Introduction
Reduction in low-density lipoprotein cholesterol
(LDL-C) levels has been included in practice guidelines as a
fundamental method of reducing cardiovascular events and mortality.
Based on previous studies, statin therapy has been considered as a
first-line treatment strategy for targeting atherosclerotic
cardiovascular disease (1–4). While receiving moderate- or
high-intensity statin therapy, numerous patients were unable to
achieve LDL-C concentrations <70 mg/dl. In addition, certain
patients terminated statin therapy due to their inability to
tolerate the effective doses and experiencing adverse events.
Therefore, non-statin therapy for LDL-C reduction has been
considered, and novel effective medication and treatment strategies
for reducing LDL-C levels have become a focus of research.
During the past 3 years, monoclonal antibodies,
which inhibit proprotein convertase subtilism/kexin type 9 (PCSK9),
have emerged as a novel class of therapeutic agent that target
LDL-C levels (5). Evolocumab, a fully
human monoclonal antibody, effectively reduced LDL-C levels and has
been investigated in phase 3 trials (6–10). In these
trials, the patients were randomized to groups of placebo,
ezetimibe, 140 mg Q2W or 420 mg Q4W evolocumab. When compared with
the control group, the two different doses of evolocumab
significantly reduced the LDL-C levels. However, to the best of our
knowledge, there are no studies that demonstrate different outcomes
between the two different doses of evolocumab. In the current
adjusted indirect meta-analysis, the efficacy and safety of two
different doses of evolocumab were evaluated.
Materials and methods
Literature search strategy
PubMed (https://www.ncbi.nlm.nih.gov/pubmed) and Embase
(https://www.embase.com) were searched from
January 2000 to April 2015, utilizing the following search terms
without language restrictions: (Randomized trial OR clinical trial)
AND blind OR random AND control (placebos OR ezetimibe) NOT
(comment OR editorial OR meta-analysis OR letter) AND (proprotein
convertase subtilisin/kexin type 9 OR PCSK9) inhibitor (evolocumab
OR AMG145) AND (140 mg Q2W OR 420 mg Q4W) AND efficacy (LDL-C level
OR LDL-C concentration) OR safety OR tolerability. Two reviewers
(C.C. and S.S.) evaluated the identified titles, and the
manuscripts were retrieved when the reviewers deemed them to be
potentially relevant. The results were evaluated to determine
whether data on PCSK9 inhibitors had been reported.
Study selection
The review included high quality studies fulfilling
the following inclusion criteria: i) Randomized, controlled phase 3
trial comparing evolocumab, placebo or ezetimibe, with a follow-up
of 12 months; ii) the report supplied data on the rate of patients
who achieved LDL-C concentrations <70 mg/dl or data on the
percentage of adverse events; iii) analysis was performed on
evolocumab 140 mg Q2W and 420 mg Q4W; iv) when different studies
reported on the same population, the study that included a larger
sample size and performed evaluation using more comprehensive
methods was included.
Data extraction
The following data elements were extracted from each
report according to a fixed protocol: Author, publication year,
study design, characteristics of trial participants, median
follow-up, mean age, and ratio of males, females, diabetes cases,
smokers and hypertension cases, as well as data regarding efficacy
and safety separated by the different doses of evolocumab. For
studies with more than one control group, the most appropriate
control group was used. Two authors (C.C. and S.S.) independently
conducted the data extraction, and any disagreement was resolved by
discussion.
End-points and definitions
The efficacy end-point was the percentage of
patients who achieved LDL-C concentrations <70 mg/dl (1.8
mmol/l). The primary safety end-point was the rate of any adverse
events. The secondary safety end-point included back pain,
headache, nasopharyngitis, muscle-associated events and potential
injection-site reactions. All missing data from studies were
obtained from supplements.
Statistical analysis
Random-effect odds ratios (ORs) and 95% confidence
intervals (CIs) were calculated using Review Manager 5.3
(Copenhagen: The Nordic Cochrane Centre, The Cochrane
Collaboration, Denmark), and the outcomes that demonstrated a
smaller heterogeneity (I2<50%, χ2
test; two-tailed P<0.1) were confirmed by a fixed-effects model
to avoid small studies being overly weighted. Two-tailed P<0.05
was considered to indicate a statistically significant difference
(11,12). Secondly an Indirect Meta-analysis Tool
(Metcardio; Turin, Italy) was used for the adjusted indirect
comparison according to Song et al (13). Pooled OR (comparing evolocumab 140 mg
Q2W or evolocumab 420 mg Q4W vs. placebo) and interaction OR for
evolocumab 140 mg Q2W or evolocumab 420 mg Q4W was calculated. In
addition, pertinent 95% CI and Z scores for two-tailed hypothesis
testing were calculated (P<0.05 was considered to indicate a
statistically significant difference). The interaction
ORevolocumab 140 mg Q2W vs. evolocumab 420 mg Q4W was
calculated as follows: ln (ORevolocumab 140 mg Q2W vs.
evolocumab 420 mg Q4W)=ln (ORevolocumab 140 mg Q2W vs.
placebo) - ln (ORevolocumab 420 mg Q4W vs.
placebo), and var [ln (ORevolocumab 140 mg Q2W vs.
evolocumab 420 mg Q4W)] = var [ln (ORevolocumab 140 mg
Q2W vs. placebo)] + var [ln (ORevolocumab 420 mg Q4W vs.
placebo)], where ln is the natural logarithm, and var is the
variance.
For the direct meta-analysis, a funnel plot was
generated for assessment of publication bias; in addition,
sensitivity analysis was conducted by removing the study one by one
when a significant heterogeneity was observed.
