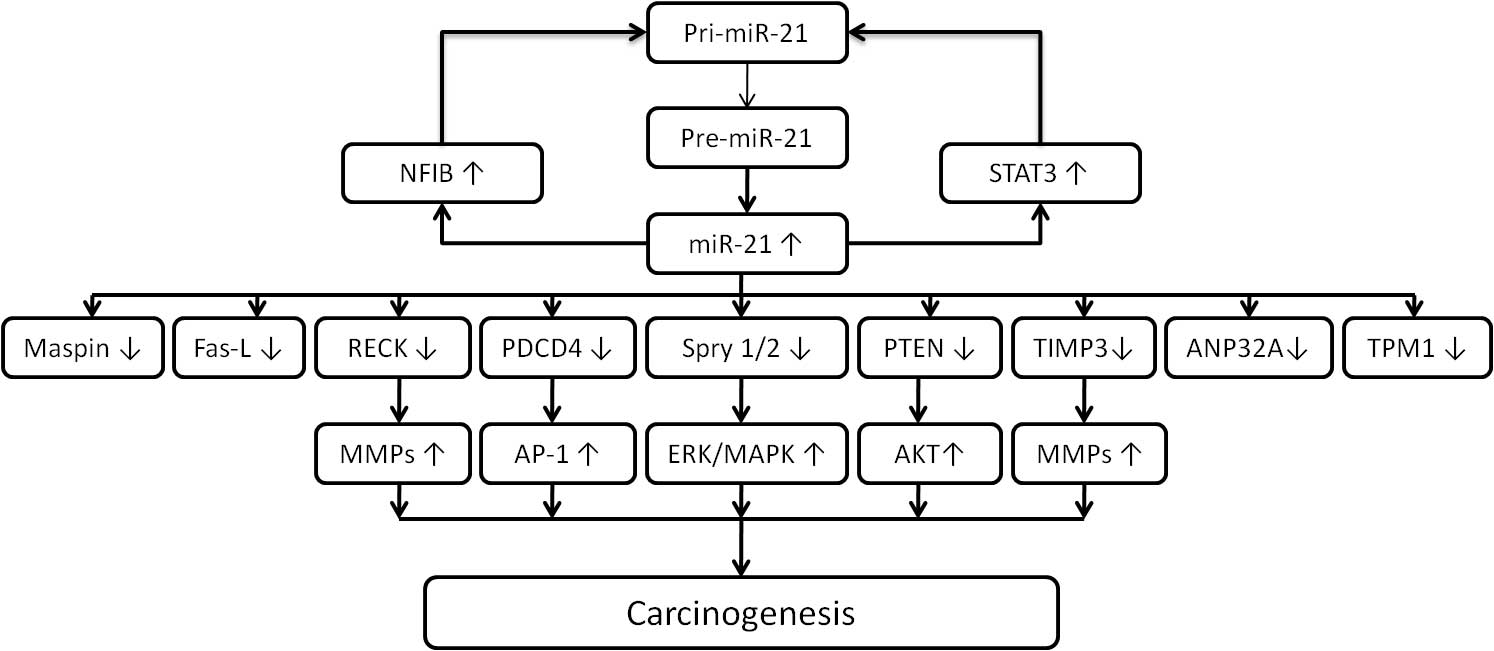
MicroRNAs (miRs) are a class of naturally occurring
short non-coding RNA molecules of 15–27 nucleotides in length that
regulate eukaryotic gene expression at the post-transcriptional
level. Almost 2,000 human miRNAs have been identified through
cloning and/or sequence analysis (1).
They have key regulatory roles in the development, differentiation
and apoptosis of normal cells, as well as in the determination of
the final phenotype of cancer cells, affecting carcinogenesis and
metastatic potential (2). miR-21 is an
abundantly expressed miRNA in mammalian cells, whose upregulation
is associated with numerous types of cancer (3,4). By
generation of a conditional miR-21 knock-in mouse, it was
demonstrated that miR-21 functions as an oncogene with its
overexpression resulting in malignant B-cell lymphoma (5). Indeed, miR-21 was found to be the only
consistently upregulated miRNA in a study that profiled 540
clinical samples from cancer patients (6). The majority of studies on miR-21 have
focused on its role in carcinogenesis and its clinical application.
miR-21 is also expressed in hematopoietic cells of the immune
system, including B/T cells, monocytes, macrophages and dendritic
cells. High miR-21 levels are, therefore, considered to be a marker
of immune cell activation (7).
Regarding pathological necrosis, miR-21 enhances cellular necrosis
by negatively regulating tumor suppressor genes associated with the
death receptor-mediated intrinsic apoptotic pathway (8).
The biological functions of various miRNAs,
including miR-21, have been extensively investigated and miR-21 is
evolutionarily conserved across a wide range of vertebrate species
(9). However, the location of the gene
encoding miR-21 in the genome is different between humans and other
vertebrate species. In rats and mice, the miR-21 gene is located on
chromosome 10 and 21, respectively, whereas in humans, it is
located on chromosome 17q23.2. The primary transcript of the miR-21
gene is independently transcribed from a conserved promoter located
within the intron of the overlapping protein-coding gene (10). Experimental data have shown that in
numerous cell types, miR-21 functions as an anti-apoptotic and
pro-survival factor (11,12). High expression levels of miR-21 may be
a characteristic of cancer cells and represent a common feature of
pathological cell growth or cell stress. For instance, miR-21 was
shown to be upregulated in a mouse model of cardiac hypertrophy and
vascular neointimal lesion formation (13,14). The
induction of miR-21 is associated with cellular de-differentiation.
