Introduction
Infections caused by carbapenem-resistant
Klebsiella pneumoniae (CR-KP) isolates are worldwide. The
prevalence of CR-KP infection in areas of endemicity may vary
between 20 and 40%. In addition, these infections often occur in
debilitated and immunocompromised patients, in association with
prolonged hospital stays (1). The
isolates are often resistant to multiple antibiotics, and the
mortality associated with infections due to CR-KP is extremely high
(2–4).
Early identification of possible CR-KP-infected patients and
implementation of proper precaution are core measures for
controlling CR-KP infections.
Risk factors involved in CR-KP infections have been
previously investigated (5–8). These factors were heterogeneous. A
retrospective study was conducted in a Chinese tertiary care
hospital to identify the main factors associated with nosocomial
CR-KP infections, and a model was established for the early
prediction of patients with such infection. The results show that
the prediction model developed in the present study might be useful
for controlling infections caused by CR-KP strains.
Materials and methods
Setting and participants
The Beijing Shijitan Hospital of the Capital Medical
University is a 1,100-bed tertiary care hospital in Beijing, China,
with a 26-bed general intensive care unit (ICU), an 8-bed
cardiology ICU, an 8-bed respiratory ICU, and a 6-bed emergency
medicine ICU.
This retrospective study was conducted based on the
hospital electronic database. During the 2-year study period (from
January 1, 2012 to December 31, 2013), patients with K.
pneumoniae nosocomial infection were evaluated. During the
study period, rectal swab screening was not a routine admission
procedure and patients were clustered within at least 48 h once
CR-KP infection was confirmed.
In one hospitalization period, each patient was
evaluated only once at the time of the index culture (nosocomial
infections were validated by trained infection management doctors
according to the criteria based on a previous study) (9), and the index culture was defined with the
K. pneumoniae strain first isolated from a clinical specimen
and then the corresponding nosocomial infection was confirmed.
Patients with CR-KP infections were defined as cases. For each case
enrolled, two matched controls with no CR-KP infection during their
hospitalization were randomly selected. Matching involved month of
admission, ward, as well as interval days (interval from admission
to confirmation of the index culture). The length of the entire
hospital stay of the controls was equal or more to the interval
days of the matched cases.
The following patient data were extracted: Age;
gender; transfer from another hospital; comorbidity (at the time of
index culture); recent admission to ICU (defined as patients
admitted to ICU for >24 h before the index culture in one
hospitalization period); with CR-KP-positive patients in nearby
beds (defined as patients with CR-KP-positive patients in the same
ward for >24 h before the index culture within the
hospitalization period); adopted invasive procedures including
central venous catheterization, urinary catheter, nasogastric tube,
surgical drain, and invasive mechanical ventilation (at the time of
the index culture); and on prior antibiotic therapy (defined as the
use of a systemic antimicrobial agent for ≥48 h within the
preceding 10 days of index culture in one hospitalization
period).
Approval for the study was obtained from the ethics
committee of the Beijing Shijitan Hospital of Capital Medical
University.
Microbiological procedures
The Vitek 2 system (bioMérieux, Marcy l'Étoile,
France) was used for identification of isolates and antimicrobial
susceptibility testing. The production of extended-spectrum
β-lactamases (ESBLs) was suggested following use of the Advanced
Expert System of the Vitek 2 system. Susceptibility results to
carbapenems (imipenem, meropenem, and ertapenem) were interpreted
according to Clinical Laboratory Standards Institute guidelines
coevally (i.e., susceptibility results of year 2012 were
interpreted according to 2012 guideline and results of year 2013
were interpreted according to 2013 standards) (10). Isolates were considered CR-KP if any of
the carbapenems (ertapenem, meropenem, and imipenem) was resistant.
If the carbapenem MIC of one isolate was intermediary, the isolate
was excluded from the analysis.
Statistical analysis
The SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA)
software was used for data analysis. Non-normally distributed
continuous variables were reported as medians (interquartile
range); and numbers and percentages were reported for categorical
variables. The Mann-Whitney U test was used to compare non-normally
distributed continuous variables (i.e., ages). Categorical
variables were evaluated with the Chi-square test.
