levels were assessed. Six RCTs
were included and no heterogeneity was found
(I2=0%; P=0.92) (10,12–14,16,17); therefore, the fixed-effects model was
used. The analysis showed no significant difference between the
reduction of TG by L-thyroxine and that in the placebo group (MD:
−0.02; 95% CI: −0.17, 0.13; P=0.78) (Fig.
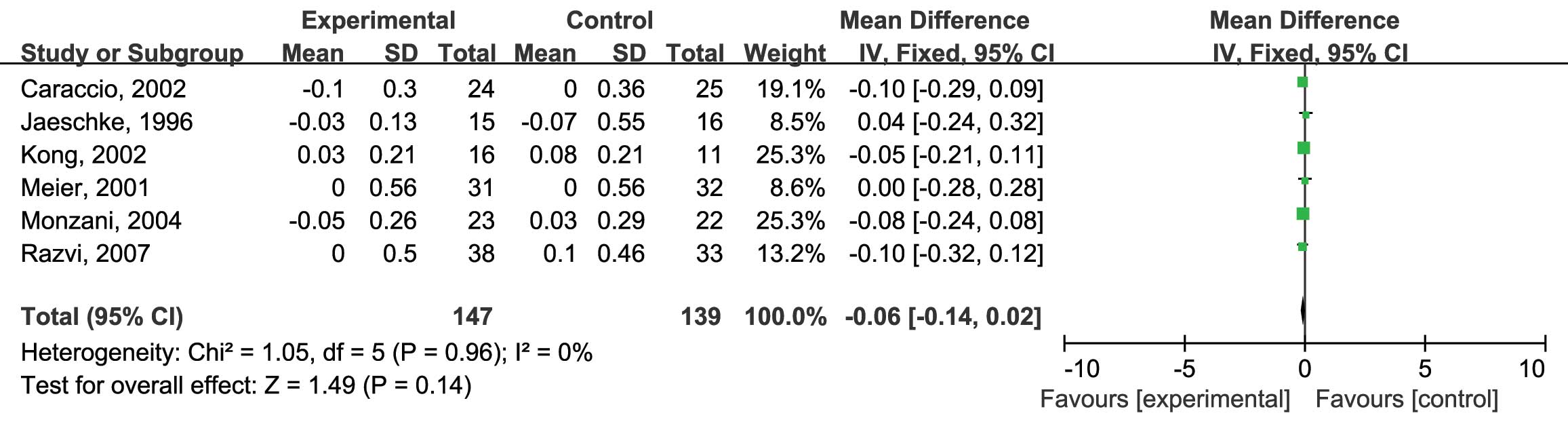
5). Finally, the HDL-C levels were assessed. Six RCTs were
included and no heterogeneity was found (I2=0%;
P=0.96) (12–17). Analysis using the fixed-effects model
revealed no significant difference between the L-thyroxine and the
placebo group (MD: −0.06; 95% CI: −0.14, 0.02; P=0.14) (Fig. 6).
Discussion
Patients with sub-clinical hypothyroidism,
particularly those with positive thyroid antibodies, are at risk of
developing overt hypothyroidism at an estimated rate of 2.0–4.3%
per year (3,22). L-thyroxine replacement treatment is
currently the major therapy for sub-clinical hypothyroidism, which
is administered with the following purposes: First, in order to
prevent progression to overt hypothyroidism and second, to reduce
symptoms of thyroid hormone deficiency (23). Replacement therapy with L-thyroxine
reverses systolic and diastolic dysfunction, arterial hypertension,
increases in carotid intima-media thickness, endothelial
dysfunction and other cardiovascular risk factors (16,24–26). However, the beneficial effect of
L-thyroxine replacement on the lipid profiles of patients with
sub-clinical hypothyroidism remains controversial (10–17,27,28).
Numerous studies have estimated the effect of L-thyroxine
replacement treatment on serum lipid levels in patients with
sub-clinical hypothyroidism; however, their results were
inconsistent. In a recent study by Anagnostis et al
(22), L-thyroxine replacement
treatment exerted no effect on serum lipid levels of patients with
mild sub-clinical hypothyroidism, whose TSH levels were <7
mIU/l. However, Tagami et al (2) reported a significant decrease of TC and
LDL-C following L-thyroxine replacement therapy. A previous
quantitative review by Danese et al (23) assessed the changes in serum lipoprotein
concentrations in patients with mild thyroid failure after
treatment with L-thyroxine, revealing that TC and LDL-C levels were
reduced subsequent to L-thyroxine treatment, whereas TG
concentrations did not change. Danese et al (23) also indicated that the serum lipid
levels at baseline and the degree of sub-clinical hypothyroidism
were the major factors affecting changes in serum lipid levels.