Results
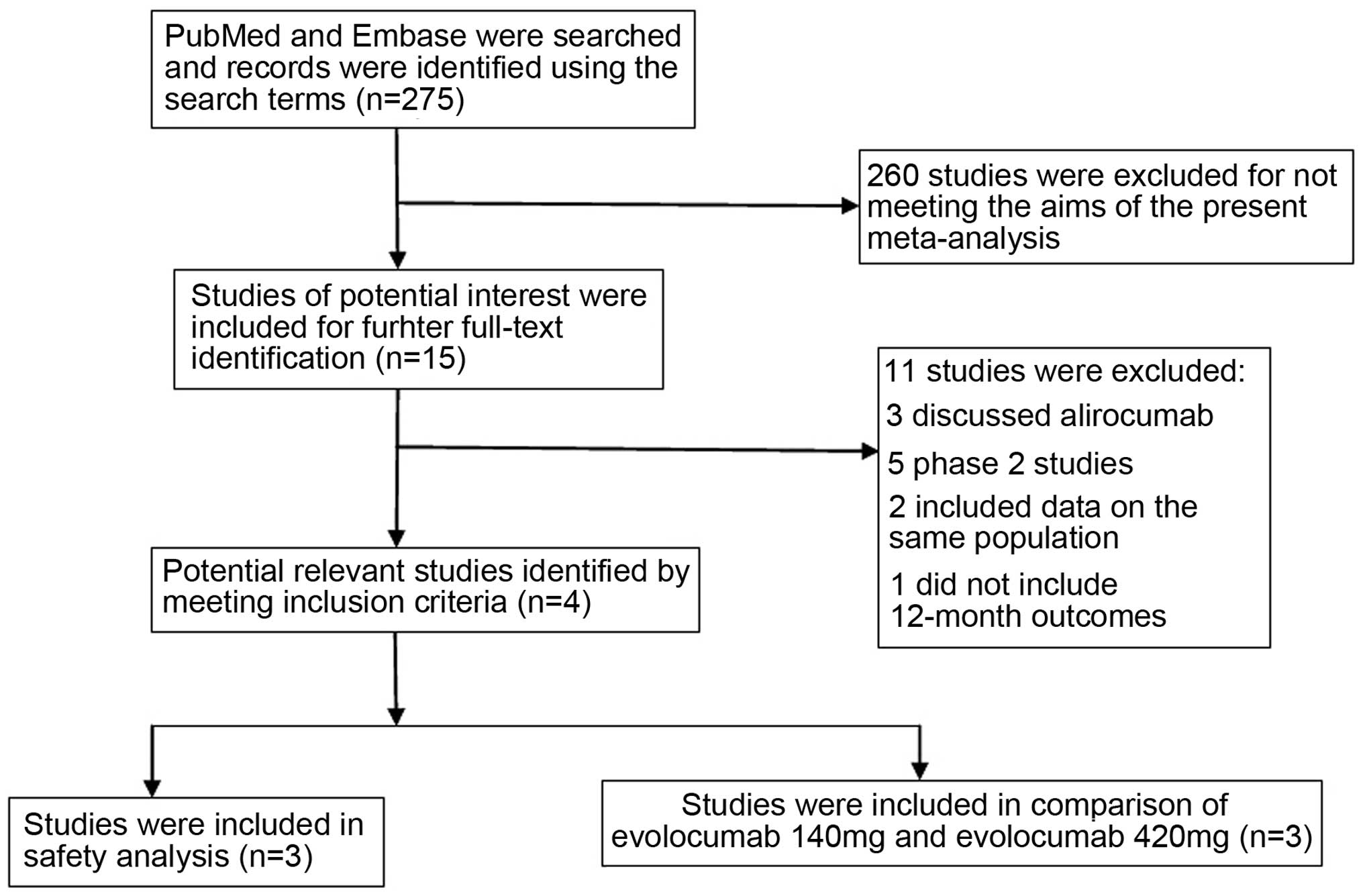
Study selection and
characteristics
The initial search identified 275 reports from
PubMed and Embase. After screening the titles and abstracts, 15
articles (6–10,14–23) were selected and underwent further
full-text identification. Subsequently five studies (14,20–23), which were phase 2 studies, were
excluded; three studies (15–17) were regarding alirocumab, two (18,19) included
data on the same population, and one study (6) did not include 12-month outcomes (Fig. 1). Four publications (7–10) were
included in the current meta-analysis. Three studies (7,8,10) were included in the indirect comparison
of evolocumab 140 mg Q2W and evolocumab 420 mg Q4W without the Goal
Achievement after Utilizing an Anti-PCSK9 Antibody in Statin
Intolerant Subjects (GAUSS-2 trial) (9), which did not include a placebo group. In
the safety analysis, the RUTHERFORD-2 Investigators (10) were excluded, as it does not contain
precise data on placebo and ezetimibe groups. The details are
presented in Tables I and II.
 | Table I.Characteristics of included
studies. |
Table I.
Characteristics of included
studies.
|
| Study |
|---|
|
|
|
|---|
| Characteristic | LAPLACE-2 | MENDEL-2 | GAUSS-2 | RUTHERFORD-2 |
|---|
| First autor
(Ref.) | Robinson et al
(7) | Koren et al
(8) | Stroes et al
(9) | Raal et al
(10) |
| Year | 2014 | 2014 | 2014 | 2015 |
| Patients (n) | 1,896 | 614 | 307 | 329 |
| Median follow-up
(weeks) | 12 | 12 | 12 | 12 |
| Age (years) | 59.8 | 53.2 | 61.7 | 51.2 |
| Males (%) | 54.2 | 31.1 | 54.1 | 42.2 |
| Diabetes (%) | 15.5 |
0.2 | 20.2 | NA |
| Smoker (%) | NA | 11.7 |
7.8 | NA |
| Hypertension
(%) | NA | 28.7 | 58.9 | NA |
 | Table II.Data extracted from the included
studies. |
Table II.
