Introduction
The great family of neurotrophins (NTs) is composed
of structurally conserved growth factors named nerve growth factor
(NGF), brain-derived neurotrophic factor (BDNF), NT and
neurotrophic factor 4 (NT4/5). Their actions are mediated by
receptors, the tropomyosin tyrosine kinase receptors (Trk) and the
common NT receptor p75NTR (1). NTs
have been extensively used as biomarkers, in particular BDNF in
nervous disorders.
In fact, BDNF levels are reduced in schizophrenia
patients (2) and may, in combination
with NGF, be used as a schizophrenia marker (3). During the treatment of catatonia in
schizophrenia patients with lorazepam, BDNF levels are reduced
(4). However, the serum levels of BDNF
are not informative with regard to Huntington's disease (5). BDNF is implicated in modified phenotypes
during alcohol withdrawal syndrome and in neuroadaptive processes
of alcohol dependence (6). In
neurological diseases, BDNF is aberrantly expressed; for instance,
its expression is low in Alzheimer's disease and high in
Parkinson's disease (7).
Antidepressant treatment is able to increase BDNF in
patients with depression (8). In major
depression, although BDNF and TrkB are decreased, proBDNF, sortilin
and p75NTR are increased in the serum (9). Recently, it has been shown that BDNF is
associated with gender in major depressive disorder, in terms of
being higher expressed in women (10).
Initially implicated in nervous system development,
the role of NTs in non-neuronal cells, including cancer cells, is
currently being investigated (11). In
colorectal cancer (CRC), BDNF and TrkB are the major factors, as
reported by a previous study by our group (12). The study indicated that the BDNF/TrkB
complex is responsible for CRC survival under stress conditions
such as nutrient starvation. Numerous studies have assessed the
levels of TrkB and BDNF in CRC tissues (13,14),
revealing that this complex has roles in CRC. In fact, local
progression, nodal and distant metastasis, clinical stage and poor
prognosis are associated with TrkB. In addition, the TrkB/BDNF
pathway enhances several biological processes in CRC, including
proliferation, invasion, migration, epithelial-mesenchymal
transition as well as resistance to apoptosis and anoikis, as
outlined in a recent review by our group (15).
While numerous studies have assessed TrkB, little is
known regarding the possible application of serum BDNF as a
biomarker. To the best of our knowledge, only one study has
assessed BDNF in the serum of CRC patients, revealing that it was
decreased in patients compared with that in controls and was not
associated with the stage of CRC (16). Among other studies, BDNF expression
shows a large variation in different cancer types. For instance, in
hepatocellular carcinoma, BDNF was shown to be increased and
associated with poor survival (17).
Furthermore, low levels of BDNF were found to be associated with
cognitive impairment and short-term memory in patients receiving
chemotherapy for advanced metastatic cancer; however no influence
on depression was identified (18). It
has been observed that a decrease in BDNF may lead to worsening of
fatigue in patients with prostate cancer due to repeated
radiotherapy; furthermore, the conveyance of a cancer diagnosis can
induce depressive disorders in patients and fatigue during cancer
treatment may be associated with depression (19). However, no difference in BDNF was found
between lung cancer patients with depression and those without.
Therefore, major depression in lung cancer patients is not
associated with BDNF levels (20).
However, the studies collectively suggested that BDNF is frequently
linked with depressive disorders in cancer patients.
To further clarify the association of BDNF with
cancer, the present study determined the serum levels of BDNF in
CRC patients receiving antidepressant treatment. Concomitantly,
NT4/5, the second ligand with the ability to activate TrkB
(1), and which has not been studied in
CRC, was assessed. To the best of our knowledge, only one study has
assessed BDNF and NT4/5 expression in breast cancer, demonstrating
that their targeting inhibits tumor cell survival (21).
Materials and methods
Patient characteristics
A total of 83 patients admitted to Limoges
University Hospital between March, 2011 and March, 2012 who had
undergone curative resection of colorectal tumors were recruited
for the present study. The present study was performed according to
the Declaration of Helsinki and the following exclusion criteria
were applied (22): Juvenile patients,
pregnant or breast-feeding women, rectal or colonic lesions that
were not histologically proven, patients with impossible follow-up,
and insufficient or unusable tissue due to inadequate
preservation.