A noteworthy example is the restricted thyroid cell line FRTL-5,
which depends on the presence of thyroid-stimulating hormone
(15). These findings led to the
hypothesis that relatively low levels of miR-21 may be temporarily
and spatially required for differentiation and development, whereas
high levels may exert oncogenic effects. Regarding the immune
system, miR-21 has been shown to regulate T-cell immunity (16). Pro-inflammatory T helper (Th)1 and
anti-inflammatory Th2 cells exist in a balanced state by
counter-regulating each other's differentiation and function.
miR-21 is induced in activated dendritic cells and directly targets
the mRNA that encodes the p35 sub-unit of Th1-promoting interleukin
(IL)-12, and in miR-21-deficient mice, increased secretion of IL-12
by dendritic cells as well as enhanced Th1 development have been
observed (17). In addition to
dendritic cell-derived miR-21, T-cell intrinsic miR-21 has been
shown to promote Th2 differentiation by inhibiting the expression
of Sprouty homolog (Spry)1 transcript, a mitogen-activated protein
kinase (MAPK) pathway inhibitor (18).
Furthermore, miR-21 has been found to be overexpressed in
CD4+ T cells derived from patients with lupus, as well
as from lupus-prone MRL/lpr mice, indicating a strong association
with autoimmune disease.
miR-21 has been shown to be the most commonly
upregulated miRNA in solid and hematological malignancies (6). Extensive studies have implicated its role
in tumor pathogenesis and during all other stages of
carcinogenesis. To date, the following functional studies have been
performed, which strongly suggest that miR-21 exerts oncogenic
activity: i) Knockdown of miR-21 in cultured glioblastoma cells
triggered the activation of caspases and led to an increase in
apoptotic cell death, suggesting that miR-21 acts as an
anti-apoptotic factor (11); ii) the
human miR-21 gene is located in the fragile site FRA17B within the
17q23.2 chromosomal region, which is one of the human papilloma
virus (HPV) integration loci. Integration of HPV into the host cell
genome caused genetic and epigenetic alterations, suggesting that
the location of the miR-21 gene at or near HPV integration sites
may contribute to its elevation in cervical cancer (29); iii) knockdown of miR-21 in hepatoma
cells increased the expression level of tumor suppressor
phosphatase and tensin homologue deleted on chromosome 10 (PTEN), a
direct target of miR-21, and decreased tumor cell proliferation,
migration and invasion (30).
Furthermore, in colorectal cancer, miR-21 expression was found to
be inversely correlated with Spry2 and PTEN leading to cancer
progression (3); and iv) miR-21 was
shown to promote hepatic lipid accumulation and cancer progression
by interacting with the HMG-box transcription factor 1-p53-sterol
regulatory element-binding transcription factor 1 pathway. An
antisense oligonucleotide specific for miR-21 impaired liver lipid
accumulation in mice and growth of xenograft tumors (31).
miR-21 has been identified as an ‘oncomiR’ in
pre-B-cell lymphoma, and inhibition of miR-21 induced biological
and behavioral alterations in diffuse large B-cell lymphoma (DLBCL)
(5,32).
DLBCLs are known to be associated with the AKT signaling pathway,
which is activated during carcinogenesis (33,34).
Furthermore, AKT activation is associated with poor prognosis of
DLBCL patients (35). miR-21 activates
the phosphoinositide-3 kinase (PI3K)/AKT signaling pathway by
directly suppressing forkhead box protein O1 expression and
downregulating PTEN expression (36).
Natural killer (NK)-cell leukemia is a cancer type derived from NK
cells, whose onset and development are, to a great extent, governed
by Epstein-Barr virus (EBV). In addition, miR-21 was found to
negatively regulate the tumor suppressors, PTEN and PDCD4 in
NK-cell leukemia. EBV infection may contribute to the upregulation
of various miRNAs, including miR-21 and miR-155, and infection with
EBV is associated with immortalization of lymphoid cells (37,38). In a
study on a Chinese cohort with DLBCL, miR-21 expression was found
to be elevated in the serum and correlated with the sub-type of
activated B-cell lymphoma, as well as with early-stage disease
(39). Jones et al (40) reported that circulating miR-21 along
with miR-494 and miR-1973 was elevated in patients with classic
Hodgkin lymphoma and indicated the disease response to therapy.
These results suggest the potential use of miR-21 as non-invasive
diagnostic markers.
Patients with chronic myeloid leukemia in the
blastic phase show a poor response to clinical treatment (41); furthermore, retinol-binding protein 2,
a histone H3 lysine 4 demethylase, was found to be underexpressed
during the blastic phase of chronic myeloid leukemia, leading to
epigenetic downregulation of miR-21 (42). Together with miR-155, miR-21 was found
to be markedly overexpressed in patients with chronic lymphocytic
leukemia (CLL) (4). IL-4 induces
B-cell differentiation and survival of CLL cells, and regulation of
miR-21 by IL-4 contributes to evasion of apoptosis of CLL cells
(43). A study using an miR-21-based
scoring system showed that miR-21 expression levels were
significantly higher in CLL patients with chromosome 17 deletion
(compared with CLL patients without chromosome 17 deletion), which
was associated with poor prognosis (44). Although the investigation of miR-21 in
acute leukemia is relatively insufficient, miR-21 is frequently
overexpressed in myeloid blasts of patients with
nucleophosmin-mutant acute myeloid leukemia and associated with a
marked downregulation of PDCD4 protein (45). Lineage-tracing experiments revealed
that Dicer1 deficiency led to apoptosis of T-cell acute
lymphoblastic leukemia (T-ALL) cells. Microarray-based miRNA
profiling revealed that miR-21 was deregulated in mouse and human
T-ALL cells. Furthermore, miR-21 was shown to regulate T-ALL cell
survival via repression of the tumor suppressor PDCD4 (46). In addition, the tumor suppressor Spry2
was revealed to be negatively correlated with miR-21 expression in
myeloma cells. Inhibition of miR-21 led to upregulation of PTEN and
downregulation of phosphorylated AKT in xenografts of myeloma
(47,48). These results suggested that miR-21 is
important in hematological malignancies.