Data extracted from the included
studies.
|
| LAPLACE-2 trial
(Robinson et al 2014) | MENDEL-2 trial
(Koren et al 2014) | GAUSS-2 trial
(Stroes et al 2014) | RUTHERFORD-2 trial
(Raal et al 2015) |
|---|
|
|
|
|
|
|
|---|
|
| Placebo | Evolocumab | Placebo | Evolocumab |
|
|
| Placebo | Evolocumab |
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Variable | Q2W (n=281) | Q4W (n=277) | Q2W (n=555) | Q4W (n=562) | Q2W (n=76) | Q4W (n=78) | Q2W (n=153) | Q4W (n=153) | Ezetimibe
(n=154) | Ezetimibe
(n=102) | Evolocumab
(n=205) | Q2W (n=54) | Q4W (n=55) | Q2W (n=104) | Q4W (n=103) |
|---|
| Adverse events | 25 | 13 | 44 | 34 | 34 | 34 | 73 | 61 | 70 | 74 | 135 | 23 | 30 | 61 | 63 |
| Headache | 10 | 5 | 10 | 10 | 3 | 1 | 5 | 5 | 5 | 9 | 16 | 1 | 3 | 4 | 5 |
|
Nasopharyngitis | NA | NA | NA | NA | 1 | 2 | 3 | 3 | 3 | 3 | 7 | 2 | 3 | 8 | 11 |
| Muscle-associated
events | NA | NA | NA | NA | 3 | 3 | 6 | 2 | 5 | 23 | 25 | 0 | 1 | 8 | 2 |
| Potential
injection-site reactions | NA | NA | NA | NA | 2 | 6 | 10 | 6 | 7 | 8 | 6 | 2 | 2 | 5 | 8 |
| Efficacy | 35 | 30 | 499 | 493 | 1 | 0 | 97 | 89 | NA | NA | NA | 1 | 1 | 71 | 65 |
| Back pain | NA | NA | NA | NA | 6 | 8 | 14 | 6 | NA | NA | NA | 0 | 1 | 2 | 6 |
Statistical analysis
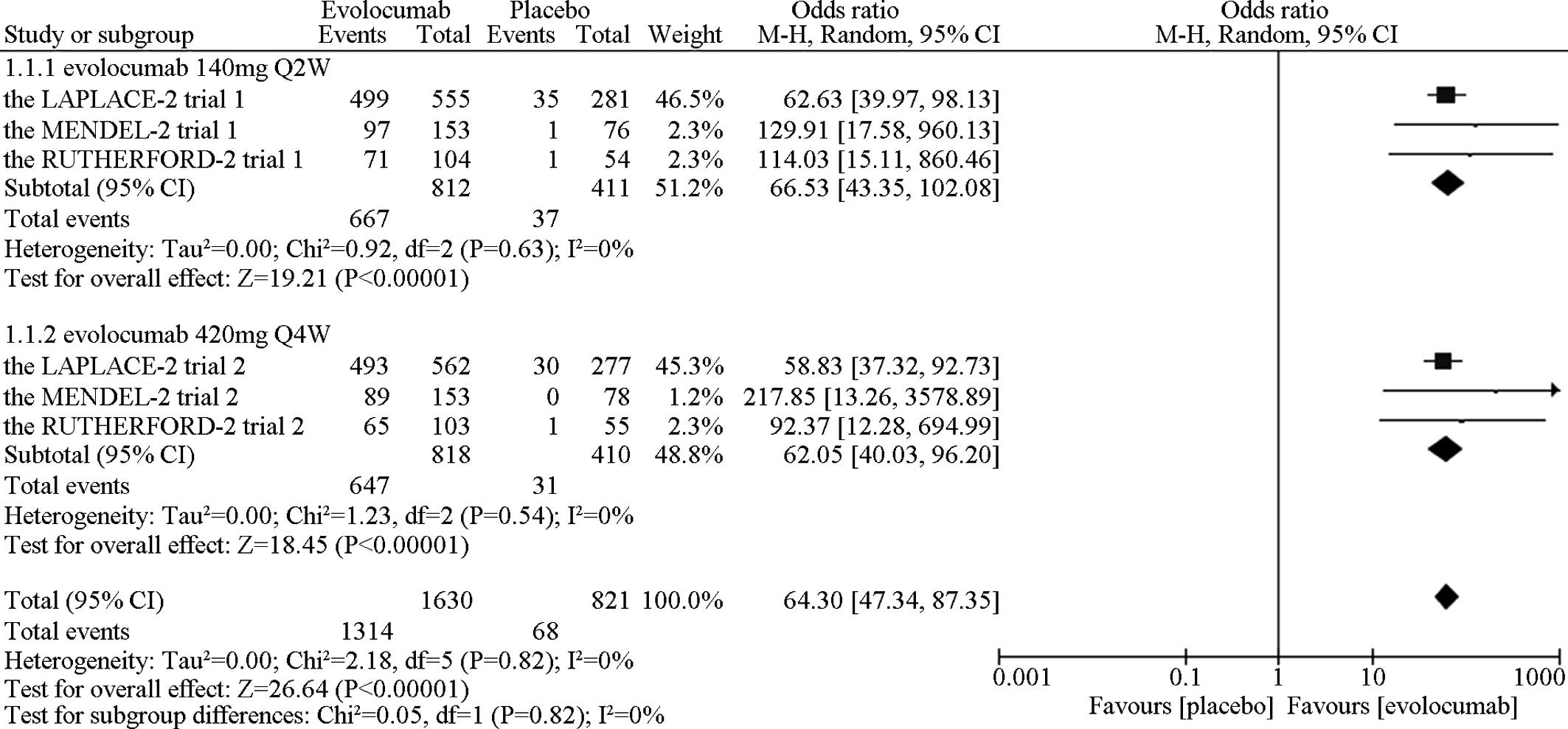
In the direct comparison, the meta-analytic pooling
implied that evolocumab markedly reduced the LDL-C level to <70