A total of 83 patients (37 women and 46 men) were
prospectively included, of which 8 had dysplastic lesions and 75
had CRC. Tumors were graded according to the pathological tumor,
nodes and metastasis international classification (23). A total of 37 patients had local disease
(stage I, T1/2-N0, n=16; stage II, T3/4, n=21), 23 had regional
lymph-node involvement (stage III, any T-N1/2) and 12 had advanced
disease (stage IV, any T, any N and presence of metastasis).
Clinical patient characteristics are summarized in Tables I and II.
 | Table I.Clinical characteristics of the
patients (n=83). |
Table I.
Clinical characteristics of the
patients (n=83).
| Clinical
characteristic | Patients, n (%) |
|---|
| Age (years) |
|
| Mean | 69±13 |
|
Range | 35–88 |
| Gender |
|
| Male | 46 (55) |
|
Female | 37 (45) |
| Performance
statusa |
|
| 0 | 46 (55.4) |
| 1 | 16 (19.3) |
| 2 | 4 (4.8) |
| 3 | 2 (2.4) |
| 4 | 5 (6.0) |
| Not
available | 10 (12.0) |
| Mental disorders |
|
| Anxiety
disorder | 7 (8.4) |
|
Depressive disorder | 8 (9.6) |
| Bipolar
disorder | 1 (1.2) |
|
Alzheimer's disease | 4 (4.8) |
|
Total | 20 |
| Psychotropic
medications |
|
|
Benzodiazepine | 13 (15.7) |
| Serotonin
uptake inhibitor | 5 (6.0) |
|
Anti-epileptic medication | 1 (1.2) |
|
Acetylcholinestase
inhibitor | 1 (1.2) |
| Anti-NMDA
receptor | 1 (1.2) |
|
Total | 21 |
 | Table II.Tumor characteristics of the patients
(n=83). |
Table II.
Tumor characteristics of the patients
(n=83).
| Tumor
characteristic | Patients, n (%) |
|---|
| CRC
stagea (n=75) |
|
| 0 | 4 (5.3) |
| 1 | 12 (16.0) |
| 2 | 21 (28.0) |
| 3 | 23 (30.7) |
| 4 | 12 (16.0) |
| Not
available | 3 (4.0) |
| Localized cancer | 75 (90.4) |
| Lower 2/3
rectum | 10 (12.0) |
| Upper 1/3
rectum and left colon | 40 (48.2) |
| Right and
transverse colon | 24 (28.9) |
| Not
available | 1 (1.2) |
| Adenomatous
polyps | 8 (9.6) |
| KRAS status |
|
|
Available | 20 |
|
Mutated | 8 (40.0) |
|
Wild-type | 12 (60.0) |
| CEA at diagnosis
(ng/ml) |
|
|
Available | 55 |
|
<5 | 39 (70.9) |
|
>5 | 16 (29.1) |
Blood collection was approved by the local Ethics
Committee (‘Comité de Protection des Personnes, Sud Ouest Outre
Mer’; CPP SOOM4; number DC-2008-604). Routine laboratory tests,
including complete blood cell count and carcinoembryonic antigen
(CEA) quantification in serum, were performed prior to and during
the follow-up period.
Follow-up
Clinical and paraclinical (biology and imaging)
parameters of all patients were collected. The median follow-up
time was 14,3 months (range, 0,2–51,7 months). The last follow-up
evaluation was performed on May, 30th 2013. Recurrence-free and
disease-specific survivals were analyzed and are presented in
Table III.
 | Table III.Management and follow-up of the
patients with colorectal cancer. |
Table III.