However, a previous meta-analysis by Villar et al (29) showed that after thyroid hormone
replacement therapy, serum TC, TG, LDL-C and HDL-C levels in
patients with sub-clinical hypothyroidism did not significantly
improve.
To further clarify the effect of L-thyroxine
treatment on serum lipid levels in patients with sub-clinical
hypothyroidism, changes in TC, LDL-C, TG and HDL-C levels between
baseline and the study end-point were compared with those in
patients receiving a placebo and statistically analyzed. The
quality of the RCTs included was good and, according to Cochrane,
the risk of bias of the studies was limited. Since all of the
studies included showed no significant heterogeneity, the
fixed-effects model was applied to all analyses. The present study
revealed that serum LDL-C levels were significantly decreased
following L-thyroxine treatment (P=0.02). However, changes in serum
TC, TG and HDL-C levels were not significantly different from those
in the placebo group (P=0.09, 0.78 and 0.14, respectively). These
results were not consistent with those of the two above-mentioned
studies (23,29). The reason for this discrepancy is
primarily due to more RCT data being included in the present
meta-analysis. The majority of the trials reviewed by Danese et
al (23) were non-randomized
without a control group and a considerable number of the studies
had a small sample size, while only two of them were RCTs. Of
these, one was included in the present meta-analysis (10), while the other one was excluded due to
incomplete data (11). The
meta-analysis by Villar et al (29) included 12 RCTs to assess the effects of
thyroid hormone replacement in patients with sub-clinical
hypothyroidism (10–14,16,17,24,30–33).
However, the objectives were broad, including reduction of
cardiovascular mortality and morbidity, improvement of symptoms,
health-associated quality of life, cognitive function, serum lipid
levels as well as improvement in TSH and adverse effects, while the
present meta-analysis focused on changes in lipid levels alone. The
analysis of changes in serum TG, in the present study included all
of the RCTs focusing on lipid levels that were included in the
meta-analysis by Villar et al (29) and an additional RCT (15). For the analysis of LDL-C and HDL-C, the
data of three further RCTs were used (15–17). With
more data included than in previous studies, the present
meta-analysis determined that, compared with placebo treatment,
L-thyroxine treatment significantly improvement serum LDL-C levels
in patients with sub-clinical hypothyroidism.
There were certain limitations of the present study.
First, the limited number of studies and sample sizes included were
the major restriction of this meta-analysis (one of the eight
articles retrieved was not included in the present meta-analysis
due to insufficient data (11) and
novel RCTs were scarce). Furthermore, the baseline levels of TSH
and serum lipids were not identical among the studies included. The
above-mentioned limitations may have engendered bias and affected
the quality of this study.
In conclusion, a meta-analysis of complete data from
RCTs published until July 2015 on the effect of L-thyroxine
treatment on serum lipids in sub-clinical hypothyroidism was
performed in the present study. A significant improvement of serum
LDL-C was revealed; however, compared with placebo treatment, serum
TC, TG and HDL-C levels were not affected by L-thyroxine. High
levels of plasma LDL-C levels is associated with occurrence of
coronary artery disease; therefore, L-thyroxine may be used to
reduce the risk of coronary artery disease. However, further
research is required.
Acknowledgements
The present study was supported by the National Key
Clinical Specialty Project awarded to the Departments of Nuclear
Medicine and Radiology of Tianjin Medical University General
Hospital (Tianjin, China). Support was also received from the
Tianjin Medical University General Hospital New Century Excellent
Talent Program, the Young and Middle-aged Innovative Talent
Training Program of the Tianjin Education Committee and the Talent
Fostering Program (the 131 Project) of the Tianjin Education
Committee, Tianjin Human Resources and Social Security Bureau
(awarded to Z.M.).