mg/dl (1.8 mmol/l) when compared with the placebo [OR=70.86, 95%
CI, 51.28–97.91; P (Z)<0.01, P (Q)=0.82,
I2=0%] (Fig. 2),
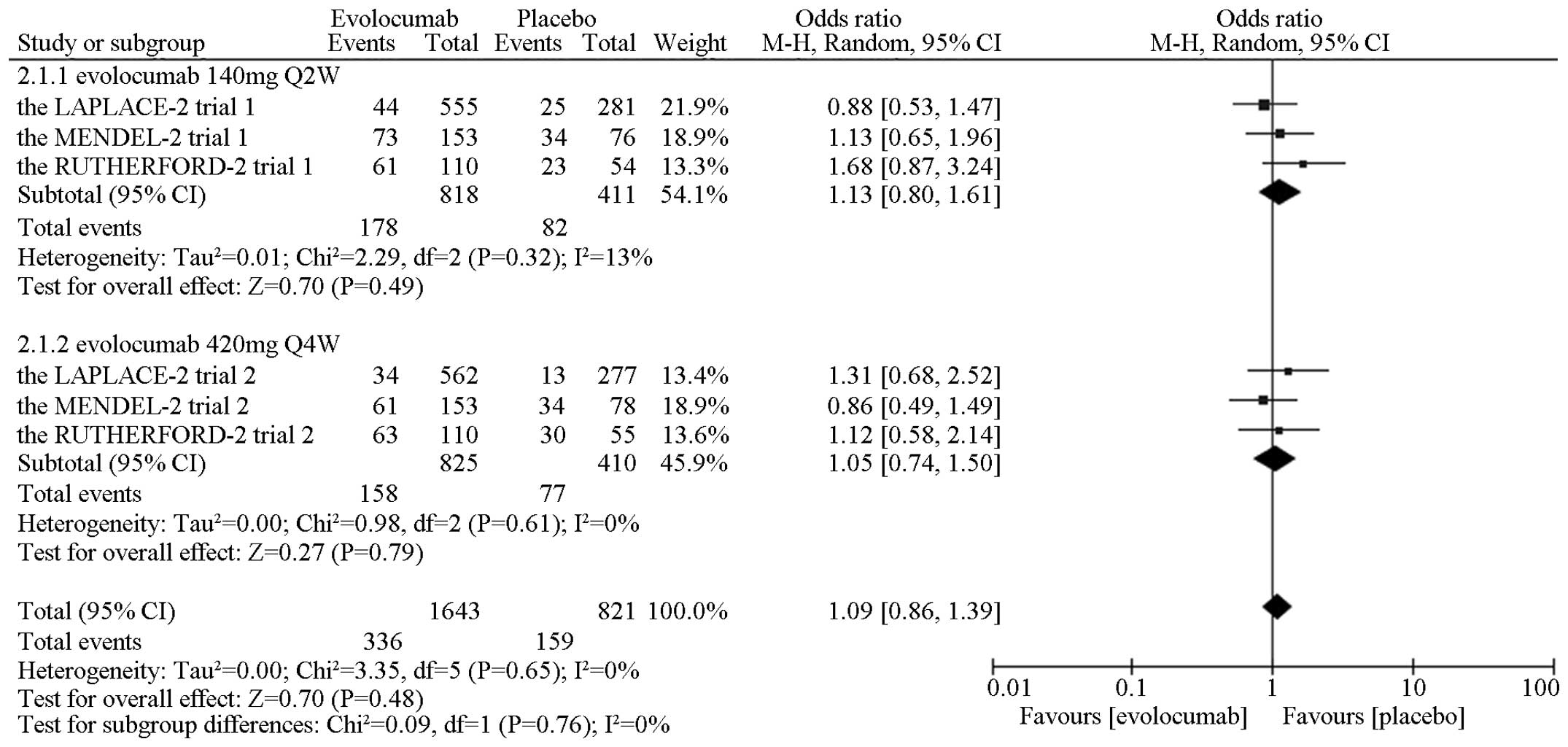
without significant differences noted in adverse events [OR=1.09,
95% CI, 0.86–1.39; P (Z)=0.47, P (Q)=0.65,
I2=0%] (Fig. 3),
back pain [OR=0.92, 95% CI, 0.49–1.72; P (Z)=0.79, P
(Q)=0.18, I2=38%], headache [OR=0.84, 95% CI,
0.50–1.42; P (Z)=0.52, P (Q)=0.69,
I2=0%], nasopharyngitis [OR=1.60, 95% CI,
0.71–3.60; P (Z)=0.26, P (Q)=0.85,
I2=0%], muscle-associated event [OR=1.24, 95% CI,
0.52–2.93; P (Z)=0.63, P (Q)=0.26,
I2=25%] and potential injection-site reactions
[OR=1.22, 95% CI, 0.61–2.43; P (Z)=0.57, P (Q)=0.30,
I2=19%]. All the data on comparison of evolocumab
140 mg Q2W vs. placebo or evolocumab 420 mg Q4W vs. placebo are
presented in Table III. A
head-to-head comparison of evolocumab 140 mg Q2W vs. evolocumab 420
mg Q4W demonstrated no significant differences in the efficacy
[OR=1.04, 95% CI, 0.55–1.99; P (Z)=0.90] and the risk of
back pain [OR=1.48, 95% CI, 0.15–14.53; P (Z)=0.73],
headache [OR=0.68, 95% CI, 0.23–2.06; P (Z)=0.50],
nasopharyngitis [OR=1.28, 95% CI, 0.24–6.82; P (Z)=0.77],
muscle-associated events [OR=4.43, 95% CI, 0.32–59.31; P
(Z)=0.27], potential injection-site reactions [OR=2.09, 95% CI,
0.34–12.71; P (Z)=0.42] and any adverse events [OR=1.08, 95%
CI, 0.66–1.74; P (Z)=0.76] (Table
IV).