Management and follow-up of the
patients with colorectal cancer.
| Management and
follow-up | Patients, n
(%) |
|---|
| Perioperative
chemo- and radiochemotherapies |
|
|
Pre-operative therapies | 11 (14.6) |
|
Post-operative therapies | 24 (32.0) |
| Follow-up period
(months) |
|
|
Mean | 14.3 |
|
Range | 0.2–51.7 |
| Disease status |
|
|
Remission | 60 (80.0) |
| Alive
with disease | 11 (14.7) |
|
Disease-associated death | 12 (16.0) |
|
Post-operative death (within
30 days) | 2 (2.7) |
ELISA for serum BDNF and NT4
levels
Immediately prior to surgery, peripheral blood
samples were taken from all patients, filled into serum separator
tubes and centrifuged for 10 min at 1,650 × g. The serum samples
were collected and stored at −80°C.
BDNF and NT4/5 serum levels were detected by ELISA.
Assays exhibited no cross-reactivity with others members of the NGF
family. All samples were analyzed in duplicate experiments. The
procedures were performed according to the manufacturer's
instructions (cat. nos. CSB-E04501h and CSB-E04689h; Cusabio
Biotech Co., Ltd., Wuhan, China). The minimum detectable doses were
0.08 ng/ml for BDNF and 0.15 ng/ml for NT4/5.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Comparisons between groups with high and low expression
of the serum BDNF and NT4/5 rates were performed using the
Mann-Whitney U test. Comparisons of the serum BDNF and NT4/5 levels
between different CRC stages were performed using the
paired-samples t-test.
For statistical analyses, StatView 5.0 software
(Stat Crew Software, Inc., Cincinnati, OH, USA) was used.
Disease-free survival was analyzed by the log-rank test using
GraphPad Prism 7 software (GraphPad Software Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The mean age in the patient cohort was 69 years