Glossary
Abbreviations
Abbreviations:
|
TSH
|
thyroid-stimulating hormone
|
|
TC
|
total cholesterol
|
|
H/LDL-C
|
high/low-density
lipoprotein-cholesterol
|
|
RCT
|
randomized controlled trial
|
|
MD
|
mean difference
|
|
CI
|
confidence interval
|
|
TG
|
triglycerides
|
References
|
1
|
Cooper DS and Biondi B: Subclinical
thyroid disease. Lancet. 379:1142–1154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tagami T, Tamanaha T, Shimazu S, Honda K,
Nanba K, Nomura H, Yoriko SU, Usui T, Shimatsu A and Naruse M:
Lipid profiles in the untreated patients with Hashimoto thyroiditis
and the effects of thyroxine treatment on subclinical
hypothyroidism with Hashimoto thyroiditis. Endocr J. 57:253–258.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Biondi B and Cooper DS: The clinical
significance of subclinical thyroid dysfunction. Endocr Rev.
29:76–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klein I and Ojamaa K: Thyroid hormone and
the cardiovascular system. N Engl J Med. 344:501–509. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isles CG and Paterson JR: Identifying
patients at risk for coronary heart disease: implications from
trials of lipid-lowering drug therapy. QJM. 93:567–574. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
LaRosa JC, He J and Vupputuri S: Effect of
statins on risk of coronary disease: a meta-analysis of randomized
controlled trials. JAMA. 282:2340–2346. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walsh JP, Bremner AP, Bulsara MK, O'leary
P, Leedman PJ, Feddema P and Michelangeli V: Thyroid dysfunction
and serum lipids: A community-based study. Clin Endocrinol (Oxf).
63:670–675. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kvetny J, Heldgaard PE, Bladbjerg EM and
Gram J: Subclinical hypothyroidism is associated with a low-grade
inflammation, increased triglyceride levels and predicts
cardiovascular disease in males below 50 years. Clin Endocrinol
(Oxf). 61:232–238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iqbal A, Jorde R and Figenschau Y: Serum
lipid levels in relation to serum thyroid-stimulating hormone and
the effect of thyroxine treatment on serum lipid levels in subjects
with subclinical hypothyroidism: The Tromsø Study. J Intern Med.
260:53–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cooper DS, Halpern R, Wood LC, Levin AA
and Ridgway EC: L-Thyroxine therapy in subclinical hypothyroidism.
A double-blind, placebo-controlled trial. Ann Intern Med.
101:18–24. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nyström E, Caidahl K, Fager G, Wikkelsö C,
Lundberg PA and Lindstedt G: A double-blind cross-over 12-month
study of L-thyroxine treatment of women with ‘subclinical’
hypothyroidism. Clin Endocrinol (Oxf). 29:63–75. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jaeschke R, Guyatt G, Gerstein H,
Patterson C, Molloy W, Cook D, Harper S, Griffith L and Carbotte R:
Does treatment with L-thyroxine influence health status in
middle-aged and older adults with subclinical hypothyroidism? J Gen
Intern Med. 11:744–749. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meier C, Staub JJ, Roth CB, Guglielmetti
M, Kunz M, Miserez AR, Drewe J, Huber P, Herzog R and Müller B:
TSH-controlled L-thyroxine therapy reduces cholesterol levels and
clinical symptoms in subclinical hypothyroidism: a double blind,
placebo-controlled trial (Basel Thyroid Study). J Clin Endocrinol
Metab. 86:4860–4866. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong WM, Sheikh MH, Lumb PJ, Naoumova RP,
Freedman DB, Crook M, Doré CJ and Finer N: A 6-month randomized
trial of thyroxine treatment in women with mild subclinical
hypothyroidism. Am J Med. 112:348–354. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Razvi S, Ingoe L, Keeka G, Oates C,
McMillan C and Weaver JU: The beneficial effect of L-thyroxine on
cardiovascular risk factors, endothelial function, and quality of
life in subclinical hypothyroidism: randomized, crossover trial. J
Clin Endocrinol Metab. 92:1715–1723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monzani F, Caraccio N, Kozàkowà M, Dardano
A, Vittone F, Virdis A, Taddei S, Palombo C and Ferrannini E:
Effect of levothyroxine replacement on lipid profile and
intima-media thickness in subclinical hypothyroidism: A
double-blind, placebo-controlled study. J Clin Endocrinol Metab.