Extensive studies have implicated the integral role
of miR-21 in tumor pathogenesis and during all other stages of
carcinogenesis. Growing evidence supports miR-21 expression as an
important biomarker of poor prognosis in human malignancies
(49). Generally, the expression level
of miR-21 has been found to be higher in more advanced
malignancies. A previous study indicated that high-grade glioma
tended to have higher expression levels of miR-21 than low-grade
glioma (11). In breast cancer,
overexpression of miR-21 was significantly correlated with advanced
clinical stage, lymph node metastasis and poor prognosis (50). Increased expression of miR-21 has been
found in human breast cancer cell lines in vitro as well as
in tissue samples, with a key role in all phases of breast cancer
pathogenesis (51). Overexpression of
the receptor tyrosine kinase HER2 accounts for a clinically
aggressive breast cancer sub-type with an increased incidence of
metastasis. STAT3 co-opts the function of nuclear HER2 by
recruiting it as its co-activator at the response elements in the
promoter of miR-21. miR-21, in turn, was found to downregulate the
expression of the metastasis suppressor protein PDCD4 in breast
cancer (52). Further studies
disclosed that serum and urine miR-21 may be a potential diagnostic
biomarker for breast cancer; however, prior to its implementation
in the clinic, further investigation is warranted (53,54).
The expression of miR-21 is also critical in colon
cancer. During the carcinogenesis of colon cancer, miR-21 induces
stemness by downregulating transforming growth factor β receptor 2
and stimulating invasion, as well as metastasis by suppressing
PDCD4 (55,56). miR-21 has been extensively investigated
for its prognostic potential in at least ten independent trials
involving 2,039 patients since 2008 (57). Nielsen et al (58) evaluated the expression of miR-21 using
semi-quantitative in situ hybridization analysis of
formalin-fixed, paraffin-embedded tissue samples from 197 patients
with stage II colorectal cancer. Strong staining for miR-21 was
significantly associated with shorter disease-free survival and
overall survival. Furthermore, a large multicenter retrospective
trial assessed the association between miRNAs and stage II colon
cancer in a Chinese population. Six selected indicator miRNAs,
comprising four upregulated miRNAs (miR-21, miR-20, miR-103 and
miR-106) and two downregulated miRNAs (miR-143 and miR-215) were
assessed to predict disease recurrence. Forty-six percent of the
high-risk patients experienced a relapse and 15% of the low-risk
group exhibited recurrence (59).
In non-small cell lung cancer (NSCLC), miR-21
enhances oncogenic K-ras activation and modulates tumorigenesis by
targeting Spry2, BTG2 and PDCD4 (24).
The epidermal growth factor receptor (EGFR) pathway has been
regarded as an important mechanism in lung adenocarcinoma. miR-21
expression was found to be significantly increased in cases of lung
adenocarcinoma with EGFR mutations, and activated EGFR signaling
enhanced miR-21 expression (60). A
meta-analysis of eight eligible studies revealed that miR-21
expression is a significant negative prognostic factor in Asian
populations (61). The association of
miR-21 with reduced overall survival has been evidenced in head and
neck carcinoma, as well as in carcinoma of the digestive system
(62). In addition, serum miR-21 was
found have prognostic value in hepatoma and to promote the
development of hepatoma by regulating PDCD4 and PTEN (63). Extensive studies have investigated the
role of miR-21 in prostate carcinogenesis driven by the androgen
receptor (64). Downregulation of RECK
and loss of BTG2 mediated by miR-21 was shown to contribute to
prostate cell transformation (65,66).
Furthermore, miR in prostate cancer tissues and in serum and urine
samples of prostate cancer patients was revealed to be an
independent diagnostic and prognostic biomarker (67–69).
Furthermore, miR-21 has been associated with the
resistance of cancer to drug treatments. Inhibition of miR-21 may
effectively reverse drug resistance in various cancer types
(70). miR-21 is also implicated in
drug resistance of breast cancer. In estrogen-positive breast
cancer, downregulation of PDCD4 was found to be mediated by
upregulation of HER2 via the MAPK and AKT signaling pathways, as
well as miR-21 in aromatase inhibitor-resistant breast cancer cells
(71). An in vitro study on
breast cancer reported that silencing of miR-21 conferred
sensitivity to tamoxifen and fulvestrant by enhancing autophagic
cell death through inhibition of the PI3K-AKT-mammalian target of
rapamycin pathway (72). Induction of
miR-21 by interaction of hyaluronan-CD44 with protein kinase C and
c-Jun has also been reported to contribute to chemotherapy
resistance (73). Regarding anti-HER2
therapy of breast cancer, upregulation of miR-21 conferred
resistance to trastuzumab along with a reduction of PTEN expression
(74). The Geparquinto trial showed a
negative association of circulating miR-21 with overall survival in
HER2-positive breast cancer patients treated with neoadjuvant
chemotherapy and trastuzumab or lapatinib (75). In HT-29 colon cancer cells, miR-21
targeted the human nuts homolog 2, leading to the expression of
thymidine phosphorylase and dihydropyrimidine dehydrogenase. These
mechanisms conferred resistance of colon cancer cells to
fluorouracil (76). In addition to the
well-known platinum-based chemotherapy for lung cancer, miR-21 has
been shown to be involved in the resistance to the EGFR inhibitor.