(range, 35–88 years). The gender distribution was 55% males and 45%
females. Among the patients who received psychoactive drugs (38/83;
46%), the majority was treated for depression (9.64% of the
totality) and the most common psychotropic medication was
benzodiazepine (15.6%) (Table I).
In terms of tumor characteristics, the majority of
patients had CRC of stage II or III (28 and 31%, respectively),
according to the 2015 guidelines by the Union for International
Cancer Control (Table II) (23,24).
The mean follow-up time was 14,3 months. At the last
follow-up, remission was observed in 80.0% of patients, 14.7% lived
with the disease and 16.0% had succumbed to CRC. Furthermore, 2.7%
of the patients had succumbed within 30 days of surgery (Table III).
BDNF and NT4 levels in CRC
patients
Fig. 1 illustrates the
serum levels of BDNF and NT4/5 in CRC patients. BDNF levels were
8.2±1.7 ng/ml and NT4/5 levels were 15.1±6.9 ng/ml, demonstrating
reliable quantitative analysis, in particular for BDNF detection.
Stratified analyses by CRC stage revealed no significant
differences between groups (data not shown), suggesting that BDNF
and NT4/5 are not implicated in CRC development. Moreover, no
association between CEA and the levels of the two NTs was
identified (data not shown).
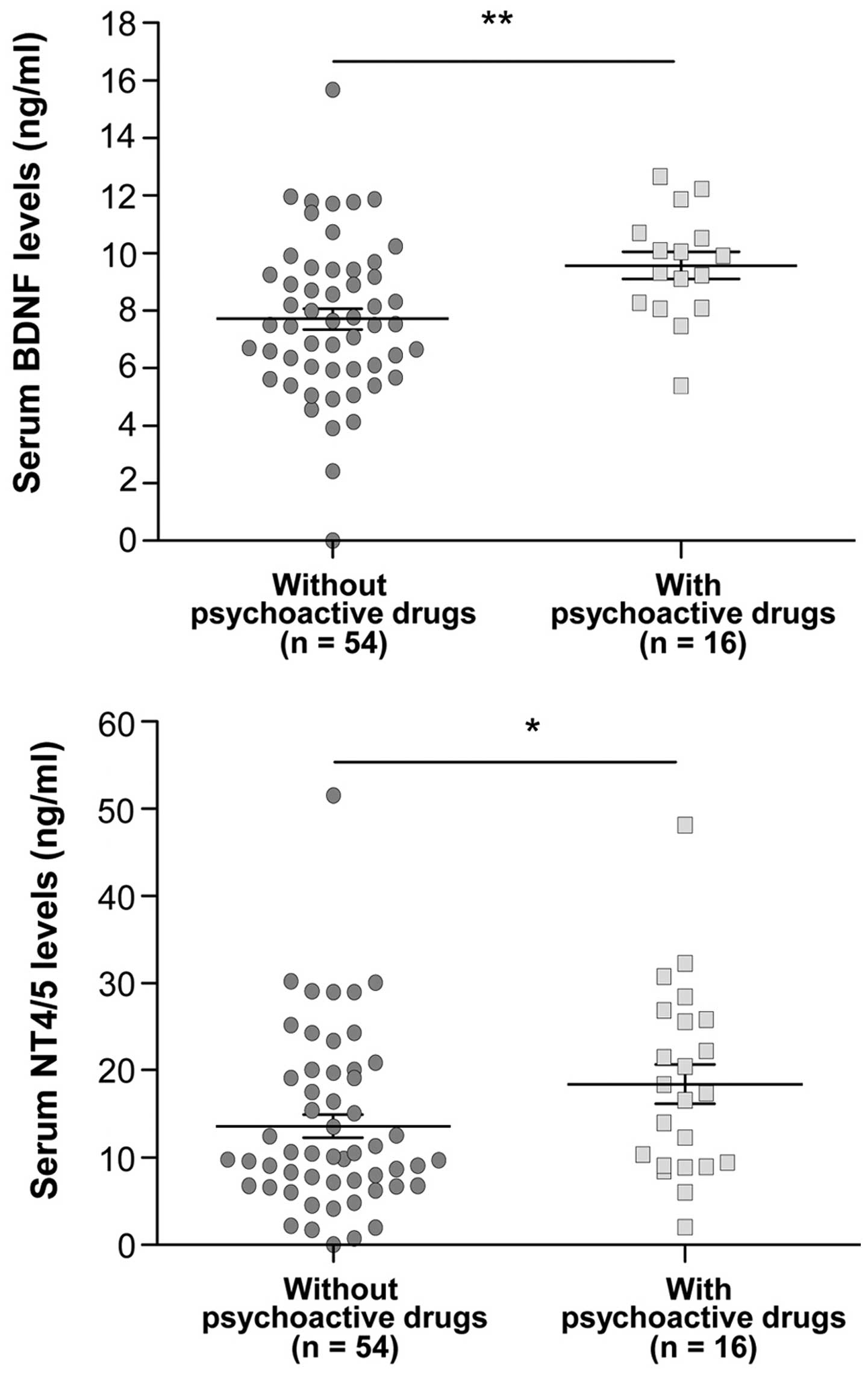
Influence of psychoactive drug intake
on serum BDNF and NT4/5 levels
The psychoactive drugs administered to the patients
were benzodiazepine and serotonin capture inhibitors (Table I). Significant differences in BDNF and
NT4/5 serum levels were observed between the patients who took
psychoactive drugs and those who did not (Fig. 2). In fact, the intake of psychoactive
drugs in CRC patients was associated with high serum levels of BDNF
(P=0.0059) and NT4/5 (P=0.0457), the two specific ligands of TrkB
receptor.