 | Table III.All pooled ORs comparing evolocumab
140 mg Q2W or evolocumab 420 mg Q4W vs. placebo [the fixed-model
was used for smaller heterogeneity (I2<50%,
χ2 test; two-tailed P<0.1), otherwise the random
model was used]. |
Table III.
All pooled ORs comparing evolocumab
140 mg Q2W or evolocumab 420 mg Q4W vs. placebo [the fixed-model
was used for smaller heterogeneity (I2<50%,
χ2 test; two-tailed P<0.1), otherwise the random
model was used].
| Variable | OR | 95% CI | χ2 | Freedom | OR | 95% CI | χ2 | Freedom |
|---|
| Efficacy | 72.35 | (46.09–113.97) | 0.92 | 2 | 69.39 | (43.65–110.31) | 1.23 | 2 |
| Adverse events |
1.13 | (0.81–1.56) | 2.29 | 2 |
1.05 | (0.74–1.50) | 0.98 | 2 |
| Back pain |
1.29 | (0.51–3.23) | 0.21 | 1 |
0.87 | (0.11–7.15) | 3.21 | 1 |
| Headache |
0.76 | (0.33–1.36) | 1.45 | 2 |
1.11 | (0.50–2.46) | 0.80 | 2 |
|
Nasopharyngitis |
1.85 | (0.51–6.80) | 0.05 | 1 |
1.44 | (0.50–4.10) | 0.66 | 1 |
| Muscle-associated
events |
2.17 | (0.24–19.59) | 2.07 | 1 |
0.50 | (0.12–2.02) | 0.51 | 1 |
| Potential
injection-site reactions |
1.90 | (0.62–5.85) | 0.41 | 1 |
0.91 | (0.22–3.73) | 2.10 | 1 |
 | Table IV.Adjusted indirect comparison of
evolocumab 140 mg Q2W vs. evolocumab 420 mg Q4W. |
Table IV.
Adjusted indirect comparison of
evolocumab 140 mg Q2W vs. evolocumab 420 mg Q4W.
| Variable | Odds ratio | 95% confidence
interval | Z score | Two-tailed
P-value |
|---|
| Efficacy | 1.04 | (0.55–1.99) | 0.13 | 0.90 |
| Adverse events | 1.08 | (0.66–1.74) | 0.30 | 0.76 |
| Back pain | 1.48 |
(0.15–14.53) | 0.34 | 0.73 |
| Headache | 0.68 | (0.23–2.06) | 0.67 | 0.50 |
|
Nasopharyngitis | 1.28 | (0.24–6.82) | 0.29 | 0.77 |
| Muscle-associated
events | 4.43 |
(0.32–59.31) | 1.10 | 0.27 |
| Potential
injection-site reactions | 2.09 |
(0.34–12.71) | 0.80 | 0.42 |
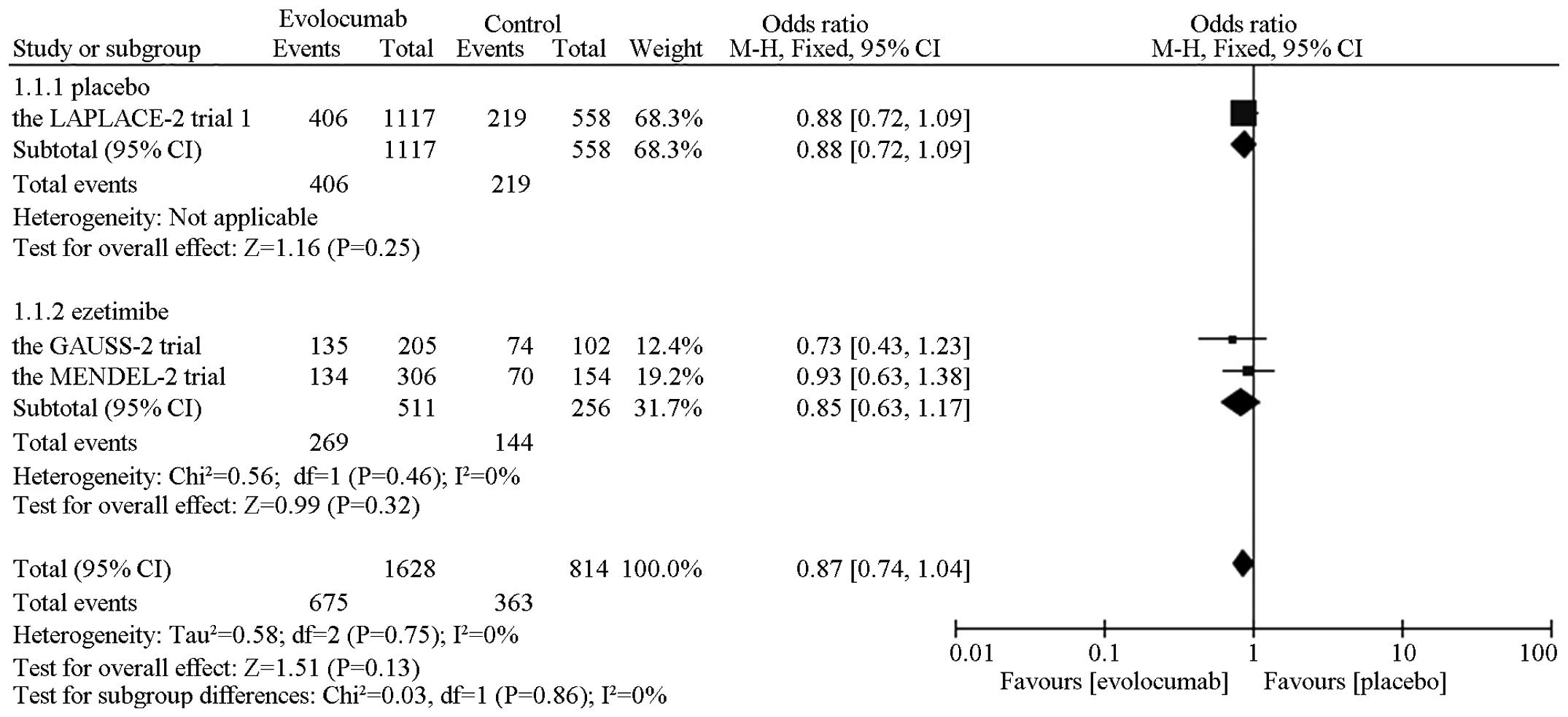
In the overall safety analysis, a significant
reduction was observed in the muscle-associated events compared
with ezetimibe [OR=0.54, 95% CI, 0.31–0.93; P (Z)=0.03; P
(Q)=0.43, I2=0%]. No obvious difference in
the risk of headache [OR=0.77, 95% CI, 0.48–1.24; P
(Z)=0.27, P (Q)=0.72, I2=0%],
nasopharyngitis [OR=1.09, 95% CI, 0.41–2.89; P (Z)=0.87,
P (Q)=0.88, I2=0%], potential
injection-site reactions [OR=0.80, 95% CI, 0.47–1.36; P
(Z)=0.41, P (Q)=0.23, I2=32%] and any