EGFR-tyrosine kinase inhibitor (TKI) has been regarded as an
important treatment option for NSCLC and miR-21 overexpression was
found to be associated with acquired resistance to EGFR-TKI
(77,78). A pilot study using plasma miRNA
profiles identified miR-21 to be involved in the primary resistance
to EGFR-TKI in patients with advanced NSCLC with an activating EGFR
mutation. The application of this non-invasive approach may be
considered for monitoring responses of lung cancer patients to
EGFR-TKI treatment (79). Furthermore,
the implication of miR-21 in chemotherapy resistance has been
investigated for a wide range of solid cancer types, including
pancreatic and prostate cancer, hepatoma, ovarian cancer, glioma,
and head and neck, stomach and bladder cancer (Table I) (68–71,75,77–94). These results support the clinical
application of miR-21 inhibition in cancer treatments in the
future.
Evidence supports that miR-21 is an oncogenic miRNA
and regulates various downstream effectors associated with cancer.
Overexpression of miR-21 is strongly associated with hematological
and solid malignancies. miR-21 may be utilized as a diagnostic and
prognostic biomarker for various types of cancer and as a potential
therapeutic target. Based on this concept, further research on
miRNA signaling pathways has begun with the aim of elucidating
their effects on conventional protein signaling pathways. The
implication of miR-21 in resistance to anticancer agents highlights
the possible clinical application of miR-21 inhibition for reducing
the resistance of cancer to drugs, with the potential to use
targeted therapeutic strategies in addition to conventional
cytotoxic agents. In 2013, a clinical trial using an miRNA was
launched (NCT01829971; ClinicalTrial.gov). The study evaluates the safety of
an miRNA-RX34 liposomal injection in patients with primary liver
cancer as well as other selected solid tumor types and
hematological malignancies. To the best of our knowledge there are
no ongoing miR-21-based clinical trials on cancer patients. Further
studies are required prior to implementing miRNA-based cancer
therapeutic strategies into clinical practice. Furthermore,
development of effective delivery methods of synthetic therapeutic
miRNAs to desired target tissues may enhance the efficacy of
miRNA-mediated treatments, enabling the adoption of this type of
therapy in cancer medicine in future.
The present study was supported by a grant from the
Chi-Mei Medical Center (Tainan, Taiwan ROC; grant no. CMNCKU 10113
to Y.F.).
|
1
|
Griffiths-Jones S, Grocock RJ, van Dongen
S, Bateman A and Enright AJ: miRBase: microRNA sequences, targets
and gene nomenclature. Nucleic Acids Res. 34:D140–D144. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim VN: MicroRNA biogenesis: Coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng YH, Wu CL, Tsao CJ, Chang JG, Lu PJ,
Yeh KT, Uen YH, Lee JC and Shiau AL: Deregulated expression of
sprouty2 and microRNA-21 in human colon cancer: Correlation with
the clinical stage of the disease. Cancer Biol Ther. 11:111–121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fulci V, Chiaretti S, Goldoni M, Azzalin
G, Carucci N, Tavolaro S, Castellano L, Magrelli A, Citarella F,
Messina M, et al: Quantitative technologies establish a novel
microRNA profile of chronic lymphocytic leukemia. Blood.
109:4944–4951. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Medina PP, Nolde M and Slack FJ: OncomiR
addiction in an in vivo model of microRNA-21-induced pre-B-cell
lymphoma. Nature. 467:86–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sheedy FJ: Turning 21: Induction of miR-21
as a key switch in the inflammatory response. Front Immunol.
6:192015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma X, Conklin DJ, Li F, Dai Z, Hua X, Li
Y, Xu-Monette ZY, Young KH, Xiong W, Wysoczynski M, et al: The
oncogenic microRNA miR-21 promotes regulated necrosis in mice. Nat
Commun. 6:71512015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krichevsky AM and Gabriely G: miR-21: A
small multi-faceted RNA. J Cell Mol Med. 13:39–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Selcuklu SD, Donoghue MT and Spillane C:
miR-21 as a key regulator of oncogenic processes. Biochem Soc
Trans. 37:918–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roldo C, Missiaglia E, Hagan JP, Falconi
M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, et
al: MicroRNA expression abnormalities in pancreatic endocrine and
acinar tumors are associated with distinctive pathologic features
and clinical behavior. J Clin Oncol. 24:4677–4684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tatsuguchi M, Seok HY, Callis TE, Thomson
JM, Chen JF, Newman M, Rojas M, Hammond SM and Wang DZ: Expression
of microRNAs is dynamically regulated during cardiomyocyte
hypertrophy. J Mol Cell Cardiol. 42:1137–1141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen
H, Dean DB and Zhang C: MicroRNA expression signature and
antisense-mediated depletion reveal an essential role of MicroRNA
in vascular neointimal lesion formation. Circ Res. 100:1579–1588.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Landgraf P, Rusu M, Sheridan R, Sewer A,
Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M,
et al: A mammalian microRNA expression atlas based on small RNA
library sequencing. Cell. 129:1401–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu TX, Munitz A and Rothenberg ME:
MicroRNA-21 is up-regulated in allergic airway inflammation and
regulates IL-12p35 expression. J Immunol. 182:4994–5002. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu TX, Hartner J, Lim EJ, Fabry V, Mingler
MK, Cole ET, Orkin SH, Aronow BJ and Rothenberg ME: MicroRNA-21
limits in vivo immune response-mediated activation of the
IL-12/IFN-gamma pathway, Th1 polarization, and the severity of
delayed-type hypersensitivity. J Immunol. 187:3362–3373. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murugaiyan G, Garo LP and Weiner HL:
MicroRNA-21, T helper lineage and autoimmunity. Oncotarget.