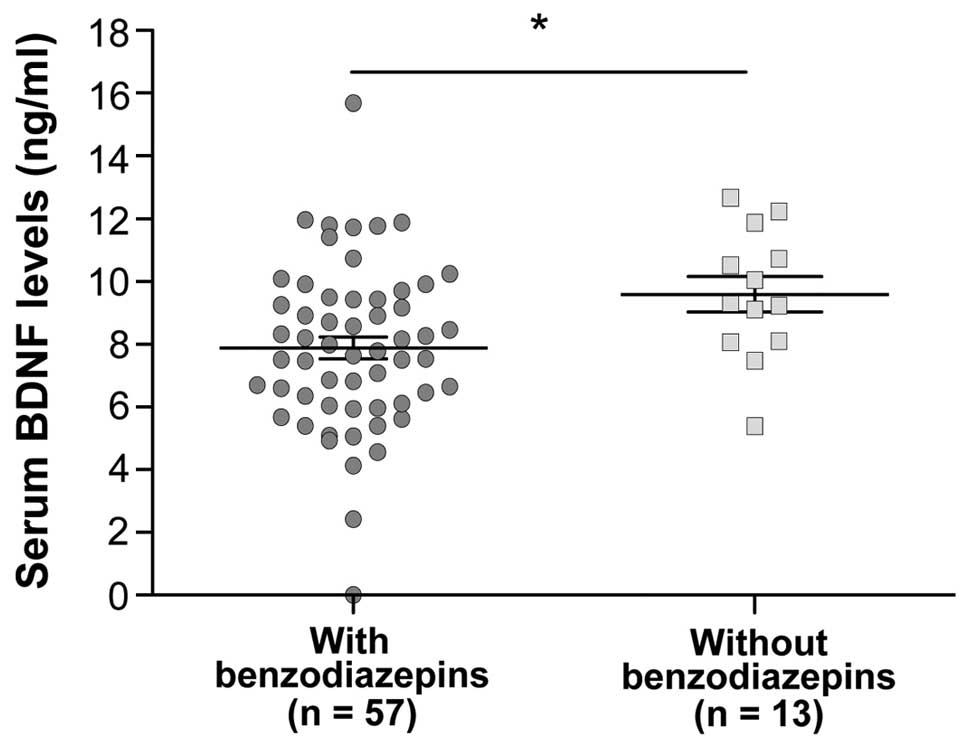
Regarding specific psychoactive drugs,
benzodiazepine intake was significantly associated with an increase
in serum BDNF levels (P=0.0221) (Fig.
3).
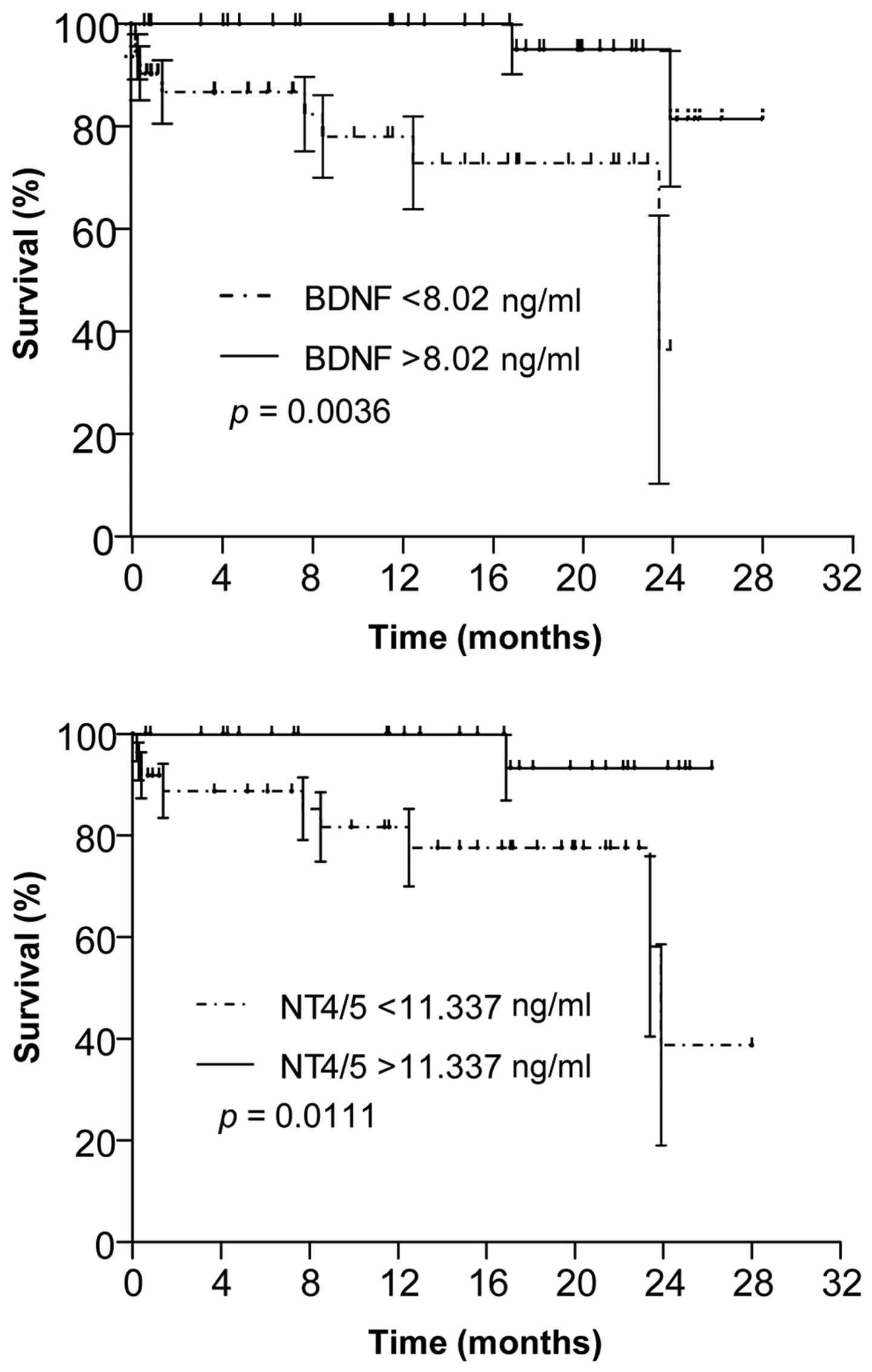
Serum BDNF and NT4/5 levels affect the
survival of CRC patients
Kaplan-Meier survival curves with stratification by
high and low serum BDNF (above or below the median of 8.0 ng/ml)
and NT4/5 (above or below the median of 11.3 ng/ml) levels revealed
that, irrespective of their treatment, survival of patients with
high serum BDNF and/or NT4/5 levels was longer than that of
patients with low levels (P=0.0036 and 0.0111, respectively)
(Fig. 4).
Discussion
CRC is the second highest cause of cancer-associated
mortality worldwide (25). It is
required to identify non-invasive serum markers for more precise
follow-up of this disease. As demonstrated by a previous study by
our group and others, NTs are promising cancer markers requiring
further study. In particular, the major role of the TrkB/BDNF axis
on the survival of CRC cells in vitro and in patient's
tissues has been demonstrated in a previous study by our group
(12).
In the present study, the serum levels of BDNF and
NT4/5 were analyzed in a cohort of 75 CRC patients. Although no
association with the CRC stage was detected, which was in
accordance with a previous study (16), a significant increase of these rates
(P=0.0059 for BDNF and P=0.0457 for NT4/5) was observed in patients
who were under psychotropic treatments, in particular for BDNF in
patients treated with benzodiazepine (P=0.0221). As benzodiazepine