The multivariate logistic regression model was used
to adjust for potential confounding. Variables associated with
CR-KP infection in the univariate analysis (P≤0.05) were included
in a logistic regression analysis to identify those independently
associated with the development of CR-KP nosocomial infection.
Statistical tests were two-tailed. P≤0.05 was considered
statistically significant.
The power of age and cumulative risk factors to
discriminate cases and controls were expressed as the area under
the receiver-operating characteristic curve.
Results
Characteristics of isolates
During the 2-year study period, 370 non-repetitive
episodes of nosocomial infections due to CR-KP were found. The
characteristics of the cases and controls are shown in Table I. The median (IQR) time of interval
days in the case cohort was 17 days (13–21 days). No active
outbreak of CR-KP during the 2-year study period was recorded.
 | Table I.The characteristics of cases and
controls. |
Table I.
The characteristics of cases and
controls.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | Case (n=370) | Control (n=740) | P-value | OR | 95% CI | P-value |
|---|
| Demographics |
|
|
|
|
|
|
| Age
(year) | 85
(80–87) | 74
(59–84) | <0.001 | 1.08 | 1.06–1.10 | <0.001 |
| Male | 321 (86.8) | 434 (58.6) | <0.001 |
3.839 | 2.36–6.26 | <0.001 |
| Transfer
from another hospital | 23 (6.2) | 16 (2.2) |
0.001 |
0.750 | 0.29–1.94 |
0.553 |
|
Comorbiditiesa |
|
|
|
|
|
|
|
Cardiovascular disease | 80
(21.6) | 32
(15.0) | <0.001 | 10.00 | 4.52–22.15 | <0.001 |
| Chronic
pulmonary disease | 162 (43.8) | 303 (40.9) |
0.366 |
|
|
|
|
Cerebrovascular disease | 88
(23.8) | 192 (25.9) |
0.434 |
|
|
|
| Liver
disease | 22 (5.9) | 50 (6.8) |
0.605 |
|
|
|
| Chronic
renal failure | 27 (7.3) | 51 (6.9) |
0.803 |
|
|
|
| Diabetes
mellitus | 23 (6.2) | 43 (5.8) |
0.788 |
|
|
|
| Peptic
ulcer disease | 8
(3.0) | 32 (4.3) |
0.068 |
|
|
|
|
Malignancy | 26 (7.0) | 56 (7.6) |
0.746 |
|
|
|
| Recent
surgeryb | 36 (9.7) | 101 (13.6) |
0.061 |
|
|
|
| Hospitalization
data |
|
|
|
|
|
|
| Admission
to ICUc | 225 (60.8) | 75
(10.1) | <0.001 | 2.06 | 1.03–4.10 |
0.040 |
|
CR-KP-positive patients in
nearby bedsd | 251 (67.8) | 111 (15.0) | <0.001 | 1.18 | 0.60–2.29 |
0.636 |
| Invasive
proceduresa |
|
|
|
|
|
|
| Central
venous catheter | 198 (53.5) | 109 (14.7) | <0.001 | 1.64 | 0.99–2.71 |
0.052 |
| Urinary
catheter | 297 (80.2) | 240 (32.4) | <0.001 | 2.21 | 1.36–3.59 |
0.001 |
|
Nasogastric tube | 207 (55.9) | 65 (8.8) | <0.001 | 0.95 | 0.41–2.23 |
0.904 |
| Surgical
drain | 10 (2.7) | 22 (3.0) |
0.800 |
|
|
|
|
Mechanical ventilation | 193 (52.2) | 56 (7.6) | <0.001 | 2.80 | 1.15–6.86 |
0.024 |
| Prior antibiotic
therapye |
|
|
|
|
|
|
|
Second-generation
cephalosporins | 40
(10.8) | 335 (45.2) | <0.001 |
0.787 | 0.43–1.43 |
0.433 |
|
Third-generation
cephalosporins | 65
(17.6) | 102 (13.8) |
0.096 |
|
|
|
|
Fourth-generation
cephalosporins | 27 (7.3) | 18 (2.4) | <0.001 |
3.79 | 1.44–10.03 |
0.007 |
|
β-lactam-β-lactamase
inhibitors | 156 (42.2) | 111 (15.0) | <0.001 | 10.65 | 5.93–19.10 | <0.001 |
|
Carbapenems | 194 (52.4) | 123 (16.6) | <0.001 |
7.75 | 4.21–14.27 | <0.001 |
|
Fluoroquinolones | 47
(12.7) | 106 (14.3) |
0.460 |
|
|
|
|
Aminoglycosides | 25 (6.8) | 38 (5.1) |
0.271 |
|
|
|
|
Othersf | 55
(14.9) | 137 (18.5) |
0.130 |
|
|
|
CR-KP isolates were recovered from the
sputum/bronchoalveolar lavage fluid (n=268), urine (n=48), blood
(n=35), skin/mucosa (n=10), surgical site (n=7), and cerebrospinal
fluid (n=2). ESBLs were produced by 61% of CR-KP strains
(n=225).