6:9644–9645. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujita S, Ito T, Mizutani T, Minoguchi S,
Yamamichi N, Sakurai K and Iba H: miR-21 Gene expression triggered
by AP-1 is sustained through a double-negative feedback mechanism.
J Mol Biol. 378:492–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan X, Wang ZX and Wang R: MicroRNA-21: A
novel therapeutic target in human cancer. Cancer Biol Ther.
10:1224–1232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Löffler D, Brocke-Heidrich K, Pfeifer G,
Stocsits C, Hackermüller J, Kretzschmar AK, Burger R, Gramatzki M,
Blumert C, Bauer K, et al: Interleukin-6 dependent survival of
multiple myeloma cells involves the Stat3-mediated induction of
microRNA-21 through a highly conserved enhancer. Blood.
110:1330–1333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iorio MV, Visone R, Di Leva G, Donati V,
Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et
al: MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang HS, Knies JL, Stark C and Colburn NH:
Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1
transactivation. Oncogene. 22:3712–3720. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hatley ME, Patrick DM, Garcia MR,
Richardson JA, Bassel-Duby R, van Rooij E and Olson EN: Modulation
of K-Ras-dependent lung tumorigenesis by MicroRNA-21. Cancer Cell.
18:282–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu S, Si ML, Wu H and Mo YY: MicroRNA-21
targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol
Chem. 282:14328–14336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schramedei K, Mörbt N, Pfeifer G, Läuter
J, Rosolowski M, Tomm JM, von Bergen M, Horn F and Brocke-Heidrich
K: MicroRNA-21 targets tumor suppressor genes ANP32A and SMARCA4.
Oncogene. 30:2975–2985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gabriely G, Wurdinger T, Kesari S, Esau
CC, Burchard J, Linsley PS and Krichevsky AM: MicroRNA 21 promotes
glioma invasion by targeting matrix metalloproteinase regulators.
Mol Cell Biol. 28:5369–5380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thorland EC, Myers SL, Gostout BS and
Smith DI: Common fragile sites are preferential targets for HPV16
integrations in cervical tumors. Oncogene. 22:1225–1237. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu H, Ng R, Chen X, Steer CJ and Song G:
MicroRNA-21 is a potential link between non-alcoholic fatty liver
disease and hepatocellular carcinoma via modulation of the
HBP1-p53-Srebp1c pathway. Gut gutjnl-2014-308430. 2015.
|
|
32
|
Gu L, Song G, Chen L, Nie Z, He B, Pan Y,
Xu Y, Li R, Gao T, Cho WC, et al: Inhibition of miR-21 induces
biological and behavioral alterations in diffuse large B-cell
lymphoma. Acta Haematol. 130:87–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Davis RE, Ngo VN, Lenz G, Tolar P, Young
RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, et al:
Chronic active B-cell-receptor signalling in diffuse large B-cell
lymphoma. Nature. 463:88–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pfeifer M, Grau M, Lenze D, Wenzel SS,
Wolf A, Wollert-Wulf B, Dietze K, Nogai H, Storek B, Madle H, et
al: PTEN loss defines a PI3K/AKT pathway-dependent germinal center
subtype of diffuse large B-cell lymphoma. Proc Natl Acad Sci USA.
110:12420–12425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong JY, Hong ME, Choi MK, Kim YS, Chang
W, Maeng CH, Park S, Lee SJ, Do IG, Jo JS, et al: The impact of
activated p-AKT expression on clinical outcomes in diffuse large
B-cell lymphoma: A clinicopathological study of 262 cases. Ann
Oncol. 25:182–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Go H, Jang JY, Kim PJ, Kim YG, Nam SJ,
Paik JH, Kim TM, Heo DS, Kim CW and Jeon YK: MicroRNA-21 plays an
oncogenic role by targeting FOXO1 and activating the PI3K/AKT
pathway in diffuse large B-cell lymphoma. Oncotarget.
6:15035–15049. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karube K, Nakagawa M, Tsuzuki S, Takeuchi
I, Honma K, Nakashima Y, Shimizu N, Ko YH, Morishima Y, Ohshima K,
et al: Identification of FOXO3 and PRDM1 as tumor-suppressor gene
candidates in NK-cell neoplasms by genomic and functional analyses.
Blood. 118:3195–3204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin Q, McBride J, Fewell C, Lacey M, Wang
X, Lin Z, Cameron J and Flemington EK: MicroRNA-155 is an
Epstein-Barr virus-induced gene that modulates Epstein-Barr
virus-regulated gene expression pathways. J Virol. 82:5295–5306.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen W, Wang H, Chen H, Liu S, Lu H, Kong
D, Huang X, Kong Q and Lu Z: Clinical significance and detection of
microRNA-21 in serum of patients with diffuse large B-cell lymphoma
in Chinese population. Eur J Haematol. 92:407–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jones K, Nourse JP, Keane C, Bhatnagar A
and Gandhi MK: Plasma microRNA are disease response biomarkers in
classical Hodgkin lymphoma. Clin Cancer Res. 20:253–264. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Anger B, Carbonell F, Braunger I, Heinze
B, Gutensohn W, Thiel E and Heimpel H: Blast crisis of Philadelphia
chromosome-positive chronic myelocytic leukemia (CML). Treatment
results of 69 patients. Blut. 57:131–137. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou M, Zeng J, Wang X, Wang X, Huang T,
Fu Y, Sun T, Jia J and Chen C: Histone demethylase RBP2 decreases
miR-21 in blast crisis of chronic myeloid leukemia. Oncotarget.