is able to act on the central nervous system, it is expected to
affect the levels of BDNF, as previously demonstrated in patients
treated for depression with diazepam (5 mg), or tandospirone (20
mg) or paroxetine (10 mg), versus matched placebo (8). Thus, in future studies on NT expression
in cancer patients, psychotropic treatments must be taken into
account.
Finally, the present study revealed that patients
with high serum levels of BDNF and NT4/5 levels survived for longer
than those with low levels. This finding may be explained by the
psychoactive treatments administrated to the CRC patients; however,
it was in disagreement with a study on hepatocellular carcinoma
(17). Moreover, in hepatocellular
carcinoma, the quantity detected in serum was associated with the
BDNF expression found in tissues (17); this point should be further studied to
confirm the present hypothesis of the implication of NT in CRC.
It is worth mentioning that the present study had
certain limitations. For instance, only the mature form of BDNF was
detected by ELISA, while three further immature BDNF isoforms
co-exist: Uncleaved precursor, pro-BDNF and the cleaved pro-domain
(26). It may be worthwhile
investigating the other isoforms using the molecular approach
described by Zhou et al (9).
Another explanation for differences between the results of the
present study from those of previous ones may be the fact that BDNF
and NT4/5 are sequestered in exosome particles (27). It may be worthwhile examining these
specific particles, which are frequently used by cancer and other
cells to communicate.