Risk factors associated with CR-KP
infection
The main findings of the univariate and multivariate
analysis associated with CR-KP infection are shown in Table I. The multivariate analysis revealed
that age, male gender, with cardiovascular disease, recent
admission to ICU, indwelling urinary catheter and/or surgical
drain, mechanical ventilation, and prior administration of
β-lactam-β-lactamase inhibitors, fourth-generation cephalosporins,
and/or carbapenems were significantly different between CR-KP
infections and controls.
Cumulative risk factors and their
predicting power of CR-KP infection
Male gender, with cardiovascular disease, recent
admission to ICU, indwelling urinary catheter, mechanical
ventilation, and prior use of antibiotics of β-lactam-β-lactamase
inhibitors, fourth-generation cephalosporins and/or carbapenems
were included in the calculation of cumulative risk factors
(Table II).
 | Table II.Distribution of cumulative risk
factors for CR-KP infection. |
Table II.
Distribution of cumulative risk
factors for CR-KP infection.
| No. of risk
factors | Casea |
Controla | Totala | Caseb |
Controlb | Totalb |
|---|
| 0 |
4 | 154 | 158 |
4 | 93 | 97 |
| 1 | 13 | 279 | 292 | 11 | 160 | 171 |
| 2 | 39 | 171 | 210 | 35 | 102 | 137 |
| 3 | 76 | 94 | 170 | 71 | 54 | 125 |
| 4 | 86 | 29 | 115 | 86 | 19 | 105 |
| 5 | 86 | 13 | 99 | 71 |
9 | 80 |
| 6 | 66 |
0 | 66 | 60 |
0 | 60 |
| 7 |
0 |
0 |
0 |
0 |
0 |
0 |
| Total | 370 | 740 | 1,110 | 338 | 437 | 775 |
The area under the curve (AUC) was 0.739 (95% CI,
0.709–0.768; P≤0.001) for age prediction CR-KP infection. The
Yonden index was at its maximum when the patient age was 70 years.
After filtering by age (≥70 years), 338 CR-KP infection cases and
437 controls remained.
The sensitivity, specificity, and total consistency
rate for predicting CR-KP infection at different cut-off levels
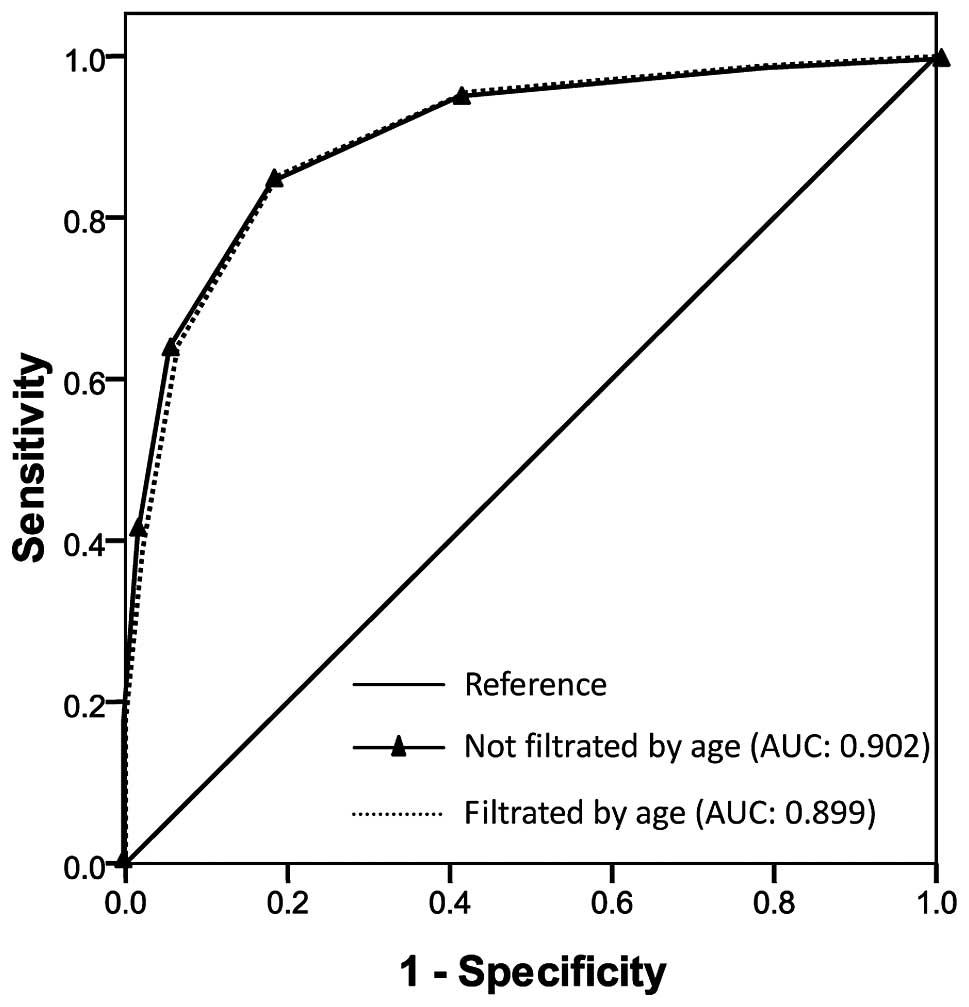
using cumulative risk factors are shown in Table III. The AUC was 0.902 (95% CI,
0.883–0.920; P≤0.001) for cumulative risk factors predicting CR-KP