6:1249–1261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ruiz-Lafuente N, Alcaraz-García MJ,
Sebastián-Ruiz S, García-Serna AM, Gómez-Espuch J, Moraleda JM,
Minguela A, García-Alonso AM and Parrado A: IL-4 up-regulates
MiR-21 and the MiRNAs hosted in the CLCN5 gene in chronic
lymphocytic leukemia. PLoS One. 10:e01249362015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rossi S, Shimizu M, Barbarotto E, Nicoloso
MS, Dimitri F, Sampath D, Fabbri M, Lerner S, Barron LL, Rassenti
LZ, et al: microRNA fingerprinting of CLL patients with chromosome
17p deletion identify a miR-21 score that stratifies early
survival. Blood. 116:945–952. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Riccioni R, Lulli V, Castelli G, Biffoni
M, Tiberio R, Pelosi E, Lo-Coco F and Testa U: miR-21 is
overexpressed in NPM1-mutant acute myeloid leukemias. Leuk Res.
39:221–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Junker F, Chabloz A, Koch U and Radtke F:
Dicer1 imparts essential survival cues in Notch-driven T-ALL via
miR-21-mediated tumor suppressor Pdcd4 repression. Blood.
126:993–1004. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Leone E, Morelli E, Di Martino MT, Amodio
N, Foresta U, Gullà A, Rossi M, Neri A, Giordano A, Munshi NC, et
al: Targeting miR-21 inhibits in vitro and in vivo multiple myeloma
cell growth. Clin Cancer Res. 19:2096–2106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang JH, Zhou WW, Cheng ST, Liu BX, Liu FR
and Song JQ: Downregulation of Sprouty homolog 2 by microRNA-21
inhibits proliferation, metastasis and invasion, however promotes
the apoptosis of multiple myeloma cells. Mol Med Rep. 12:1810–1816.
2015.PubMed/NCBI
|
|
49
|
Wang W, Li J, Zhu W, Gao C, Jiang R, Li W,
Hu Q and Zhang B: MicroRNA-21 and the clinical outcomes of various
carcinomas: A systematic review and meta-analysis. BMC Cancer.
14:8192014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang ZJ and Ma SL: miRNAs in breast
cancer tumorigenesis (Review). Oncol Rep. 27:903–910.
2012.PubMed/NCBI
|
|
52
|
Venturutti L, Romero LV, Urtreger AJ,
Chervo MF, Russo RI Cordo, Mercogliano MF, Inurrigarro G, Pereyra
MG, Proietti CJ, Izzo F, et al: Stat3 regulates ErbB-2 expression
and co-opts ErbB-2 nuclear function to induce miR-21 expression,
PDCD4 downregulation and breast cancer metastasis. Oncogene.
35:2208–2222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Erbes T, Hirschfeld M, Rücker G, Jaeger M,
Boas J, Iborra S, Mayer S, Gitsch G and Stickeler E: Feasibility of
urinary microRNA detection in breast cancer patients and its
potential as an innovative non-invasive biomarker. BMC Cancer.
15:1932015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li S, Yang X, Yang J, Zhen J and Zhang D:
Serum microRNA-21 as a potential diagnostic biomarker for breast
cancer: A systematic review and meta-analysis. Clin Exp Med.
16:29–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yu Y, Nangia-Makker P, Farhana LG,
Rajendra S, Levi E and Majumdar AP: miR-21 and miR-145 cooperation
in regulation of colon cancer stem cells. Mol Cancer. 14:982015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dong Y, Yu J and Ng SS: MicroRNA
dysregulation as a prognostic biomarker in colorectal cancer.