In conclusion, the present study demonstrated that
detection of NTs in the serum of CRC patients using ELISA systems
may be influenced by psychotropic treatments. Therefore, clinicians
should pay attention to whether cancer patients receive
antidepressants, particularly diazepam and tandopsirone (8). In future studies, a survival analysis
should be performed for patients with and without psychotropic drug
treatments separately. The present study suggested that in CRC
patients, serum levels of BDNF and NT4/5 cannot be used as markers
of disease progression or tumor stage, while they may serve as
prognostic indicators.
Acknowledgements
The present study was supported by the Comité
Orientation Recherche Cancer (Limoges, France). The funders had no
role in study design, data collection and analysis, decision to
publish, or preparation of the manuscript.
References
|
1
|
Chao MV: Neurotrophins and their
receptors: A convergence point for many signalling pathways. Nat
Rev Neurosci. 4:299–309. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rizos EN, Papadopoulou A, Laskos E,
Michalopoulou PG, Kastania A, Vasilopoulos D, Katsafouros K and
Lykouras L: Reduced serum BDNF levels in patients with chronic
schizophrenic disorder in relapse, who were treated with typical or
atypical antipsychotics. World J Biol Psychiatry. 11:251–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martinotti G, Di Iorio G, Marini S, Ricci
V, De Berardis D and Di Giannantonio M: Nerve growth factor and
brain-derived neurotrophic factor concentrations in schizophrenia:
A review. J Biol Regul Homeost Agents. 26:347–356. 2012.PubMed/NCBI
|
|
4
|
Huang TL and Hung YY: Lorazepam reduces
the serum brain-derived neurotrophic factor level in schizophrenia
patients with catatonia. Prog Neuropsychopharmacol Biol Psychiatry.
33:158–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zuccato C, Marullo M, Vitali B, Tarditi A,
Mariotti C, Valenza M, Lahiri N, Wild EJ, Sassone J, Ciammola A, et
al: Brain-derived neurotrophic factor in patients with Huntington's
disease. PLoS One. 6:e229662011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang MC, Chen CH, Liu HC, Chen CC, Ho CC
and Leu SJ: Differential patterns of serum brain-derived
neurotrophic factor levels in alcoholic patients with and without
delirium tremens during acute withdrawal. Alcohol Clin Exp Res.
35:126–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ventriglia M, Zanardini R, Bonomini C,
Zanetti O, Volpe D, Pasqualetti P, Gennarelli M and
Bocchio-Chiavetto L: Serum brain-derived neurotrophic factor levels
in different neurological diseases. Biomed Res Int.
2013.20901082
|
|
8
|
Tamaji A, Iwamoto K, Kawamura Y, Takahashi
M, Ebe K, Kawano N, Kunimoto S, Aleksic B, Noda Y and Ozaki N:
Differential effects of diazepam, tandospirone, and paroxetine on
plasma brain-derived neurotrophic factor level under mental stress.
Hum Psychopharmacol. 27:329–333. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou L, Xiong J, Lim Y, Ruan Y, Huang C,
Zhu Y, Zhong JH, Xiao Z and Zhou XF: Upregulation of blood proBDNF
and its receptors in major depression. J Affect Disord.
150:776–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kreinin A, Lisson S, Nesher E, Schneider
J, Bergman J, Farhat K, Farah J, Lejbkowicz F, Yadid G, Raskin L,
et al: Blood BDNF level is gender specific in severe depression.
PLoS One. 10:e01276432015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chopin V, Lagadec C, Toillon RA and Le
Bourhis X: Neurotrophin signaling in cancer stem cells. Cell Mol
Life Sci. 73:1859–1870. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akil H, Perraud A, Mélin C, Jauberteau M-O
and Mathonnet M: Fine-tuning roles of endogenous brain-derived
neurotrophic factor, TrkB and sortilin in colorectal cancer cell
survival. PLoS One. 6:e250972011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Farias CB, Heinen TE, dos Santos RP,
Abujamra AL, Schwartsmann G and Roesler R: BDNF/TrkB signaling
protects HT-29 human colon cancer cells from EGFR inhibition.