infection tested in the total 1,110 patients and 0.899 (95% CI,
0.877–0.921; P≤0.001) tested in the 775 patients filtered by age
(Fig. 1), respectively. The Yonden
index was at its maximum when the cumulative risk factors were ≥3
in the two prediction models. However, the sensitivity of
prediction increased from 84.8 to 85.2% and the specificity
increased from 68.1 to 81.2% with age (≥70 years),
respectively.
 | Table III.Sensitivity, specificity, and total
consistency rate for predicting CR-KP infection at different
cut-off levels. |
Table III.
Sensitivity, specificity, and total
consistency rate for predicting CR-KP infection at different
cut-off levels.
| No. of risk
factors | SEa | SPa | TCRa | SEb | SPb | TCRb |
|---|
| ≥1 | 98.9 | 20.8 | 46.8 | 98.8 | 21.3 | 55.1 |
| ≥2 | 95.4 | 58.5 | 70.8 | 95.6 | 57.9 | 774.3 |
| ≥3 | 84.8 | 68.1 | 82.7 | 85.2 | 81.2 | 83.0 |
| ≥4 | 64.3 | 94.3 | 84.3 | 64.2 | 93.6 | 80.7 |
| ≥5 | 41.1 |
98.2 | 79.2 | 38.8 |
97.9 |
72.1 |
| ≥6 | 17.8 | 100.0 | 72.6 | 17.8 | 100.0 |
64.1 |
Discussion
The results of the present study showed that age,
male gender, with cardiovascular disease, recent admission to ICU
for more than 24 h, indwelling urinary catheter, mechanical
ventilation, exposure to β-lactam-β-lactamase inhibitors,
fourth-generation cephalosporins and carbapenems were associated
with CR-KP infection.
Compared with young patients, elderly patients were
more likely to have comorbidities and susceptibility to infections
(11). In the present study, patients
with CR-KP infection were elderly compared to those with no CR-KP
infection.
Male gender was associated with CR-KP infection.
Daikos et al found that the proportion of male patients
surviving was significantly higher than those that succumbed due to
carbapenemase-producing K. pneumoniae bloodstream infections
(2). However, to the best of our
knowledge, this is the first time that the male gender and
cardiovascular disease constituted risk factors for CR-KP
infection. Regarding the latter, it may be explained by the fact
that cardiovascular disease is diagnosed more frequently in men
(12).