89:2099–2106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Caraccio N, Ferrannini E and Monzani F:
Lipoprotein profile in subclinical hypothyroidism: Response to
levothyroxine replacement, a randomized placebo-controlled study. J
Clin Endocrinol Metab. 87:1533–1538. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L and Sterne JA:
Cochrane Bias Methods Group; Cochrane Statistical Methods Group;
Cochrane Statistical Methods Group: The Cochrane Collaboration's
tool for assessing risk of bias in randomised trials. BMJ.
343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hopp L: Risk of bias reporting in Cochrane
systematic reviews. Int J Nurs Pract. 21:683–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higgins JP and Green S: Cochrane Handbook
for Systematic Reviews of Interventions: Cochrane Book Series. The
Cochrane Collaboration and John Wiley & Sons Ltd.; West Sussex:
2008, View Article : Google Scholar
|
|
21
|
Song X, Meng Z, Jia Q, Zhang L, Xu K, Tan
J, Zhang G, Zheng W, Li X and Zhang J: Different radioiodine dose
for remnant thyroid ablation in patients with differentiated
thyroid cancer: a meta-analysis. Clin Nucl Med. 40:774–779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anagnostis P, Efstathiadou ZA, Slavakis A,
Selalmatzidou D, Poulasouchidou M, Katergari S, Karathanasi E,
Dogramatzi F and Kita M: The effect of L-thyroxine substitution on
lipid profile, glucose homeostasis, inflammation and coagulation in
patients with subclinical hypothyroidism. Int J Clin Pract.
68:857–863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Danese MD, Ladenson PW, Meinert CL and
Powe NR: Clinical review 115: effect of thyroxine therapy on serum
lipoproteins in patients with mild thyroid failure: a quantitative
review of the literature. J Clin Endocrinol Metab. 85:2993–3001.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jorde R, Waterloo K, Storhaug H, Nyrnes A,
Sundsfjord J and Jenssen TG: Neuropsychological function and
symptoms in subjects with subclinical hypothyroidism and the effect
of thyroxine treatment. J Clin Endocrinol Metab. 91:145–153. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biondi B, Lombardi G and Palmieri EA:
Screening and treatment for subclinical thyroid disease. JAMA.
291:15622004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biondi B and Klein I: Hypothyroidism as a
risk factor for cardiovascular disease. Endocrine. 24:1–13. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanis BC, Westendorp GJ and Smelt HM:
Effect of thyroid substitution on hypercholesterolaemia in patients
with subclinical hypothyroidism: a reanalysis of intervention
studies. Clin Endocrinol (Oxf). 44:643–649. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Çatlı G, Anık A, Tuhan H Ünver, Böber E
and Abacı A: The effect of L-thyroxine treatment on hypothyroid
symptom scores and lipid profile in children with subclinical
hypothyroidism. J Clin Res Pediatr Endocrinol. 6:238–244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Villar HC, Saconato H, Valente O and
Atallah AN: Thyroid hormone replacement for subclinical
hypothyroidism. Cochrane Database Syst Rev. CD0034192007.PubMed/NCBI
|
|
30
|
Caraccio N, Natali A, Sironi A, Baldi S,
Frascerra S, Dardano A, Monzani F and Ferrannini E: Muscle
metabolism and exercise tolerance in subclinical hypothyroidism: a
controlled trial of levothyroxine. J Clin Endocrinol Metab.
90:4057–4062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monzani F, Di Bello V, Caraccio N, Bertini
A, Giorgi D, Giusti C and Ferrannini E: Effect of levothyroxine on
cardiac function and structure in subclinical hypothyroidism: a
double blind, placebo-controlled study. J Clin Endocrinol Metab.
86:1110–1115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ross DS: Bone density is not reduced
during the short-term administration of levothyroxine to
postmenopausal women with subclinical hypothyroidism: a randomized,
prospective study. Am J Med. 95:385–388. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yazici M, Gorgulu S, Sertbas Y, Erbilen E,
Albayrak S, Yildiz O and Uyan C: Effects of thyroxin therapy on
cardiac function in patients with subclinical hypothyroidism: index
of myocardial performance in the evaluation of left ventricular
function. Int J Cardiol. 95:135–143. 2004. View Article : Google Scholar : PubMed/NCBI
|