adverse events [OR=0.87, 95% CI, 0.74–1.04; P (Z)=0.13, P
(Q)=0.75, I2=0%] (Fig. 4). The subgroup analysis showed the same
results, in the placebo and ezetimibe groups. The outcomes are
presented in Table V.
 | Table V.Evolocumab safety outcomes. |
Table V.
Evolocumab safety outcomes.
|
| Evolocumab vs.
placebo | Evolocumab vs.
ezetimibe | Evolocumab vs.
control |
|---|
|
|
|
|
|
|---|
|
| OR | 95% CI | P (Q) | P (Z) | OR | 95% CI | P (Q) | P (Z) | OR | 95% CI | P (Q) | P (Z) |
|---|
| Adverse events | 0.88 | (0.72–1.09) | – | 0.25 | 0.85 | (0.63–1.17) | 0.46 | 0.32 | 0.87 | (0.74–1.04) | 0.75 | 0.13 |
| Headache | 0.63 | (0.32–1.24) | – | 0.18 | 0.92 | (0.47–1.81) | 0.84 | 0.82 | 0.77 | (0.48–1.24) | 0.72 | 0.27 |
|
Nasopharyngitis | – | – | – | – | 1.09 | (0.41–2.89) | 0.88 | 0.87 | 1.09 | (0.41–2.89) | 0.88 | 0.87 |
| Muscle-associated
events | – | – | – | – | 0.54 | (0.31–0.93) | 0.43 | 0.03 | 0.54 | (0.31–0.93) | 0.43 | 0.03 |
| Potential
injection-site reactions | 0.94 | (0.39–2.22) | – | 0.88 | 0.67 | (0.21–2.12) | 0.10 | 0.49 | 0.80 | (0.47–1.36) | 0.23 | 0.41 |
Publication bias and sensitivity
analysis
The funnel plots of these direct comparison studies
indicated no evidence of publication bias, therefore are not
included in the present study. Furthermore, the sensitivity
analysis in the direct comparison was conducted by removing the
largest study when a significant result was observed, the results
remained unchanged (data not shown).
Discussion
The results of the current meta-analysis indicated
that evolocumab was associated with a reduced risk of
muscle-associated events when compared with ezetimibe, and
different doses of evolocumab resulted in the same outcomes with
regard to efficacy and safety. In the direct meta-analysis, the two
doses of evolocumab appeared to exert a significant LDL-C lowering
effect. As a fully human monoclonal antibody, evolocumab offers
promising therapeutic applications in the control of
PCSK9-regulated pathologies; inhibiting direct binding of PCSK9 and
LDL receptors, reducing the degradation of the receptor, increasing
LDL receptor activity on the hepatocyte surface and, ultimately,
improving the uptake of plasma lipoprotein (24,25). Within
the above-mentioned mechanism, evolocumab has demonstrated
significant reduction in LDL-C levels (6–10).