As expected, ICU stay was an independent risk factor
for CR-KP infections. ICUs are the principal hospital reservoirs of
MDR bacteria. Patients with ICU admission represent a population
subjected to many invasive procedures and medical devices, which
facilitate cross-transmission of resistance mechanisms (7,13,14).
In the current study, CR-KP infection was also
associated with indwelling urinary catheter and mechanical
ventilation. These invasive procedures are well-known risk factors
for MDR bacteria infection/colonization (7,13,15).
Prior administration of β-lactam-β-lactamase
inhibitors and/or carbapenems was associated with CR-KP infection,
as reported in other studies (7,8,16). This observation may be due to the fact
that treatment with broad-spectrum antibiotics potentially promotes
the selection of CR-KP strains by eliminating susceptible competing
clones. In a previous study, it was reported that a combination
therapy containing carbapenem was strongly associated with survival
in CR-KP bloodstream infections (2).
Thus, administration of carbapenem should be used prudently in
empirical therapy, and once the CR-KP infection is highly suspected
or verified, a regimen including carbapenem is likely to benefit
the outcome.
Previous findings have shown that the presence of
CR-KP-colonized patients in nearby beds was associated with CR-KP
infection/colonization (17,18). In the present study, although the
proportion of patients who had CR-KP-positive patients in nearby
beds was higher in the case group than in the control group,
CR-KP-positive patients in nearby beds was not an independent risk
factor of CR-KP infection. This discrepancy may partly be because
of the different case-control design, and remains to be
confirmed.
The model for predicting CR-KP infection developed
in this study displayed good discriminatory power (AUC=0.902),
which is similar to the model developed by Tumbarello et al
(7). The sensibility was 84.8% and
specificity was 68.1% when there were three or more risk factors
for predicting CR-KP infection. The age of patients (≥70 years)
increased the predicting specificity to 81.2%. The predicting model
is potentially useful in hospitals where CR-KP infection is
epidemic. Empirical application of infection control measures, such
as rectal swabs, may be limited to individuals with a higher
possibility of CR-KP infection, thereby reducing workloads and
costs. This prediction model may also be useful for empirical
antimicrobial therapy and potentially reduce the risk of
ineffective therapy during the initial phase of treatment. Notably,
the prevention of nosocomial acquisition of resistant bacteria is
dependent on implementation of and adherence to infection control
standards.
In conclusion, the present findings provide
information that may be useful for identifying patients at high
risk for CR-KP infection and may assist physicians to use more
effective treatments for CR-KP infections.
Glossary
Abbreviations
Abbreviations:
|
CR-KP
|
Carbapenem-resistant Klebsiella
pneumonia
|
|
CS-KP
|
Carbapenem-sensitive Klebsiella
pneumonia
|
|
ICU
|
intensive care unit
|
|
ESBLs
|
extended-spectrum β-lactamases
|
|
MIC
|
minimal inhibitory concentration
|
|
AUC
|
area under the curve
|
|
MDR
|
multidrug resistance
|
References
|
1
|
Tzouvelekis LS, Markogiannakis A,
Psichogiou M, Tassios PT and Daikos GL: Carbapenemases in
Klebsiella pneumoniae and other Enterobacteriaceae: An evolving
crisis of global dimensions. Clin Microbiol Rev. 25:682–707. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Daikos GL, Tsaousi S, Tzouvelekis LS,
Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V,
Miriagou V, Nepka M, et al: Carbapenemase-producing Klebsiella
pneumoniae bloodstream infections: Lowering mortality by antibiotic
combination schemes and the role of carbapenems. Antimicrob Agents
Chemother. 58:2322–2328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fraenkel-Wandel Y, Raveh-Brawer D,
Wiener-Well Y, Yinnon AM and Assous MV: Mortality due to blaKPC
Klebsiella peumoniae bacteraemia. J Antimicrob Chemother.