Cancer Manag Res. 6:405–422. 2014.PubMed/NCBI
|
|
58
|
Nielsen BS, Jørgensen S, Fog JU, Søkilde
R, Christensen IJ, Hansen U, Brünner N, Baker A, Møller S and
Nielsen HJ: High levels of microRNA-21 in the stroma of colorectal
cancers predict short disease-free survival in stage II colon
cancer patients. Clin Exp Metastasis. 28:27–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang JX, Song W, Chen ZH, Wei JH, Liao
YJ, Lei J, Hu M, Chen GZ, Liao B, Lu J, et al: Prognostic and
predictive value of a microRNA signature in stage II colon cancer:
A microRNA expression analysis. Lancet Oncol. 14:1295–1306. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Seike M, Goto A, Okano T, Bowman ED,
Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, et
al: miR-21 is an EGFR-regulated anti-apoptotic factor in lung
cancer in never-smokers. Proc Natl Acad Sci USA. 106:12085–12090.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ma XL, Liu L, Liu XX, Li Y, Deng L, Xiao
ZL, Liu YT, Shi HS and Wei YQ: Prognostic role of microRNA-21 in
non-small cell lung cancer: A meta-analysis. Asian Pac J Cancer
Prev. 13:2329–2334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fu X, Han Y, Wu Y, Zhu X, Lu X, Mao F,
Wang X, He X and Zhao Y and Zhao Y: Prognostic role of microRNA-21
in various carcinomas: A systematic review and meta-analysis. Eur J
Clin Invest. 41:1245–1253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wang X, Zhang J, Zhou L, Lu P, Zheng ZG,
Sun W, Wang JL, Yang XS, Li XL, Xia N, et al: Significance of serum
microRNA-21 in diagnosis of hepatocellular carcinoma (HCC):
Clinical analyses of patients and an HCC rat model. Int J Clin Exp
Pathol. 8:1466–1478. 2015.PubMed/NCBI
|
|
64
|
Ribas J, Ni X, Haffner M, Wentzel EA,
Salmasi AH, Chowdhury WH, Kudrolli TA, Yegnasubramanian S, Luo J,
Rodriguez R, et al: miR-21: An androgen receptor-regulated microRNA
that promotes hormone-dependent and hormone-independent prostate
cancer growth. Cancer Res. 69:7165–7169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Reis ST, Pontes-Junior J, Antunes AA,
Dall'Oglio MF, Dip N, Passerotti CC, Rossini GA, Morais DR,
Nesrallah AJ, Piantino C, et al: miR-21 may acts as an oncomir by
targeting RECK, a matrix metalloproteinase regulator, in prostate
cancer. BMC Urol. 12:142012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Coppola V, Musumeci M, Patrizii M,
Cannistraci A, Addario A, Maugeri-Saccà M, Biffoni M,
Francescangeli F, Cordenonsi M, Piccolo S, et al: BTG2 loss and
miR-21 upregulation contribute to prostate cell transformation by
inducing luminal markers expression and epithelial-mesenchymal
transition. Oncogene. 32:1843–1853. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li T, Li RS, Li YH, Zhong S, Chen YY,
Zhang CM, Hu MM and Shen ZJ: miR-21 as an independent biochemical
recurrence predictor and potential therapeutic target for prostate
cancer. J Urol. 187:1466–1472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Samsonov R, Shtam T, Burdakov V, Glotov A,
Tsyrlina E, Berstein L, Nosov A, Evtushenko V, Filatov M and Malek
A: Lectin-induced agglutination method of urinary exosomes
isolation followed by mi-RNA analysis: Application for prostate
cancer diagnostic. Prostate. 76:68–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Koppers-Lalic D, Hackenberg M, de Menezes
R, Misovic B, Wachalska M, Geldof A, Zini N, de Reijke T, Wurdinger
T, Vis A, et al: Non-invasive prostate cancer detection by
measuring miRNA variants (isomiRs) in urine extracellular vesicles.
Oncotarget. 7:22566–22578. 2016.PubMed/NCBI
|
|
70
|
Hong L, Han Y, Zhang Y, Zhang H, Zhao Q,
Wu K and Fan D: MicroRNA-21: A therapeutic target for reversing
drug resistance in cancer. Expert Opin Ther Targets. 17:1073–1080.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen Z, Yuan YC, Wang Y, Liu Z, Chan HJ
and Chen S: Down-regulation of programmed cell death 4 (PDCD4) is
associated with aromatase inhibitor resistance and a poor prognosis
in estrogen receptor-positive breast cancer. Breast Cancer Res
Treat. 152:29–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yu X, Li R, Shi W, Jiang T, Wang Y, Li C
and Qu X: Silencing of MicroRNA-21 confers the sensitivity to
tamoxifen and fulvestrant by enhancing autophagic cell death
through inhibition of the PI3K-AKT-mTOR pathway in breast cancer
cells. Biomed Pharmacother. 77:37–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bourguignon LY, Spevak CC, Wong G, Xia W
and Gilad E: Hyaluronan-CD44 interaction with protein kinase
C(epsilon) promotes oncogenic signaling by the stem cell marker
Nanog and the Production of microRNA-21, leading to down-regulation
of the tumor suppressor protein PDCD4, anti-apoptosis, and
chemotherapy resistance in breast tumor cells. J Biol Chem.
284:26533–26546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Gong C, Yao Y, Wang Y, Liu B, Wu W, Chen
J, Su F, Yao H and Song E: Up-regulation of miR-21 mediates
resistance to trastuzumab therapy for breast cancer. J Biol Chem.