Furthermore, in the current safety analysis, a significant
difference was observed in the muscle-associated events group when
the evolocumab group was compared with the ezetimibe group, which
suggested that evolocumab was associated with fewer
muscle-associated events than ezetimibe. Therefore, evolocumab was
demonstrated to be safe and well tolerated.
In previous phase 1 and phase 2 trials (14,26), no
significant differences were identified in efficacy between the
intravenous and subcutaneous groups, and evolocumab was
characterized by a dose-dependent LDL-C reduction in terms of
effect and duration. The higher the dosage that patients take, the
longer the duration of the effect. Therefore, two doses are
commonly administered; 140 mg evolocumab, subcutaneously, every 2
weeks and 420 mg evolocumab, subcutaneously, every 4 weeks. The
head-to-head comparison of evolocumab 140 mg Q2W vs. evolocumab 420
mg Q4W indicated no significant differences in efficacy and any
adverse events, including back pain, headache, nasopharyngitis,
muscle-associated events and potential injection-site reactions.
This high-dosage, long-interval administration of evolocumab 420 mg
Q4W is desirable. The results imply that clinicians should
preferentially administer evolocumab 420 mg Q4W in order to improve
patient compliance and convenience. As there was no direct evidence
of a comparison between evolocumab 140 mg Q2W and evolocumab 420 mg
Q4W, an adjusted indirect comparison was made in the present study
to evaluate the efficacy and safety of different doses of
evolocumab. Random errors are sources of discrepancies between the
direct and the adjusted indirect comparison. As the adjusted
indirect comparison widens the confidence interval, infrequent
significant differences may be caused. Therefore, there remains a
lack of direct evidence as to whether evolocumab 140 mg Q2W and
evolocumab 420 mg Q4W possess different efficacy and safety.
However, there are various key issues that require
attention with regard to investigating PCSK9 inhibitors. It remains
to be elucidated as to whether a reduction in LDL-C levels using a
PCSK9 inhibitor may result in a reduction in cardiovascular events.
Therefore, long-term, extensive, randomized clinical trials with
definite cardiovascular endpoints are required. In addition, a
long-term follow-up of security and tolerance is required to
establish whether PCSK9 inhibitors elicit an immune response, which
may lead to a loss of responsiveness to treatment. Furthermore,
although injectable treatment reduces the dosing frequency, patient
acceptance levels should be considered and relevant guidance is
required. Finally, monoclonal antibodies are expensive, which
presents a limitation for their clinical application.
In conclusion, according to the current
meta-analysis outcomes, evolocumab presents as an efficacious, safe
and promising therapeutic strategy for hypercholesterolemia and the
associated cardiovascular diseases. The head-to-head comparison of
evolocumab 140 mg Q2W vs. evolocumab 420 mg Q4W indicated no
significant differences in efficacy and adverse events.
Furthermore, in the safety analysis, evolocumab had a reduced risk
of muscle-associated events when compared with ezetimibe and the
placebo groups.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81170174),
the Natural Scientific Fund of Jiangsu province (grant no.
BK2011304).
References
|
1
|
Stone NJ, Robinson JG, Lichtenstein AH,
Merz CN Bairey, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D,
Lloyd-Jones DM, et al: American College of Cardiology/American
Heart Association Task Force on Practice Guidelines: 2013 ACC/AHA
guideline on the treatment of blood cholesterol to reduce
atherosclerotic cardiovascular risk in adults: A report of the
American College of Cardiology/American Heart Association Task
Force on Practice Guidelines. Circulation. 129(Suppl 2): S1–S45.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jacobson TA, Ito MK, Maki KC, Orringer CE,
Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, et al:
National Lipid Association recommendations for patient-centered
management of dyslipidemia: Part 1 - executive summary. J Clin
Lipidol. 8:473–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson TJ, Grégoire J, Hegele RA,
Couture P, Mancini GB, McPherson R, Francis GA, Poirier P, Lau DC,
Grover S, et al: 2012 update of the Canadian Cardiovascular Society
guidelines for the diagnosis and treatment of dyslipidemia for the
prevention of cardiovascular disease in the adult. Can J Cardiol.
29:151–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grundy SM: Expert Dyslipidemia Panel: An
International Atherosclerosis Society Position Paper: Global
recommendations for the management of dyslipidemia. J Clin Lipidol.