71:1083–1087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qureshi ZA, Paterson DL, Potoski BA,
Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM and
Doi Y: Treatment outcome of bacteremia due to KPC-producing
Klebsiella pneumoniae: Superiority of combination antimicrobial
regimens. Antimicrob Agents Chemother. 56:2108–2113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falagas ME, Rafailidis PI, Kofteridis D,
Virtzili S, Chelvatzoglou FC, Papaioannou V, Maraki S, Samonis G
and Michalopoulos A: Risk factors of carbapenem-resistant
Klebsiella pneumoniae infections: A matched case control study. J
Antimicrob Chemother. 60:1124–1130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwaber MJ, Klarfeld-Lidji S,
Navon-Venezia S, Schwartz D, Leavitt A and Carmeli Y: Predictors of
carbapenem-resistant Klebsiella pneumoniae acquisition among
hospitalized adults and effect of acquisition on mortality.
Antimicrob Agents Chemother. 52:1028–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tumbarello M, Trecarichi EM, Tumietto F,
Del Bono V, De Rosa FG, Bassetti M, Losito AR, Tedeschi S, Saffioti
C, Corcione S, et al: Predictive models for identification of
hospitalized patients harboring KPC-producing Klebsiella
pneumoniae. Antimicrob Agents Chemother. 58:3514–3520. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zarkotou O, Pournaras S, Voulgari E,
Chrysos G, Prekates A, Voutsinas D, Themeli-Digalaki K and Tsakris
A: Risk factors and outcomes associated with acquisition of
colistin-resistant KPC-producing Klebsiella pneumoniae: A matched
case-control study. J Clin Microbiol. 48:2271–2274. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horan TC, Andrus M and Dudeck MA: CDC/NHSN
surveillance definition of health care-associated infection and
criteria for specific types of infections in the acute care
setting. Am J Infect Control. 36:309–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clinical and Laboratory Standards
Institute (CLSI). Methods for dilution antimicrobial susceptibility
tests for bacteria that grow aerobically; Approved standard
M100CLSI; Wayne, PA: 2012
|
|
11
|
Rodríguez-Baño J, Alcalá JC, Cisneros JM,
Grill F, Oliver A, Horcajada JP, Tórtola T, Mirelis B, Navarro G,
Cuenca M, et al: Community infections caused by extended-spectrum
beta-lactamase-producing Escherichia coli. Arch Intern Med.
168:1897–1902. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pilote L, Dasgupta K, Guru V, Humphries
KH, McGrath J, Norris C, Rabi D, Tremblay J, Alamian A, Barnett T,
et al: A comprehensive view of sex-specific issues related to
cardiovascular disease. CMAJ. 176:S1–S44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trecarichi EM, Cauda R and Tumbarello M:
Detecting risk and predicting patient mortality in patients with
extended-spectrum β-lactamase-producing Enterobacteriaceae
bloodstream infections. Future Microbiol. 7:1173–1189. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lucena A, Dalla Costa LM, Nogueira KS,
Matos AP, Gales AC, Paganini MC, Castro ME and Raboni SM:
Nosocomial infections with metallo-beta-lactamase-producing
Pseudomonas aeruginosa: Molecular epidemiology, risk factors,
clinical features and outcomes. J Hosp Infect. 87:234–240. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin MY, Lyles-Banks RD, Lolans K, Hines
DW, Spear JB, Petrak R, Trick WE, Weinstein RA and Hayden MK:
Centers for Disease Control and Prevention Epicenters Program: The
importance of long-term acute care hospitals in the regional
epidemiology of Klebsiella pneumoniae carbapenemase-producing
Enterobacteriaceae. Clin Infect Dis. 57:1246–1252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Papadimitriou-Olivgeris M, Marangos M,
Fligou F, Christofidou M, Bartzavali C, Anastassiou ED and Filos
KS: Risk factors for KPC-producing Klebsiella pneumoniae enteric
colonization upon ICU admission. J Antimicrob Chemother.
67:2976–2981. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Papadimitriou-Olivgeris M, Christofidou M,
Fligou F, Bartzavali C, Vrettos T, Filos KS, Marangos M and
Anastassiou ED: The role of colonization pressure in the
dissemination of colistin or tigecycline resistant KPC-producing
Klebsiella pneumoniae in critically ill patients. Infection.
42:883–890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonten MJ: Colonization pressure: A
critical parameter in the epidemiology of antibiotic-resistant
bacteria. Crit Care. 16:1422012.PubMed/NCBI
|