286:19127–19137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Müller V, Gade S, Steinbach B, Loibl S,
von Minckwitz G, Untch M, Schwedler K, Lübbe K, Schem C, Fasching
PA, et al: Changes in serum levels of miR-21, miR-210, and miR-373
in HER2-positive breast cancer patients undergoing neoadjuvant
therapy: A translational research project within the Geparquinto
trial. Breast Cancer Res Treat. 147:61–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Deng J, Lei W, Fu JC, Zhang L, Li JH and
Xiong JP: Targeting miR-21 enhances the sensitivity of human colon
cancer HT-29 cells to chemoradiotherapy in vitro. Biochem Biophys
Res Commun. 443:789–795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li B, Ren S, Li X, Wang Y, Garfield D,
Zhou S, Chen X, Su C, Chen M, Kuang P, et al: miR-21 overexpression
is associated with acquired resistance of EGFR-TKI in non-small
cell lung cancer. Lung Cancer. 83:146–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shen H, Zhu F, Liu J, Xu T, Pei D, Wang R,
Qian Y, Li Q, Wang L, Shi Z, et al: Alteration in Mir-21/PTEN
expression modulates gefitinib resistance in non-small cell lung
cancer. PLoS One. 9:e1033052014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wang S, Su X, Bai H, Zhao J, Duan J, An T,
Zhuo M, Wang Z, Wu M, Li Z, et al: Identification of plasma
microRNA profiles for primary resistance to EGFR-TKIs in advanced
non-small cell lung cancer (NSCLC) patients with EGFR activating
mutation. J Hematol Oncol. 8:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wu ZH, Tao ZH, Zhang J, Li T, Ni C, Xie J,
Zhang JF and Hu XC: miRNA-21 induces epithelial to mesenchymal
transition and gemcitabine resistance via the PTEN/AKT pathway in
breast cancer. Tumour Biol. 37:7245–7254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yu Y, Kanwar SS, Patel BB, Oh PS, Nautiyal
J, Sarkar FH and Majumdar AP: MicroRNA-21 induces stemness by
downregulating transforming growth factor beta receptor 2 (TGFβR2)
in colon cancer cells. Carcinogenesis. 33:68–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Feng YH, Wu CL, Shiau AL, Lee JC, Chang
JG, Lu PJ, Tung CL, Feng LY, Huang WT and Tsao CJ:
MicroRNA-21-mediated regulation of Sprouty2 protein expression
enhances the cytotoxic effect of 5-fluorouracil and metformin in
colon cancer cells. Int J Mol Med. 29:920–926. 2012.PubMed/NCBI
|
|
83
|
Liu ZL, Wang H, Liu J and Wang ZX:
MicroRNA-21 (miR-21) expression promotes growth, metastasis, and
chemo- or radioresistance in non-small cell lung cancer cells by
targeting PTEN. Mol Cell Biochem. 372:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wei X, Wang W, Wang L, Zhang Y, Zhang X,
Chen M, Wang F, Yu J, Ma Y and Sun G: MicroRNA-21 induces
5-fluorouracil resistance in human pancreatic cancer cells by
regulating PTEN and PDCD4. Cancer Med. 5:693–702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Paik WH, Kim HR, Park JK, Song BJ, Lee SH
and Hwang JH: Chemosensitivity induced by down-regulation of
microRNA-21 in gemcitabine-resistant pancreatic cancer cells by
indole-3-carbinol. Anticancer Res. 33:1473–1481. 2013.PubMed/NCBI
|
|
86
|
Shi GH, Ye DW, Yao XD, Zhang SL, Dai B,
Zhang HL, Shen YJ, Zhu Y, Zhu YP, Xiao WJ, et al: Involvement of
microRNA-21 in mediating chemo-resistance to docetaxel in
androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin.
31:867–873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tomimaru Y, Eguchi H, Nagano H, Wada H,
Tomokuni A, Kobayashi S, Marubashi S, Takeda Y, Tanemura M,
Umeshita K, et al: MicroRNA-21 induces resistance to the
anti-tumour effect of interferon-α/5-fluorouracil in hepatocellular
carcinoma cells. Br J Cancer. 103:1617–1626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
He C, Dong X, Zhai B, Jiang X, Dong D, Li
B, Jiang H, Xu S and Sun X: miR-21 mediates sorafenib resistance of
hepatocellular carcinoma cells by inhibiting autophagy via the
PTEN/Akt pathway. Oncotarget. 6:28867–28881. 2015.PubMed/NCBI
|
|
89
|
Echevarría-Vargas IM, Valiyeva F and
Vivas-Mejía PE: Upregulation of miR-21 in cisplatin resistant
ovarian cancer via JNK-1/c-Jun pathway. PLoS One. 9:e970942014.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Xie Z, Cao L and Zhang J: miR-21 modulates
paclitaxel sensitivity and hypoxia-inducible factor-1α expression
in human ovarian cancer cells. Oncol Lett. 6:795–800.
2013.PubMed/NCBI
|
|
91
|
Lan F, Pan Q, Yu H and Yue X: Sulforaphane
enhances temozolomide-induced apoptosis because of down-regulation
of miR-21 via Wnt/β-catenin signaling in glioblastoma. J Neurochem.
134:811–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shi L, Chen J, Yang J, Pan T, Zhang S and
Wang Z: miR-21 protected human glioblastoma U87MG cells from
chemotherapeutic drug temozolomide induced apoptosis by decreasing
Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 1352:255–264.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhou X, Ren Y, Liu A, Jin R, Jiang Q,
Huang Y, Kong L, Wang X and Zhang L: WP1066 sensitizes oral
squamous cell carcinoma cells to cisplatin by targeting
STAT3/miR-21 axis. Sci Rep. 4:74612014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bourguignon LY, Earle C, Wong G, Spevak CC
and Krueger K: Stem cell marker (Nanog) and Stat-3 signaling
promote MicroRNA-21 expression and chemoresistance in
hyaluronan/CD44-activated head and neck squamous cell carcinoma
cells. Oncogene. 31:149–160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yang GD, Huang TJ, Peng LX, Yang CF, Liu
RY, Huang HB, Chu QQ, Yang HJ, Huang JL, Zhu ZY, et al:
Epstein-Barr Virus_Encoded LMP1 upregulates microRNA-21 to promote
the resistance of nasopharyngeal carcinoma cells to
cisplatin-induced Apoptosis by suppressing PDCD4 and Fas-L. PLoS
One. 8:e783552013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yang SM, Huang C, Li XF, Yu MZ, He Y and
Li J: miR-21 confers cisplatin resistance in gastric cancer cells
by regulating PTEN. Toxicology. 306:162–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tao J, Lu Q, Wu D, Li P, Xu B, Qing W,
Wang M, Zhang Z and Zhang W: microRNA-21 modulates cell
proliferation and sensitivity to doxorubicin in bladder cancer
cells. Oncol Rep. 25:1721–1729. 2011.PubMed/NCBI
|