7:561–565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noto D, Cefalù AB and Averna MR: Beyond
statins: New lipid lowering strategies to reduce cardiovascular
risk. Curr Atheroscler Rep. 16:4142014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blom DJ, Hala T, Bolognese M, Lillestol
MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM,
et al: DESCARTES Investigators: A 52-week placebo-controlled trial
of evolocumab in hyperlipidemia. N Engl J Med. 370:1809–1819. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Robinson JG, Nedergaard BS, Rogers WJ,
Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P,
Scott R, et al: LAPLACE-2 Investigators: Effect of evolocumab or
ezetimibe added to moderate- or high-intensity statin therapy on
LDL-C lowering in patients with hypercholesterolemia: The LAPLACE-2
randomized clinical trial. JAMA. 311:1870–1882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koren MJ, Lundqvist P, Bolognese M, Neutel
JM, Monsalvo ML, Yang J, Kim JB, Scott R, Wasserman SM and Bays H:
MENDEL-2 Investigators: Anti-PCSK9 monotherapy for
hypercholesterolemia: The MENDEL-2 randomized, controlled phase III
clinical trial of evolocumab. J Am Coll Cardiol. 63:2531–2540.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stroes E, Colquhoun D, Sullivan D, Civeira
F, Rosenson RS, Watts GF, Bruckert E, Cho L, Dent R, Knusel B, et
al: GAUSS-2 Investigators: Anti-PCSK9 antibody effectively lowers
cholesterol in patients with statin intolerance: The GAUSS-2
randomized, placebo-controlled phase 3 clinical trial of
evolocumab. J Am Coll Cardiol. 63:2541–2548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raal FJ, Stein EA, Dufour R, Turner T,
Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D,
et al: RUTHERFORD-2 Investigators: PCSK9 inhibition with evolocumab
(AMG 145) in heterozygous familial hypercholesterolaemia
(RUTHERFORD-2): A randomised, double-blind, placebo-controlled
trial. Lancet. 385:331–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of observational studies in epidemiology: A
proposal for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song F, Altman DG, Glenny AM and Deeks JJ:
Validity of indirect comparison for estimating efficacy of
competing interventions: Empirical evidence from published
meta-analyses. BMJ. 326:4722003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giugliano RP, Desai NR, Kohli P, Rogers
WJ, Somaratne R, Huang F, Liu T, Mohanavelu S, Hoffman EB, McDonald
ST, et al: Efficacy, safety, and tolerability of a monoclonal
antibody to proprotein convertase subtilisin/kexin type 9 in
combination with a statin in patients with hypercholesterolaemia
(LAPLACE-TIMI 57): A randomised, placebo-controlled, dose-ranging,
phase 2 study. Lancet. 380:2007–2017. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roth EM, Taskinen MR, Ginsberg HN,
Kastelein JJ, Colhoun HM, Robinson JG, Merlet L, Pordy R and
Baccara-Dinet MT: Monotherapy with the PCSK9 inhibitor alirocumab
versus ezetimibe in patients with hypercholesterolemia: Results of
a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol.
176:55–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robinson JG, Farnier M, Krempf M, Bergeron
J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M,
et al: ODYSSEY LONG TERM Investigators: Efficacy and safety of
alirocumab in reducing lipids and cardiovascular events. N Engl J
Med. 372:1489–1499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moriarty PM, Jacobson TA, Bruckert E,
Thompson PD, Guyton JR, Baccara-Dinet MT and Gipe D: Efficacy and
safety of alirocumab, a monoclonal antibody to PCSK9, in
statin-intolerant patients: Design and rationale of ODYSSEY
ALTERNATIVE, a randomized phase 3 trial. J Clin Lipidol. 8:554–561.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raal FJ, Giugliano RP, Sabatine MS, Koren
MJ, Langslet G, Bays H, Blom D, Eriksson M, Dent R, Wasserman SM,
et al: Reduction in lipoprotein(a) with PCSK9 monoclonal antibody
evolocumab (AMG 145): A pooled analysis of more than 1,300 patients
in 4 phase II trials. J Am Coll Cardiol. 63:1278–1288. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
1Sabatine MS, Giugliano RP, Wiviott SD,
Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J,
Wasserman SM, et al: Open-Label Study of Long-Term Evaluation
against LDL Cholesterol (OSLER) Investigators: Efficacy and safety
of evolocumab in reducing lipids and cardiovascular events. N Engl
J Med. 372:1500–1509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raal F, Scott R, Somaratne R, Bridges I,
Li G, Wasserman SM and Stein EA: Low-density lipoprotein
cholesterol-lowering effects of AMG 145, a monoclonal antibody to
proprotein convertase subtilisin/kexin type 9 serine protease in
patients with heterozygous familial hypercholesterolemia: The
Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial
Hypercholesterolemia Disorder (RUTHERFORD) randomized trial.
Circulation. 126:2408–2417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koren MJ, Scott R, Kim JB, Knusel B, Liu
T, Lei L, Bolognese M and Wasserman SM: Efficacy, safety, and
tolerability of a monoclonal antibody to proprotein convertase
subtilisin/kexin type 9 as monotherapy in patients with
hypercholesterolaemia (MENDEL): A randomised, double-blind,
placebo-controlled, phase 2 study. Lancet. 380:1995–2006. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sullivan D, Olsson AG, Scott R, Kim JB,
Xue A, Gebski V, Wasserman SM and Stein EA: Effect of a monoclonal
antibody to PCSK9 on low-density lipoprotein cholesterol levels in
statin-intolerant patients: The GAUSS randomized trial. JAMA.
308:2497–2506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirayama A, Honarpour N, Yoshida M,
Yamashita S, Huang F, Wasserman SM and Teramoto T: Effects of
evolocumab (AMG 145), a monoclonal antibody to PCSK9, in
hypercholesterolemic, statin-treated Japanese patients at high
cardiovascular risk - primary results from the phase 2 YUKAWA
study. Circ J. 78:1073–1082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Urban D, Pöss J, Böhm M and Laufs U:
Targeting the proprotein convertase subtilisin/kexin type 9 for the
treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol.
62:1401–1408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Banach M, Rizzo M, Obradovic M, Montalto
G, Rysz J, Mikhailidis DP and Isenovic ER: PCSK9 inhibition - a
novel mechanism to treat lipid disorders? Curr Pharm Des.
19:3869–3877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dias CS, Shaywitz AJ, Wasserman SM, Smith
BP, Gao B, Stolman DS, Crispino CP, Smirnakis KV, Emery MG, Colbert
A, et al: Effects of AMG 145 on low-density lipoprotein cholesterol
levels: Results from 2 randomized, double-blind,
placebo-controlled, ascending-dose phase 1 studies in healthy
volunteers and hypercholesterolemic subjects on statins. J Am Coll
Cardiol. 60:1888–1898. 2012. View Article : Google Scholar : PubMed/NCBI
|