Biochem Biophys Res Commun. 425:328–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka K, Okugawa Y, Toiyama Y, Inoue Y,
Saigusa S, Kawamura M, Araki T, Uchida K, Mohri Y and Kusunoki M:
Brain-derived neurotrophic factor (BDNF)-induced
tropomyosin-related kinase B (Trk B) signaling is a potential
therapeutic target for peritoneal carcinomatosis arising from
colorectal cancer. PLoS One. 9:e964102014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akil H, Perraud A, Jauberteau MO and
Mathonnet M: Tropomyosin-related kinase B/brain
derived-neurotrophic factor signaling pathway as a potential
therapeutic target for colorectal cancer. World J Gastroenterol.
22:490–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brierley GV, Priebe IK, Purins L, Fung
KYC, Tabor B, Lockett T, Nice E, Gibbs P, Tie J, McMurrick P, et
al: Serum concentrations of brain-derived neurotrophic factor
(BDNF) are decreased in colorectal cancer patients. Cancer Biomark.
13:67–73. 2013.PubMed/NCBI
|
|
17
|
Yang ZF, Ho DW, Lau CK, Tam KH, Lam CT, Yu
WC, Poon RTP and Fan ST: Significance of the serum brain-derived
neurotrophic factor and platelets in hepatocellular carcinoma.
Oncol Rep. 16:1237–1243. 2006.PubMed/NCBI
|
|
18
|
Jehn CF, Becker B, Flath B, Nogai H, Vuong
L, Schmid P and Lüftner D: Neurocognitive function, brain-derived
neurotrophic factor (BDNF) and IL-6 levels in cancer patients with
depression. J Neuroimmunol. 287:88–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saligan LN, Lukkahatai N, Holder G, Walitt
B and Machado-Vieira R: Lower brain-derived neurotrophic factor
levels associated with worsening fatigue in prostate cancer
patients during repeated stress from radiation therapy. World J
Biol Psychiatry. Mar 27–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayakawa M, Inagaki M, Fujimori M,
Hamazaki K, Hamazaki T, Akechi T, Tsugane S, Nishiwaki Y, Goto K,
Hashimoto K, et al: Serum brain-derived neurotrophic factor and
antidepressant-naive major depression after lung cancer diagnosis.
Jpn J Clin Oncol. 41:1233–1237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vanhecke E, Adriaenssens E, Verbeke S,
Meignan S, Germain E, Berteaux N, Nurcombe V, Le Bourhis X and
Hondermarck H: Brain-derived neurotrophic factor and
neurotrophin-4/5 are expressed in breast cancer and can be targeted
to inhibit tumor cell survival. Clin Cancer Res. 17:1741–1752.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perraud A, Akil H, Nouaille M, Petit D,
Labrousse F, Jauberteau MO and Mathonnet M: Implications of cleaved
caspase 3 and AIF expression in colorectal cancer based on patient
age. Oncol Rep. 27:1787–1793. 2012.PubMed/NCBI
|
|
23
|
Greene FL, Page DL, Fleming I, Fritz AG,
Balch CM, Haller DG and Morrow M: Small Intestine (Lymphomas,
carcinoid tumors, and visceral sarcomas are not included)AJCC
(American Joint Committee on Cancer) Cancer Staging Manual. 6th.
Springer-Verlag; New York: pp. 1132002
|
|
24
|
Wittekind C: Lymph nodes, tumour deposits,
and TNM: Are we getting better? 7th edition of UICC 2010 TNM
classification of malignant tumors. Strahlenther Onkol.
188:191–192. 2012.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hempstead BL: Brain-Derived Neurotrophic
Factor: Three Ligands, Many Actions. Trans Am Clin Climatol Assoc.
126:9–19. 2015.PubMed/NCBI
|
|
27
|
Théry C: Exosomes: Secreted vesicles and
intercellular communications. F1000 Biol Rep. 3:152011. View Article : Google Scholar : PubMed/NCBI
|