Introduction
The incidence of type-2 diabetes mellitus (T2DM) is
on the increase worldwide (1). The
number of people succumbing to coronary heart disease is greatest
in Europe, America, and urban areas of China. The number of T2DM
patients succumbing to coronary heart disease is 2- to 4-fold that
of non-T2DM patients (2). Previous
findings have shown that the release of inflammatory mediators is
closely associated with T2DM and myocardial ischemia-reperfusion
injury (MIRI) (3–5). Inflammatory mediators may increase MIRI
in patients with type-2 diabetes (6).
As a widely used intravenous anesthetic, propofol to
some extent (7) protects the heart
against IRI and reduces the expression of inflammatory mediators
(8–10).
Nevertheless, the protective effects of propofol against MIRI in
T2DM rats and the effect of inflammatory mediators have not been
investigated. Therefore, the aim of the study was to evaluate the
effects of propofol on MIRI in T2DM rats and to determine the role
of inflammatory mediators.
Materials and methods
A total of 50 healthy male Sprague-Dawley rats (6–8
weeks, 200–220 g) were provided by the Experimental Animal Center
of Hebei Province, Shijiazhuang, China [certificate no. SCXK (Ji)
2013-1-1003]. The experiment was approved and carried out in
accordance with the guidelines of Hebei University. The
experimental protocols were performed in adherence with the
Institutional Animal Care and Use Committee of Hebei University
(Baoding, China).
All the rats were fed a high-sugar and high-fat diet
at 22°C and humidity of 50%. The feeding was provided by the
Experimental Animal Center of Hebei Province (Shijiazhuang, China).
After a period of eight weeks, streptozotocin 30 mg/kg body weight
(Solarbio, Beijing, China) was injected into the abdomen to
establish the T2DM model. It has been detected that fasting glucose
is ≥14 mol/l for the preparation of a successful model.
The rats were randomly divided into five groups
(n=10/group): i) Sham-operated group (sham), ii)
ischemia-reperfusion (IR) and iii-v) IR plus low, middle and
high-dose propofol (IR+L, M, H Pro). MIRI was induced by ligating
the left anterior descending coronary artery for 30 min, followed
by reperfusion for 2 h. The rats in the sham group received an
intravenous infusion of physiologic (0.9%) saline (3 mg/kg/h) for
10 min without ligation. In the IR group, the rats received an
intravenous infusion of physiologic saline (3 mg/kg/h) for 10 min
before IR. In the IR+L, M, H Pro group rats, Pro (6, 12 and 24
mg/kg/h, intravenous) was respectively administered for 10 min
before IR. The rats were sacrificed after IR in the treatment
groups.
The rats were anesthetized, and a polyethylene
Millar catheter was inserted into the right common carotid artery
and then further advanced into the left ventricular chamber, after
which the cannula was connected to a pressure transducer. The heart
rate (HR), left ventricular systolic pressure (LVSP), and the rate
of left ventricular pressure increase in early systole (±
dp/dtmax) were recorded by an 8-channel polygraph system
(Powerlab 8s; ADInstruments, Castle Hill, New South Wales,
Australia). The levels of cardiac troponin T (cTnT), nitric oxide
(NO), endothelin-1 (ET-1), interleukin (IL)-1β, IL-6, and tumor
necrosis factor (TNF)-α were respectively measured using an
enzyme-linked immunosorbent assay. Myocardial lesions were observed
under light microscopy and scanning electron microscopy.
Statistical analysis
SPSS v16.0 (IBM, Armonk, NY, USA) was used for all
the analyses. Hemodynamics were compared and analyzed within groups
and between groups by multivariate analysis of variance. cTnT, NO,
ET-1, IL-1β, IL-6, and TNF-α levels were compared using one-way
ANOVA. The 95% confidence interval was used for significance.
Results
Changes in cardiac function
The HR, LVSP and ± dp/dtmax were
significantly decreased after IR compared with those before
ligation (P<0.05). No significant difference was observed in HR,
LVSP and ± dp/dtmax before ligation. Compared with that
of the sham group, HR, LVSP and ± dp/dtmax were
significantly decreased in the IR group rats (P<0.05). Compared
with those of the IR group, HR, LVSP and ± dp/dtmax were
significantly increased in the IR+L, M, H Pro group rats
(P<0.05; Table I).
 | Table I.Hemodynamic indices of myocardial
ischemia-reperfusion injury in rats with type-2 diabetes mellitus
(n=10, means ± SD). |
Table I.
Hemodynamic indices of myocardial
ischemia-reperfusion injury in rats with type-2 diabetes mellitus
(n=10, means ± SD).
| Variable | Group | Before ligation | After 30 min of
ischemia | After 2 h of
reperfusion |
|---|
| Heart rate | Sham |
424±15 |
433±27 |
421±8 |
|
| IR |
423±19 |
384±18a,d |
217±28a,d |
|
| IR+low-dose
propofol |
418±19 |
401±38a,b,d |
242±37a,b,d |
|
| IR+middle-dose
propofol |
420±18 |
411±10a,c,d |
262±39a,c,d |
|
| IR+high-dose
propofol |
419±16 |
398±45a,b,d |
245±26a,d,b |
| LVSP | Sham |
124±12 |
120±9 |
116±9 |
| (mmHg) | IR |
123±9 |
98±6a,d |
68±7a,d |
|
| IR+low-dose
propofol |
122±11 |
106±4a,b,d |
78±5a,b,d |
|
| IR+middle-dose
propofol |
123±10 |
112±10a,c,d |
80±8a,c,d |
|
| IR+high-dose
propofol |
121±15 |
110±5a,b,d |
75±10a,b,d |
| +
dp/dtmax | Sham |
3409±177 |
3384±141 |
3453±180 |
|
| IR |
3348±167 |
2583±315a,d |
2243±359a,d |
|
| IR+low-dose
propofol |
3385±246 |
2735±436a,b,d |
2696±217a,b,d |
|
| IR+middle-dose
propofol |
3392±321 |
2881±356a,c,d |
2755±312a,c,d |
|
| IR+high-dose
propofol |
3378±259 |
2801±345a,b,d |
2522±215a,b,d |
|
−dp/dtmax | Sham |
3819±144 |
3582±268 |
3548±396 |
|
| IR |
3883±93 |
2620±186a,d |
2232±427a,d |
|
| IR+low-dose
propofol |
3887±174 |
2830±484a,b,d |
2781±430a,b,d |
|
| IR+midle-dose
propofol |
3822±124 |
2845±320a,c,d |
2788±562a,c,d |
|
| IR+high-dose
propofol |
3857±152 |
2785±182a,b,d |
2635±352a,b,d |
Changes in NO, ET-1 and cTnT in the
serum
Compared with those of the sham group, NO was
reduced, and ET-1 and cTnT were significantly increased in the IR
group rats (P<0.05). Compared with those of the IR group, NO was
increased, and ET-1 and cTnT were significantly reduced in the
IR+L, M, H Pro group rats (P<0.05; Table II).
 | Table II.Serum concentrations of NO, ET-1and
cTnT (µmol/l) in the different groups (n=10, means ± SD). |
Table II.
Serum concentrations of NO, ET-1and
cTnT (µmol/l) in the different groups (n=10, means ± SD).
| Group | NO | ET-1 | cTnT |
|---|
| Sham |
83±3.2 |
3.90±0.25 |
14.60±1.0 |
| IR |
55±2.5a |
8.45±0.32a |
25.56±1.3a |
| IR+low-dose
propofol |
72±3.8a,b |
5.52±0.31a,b |
18.89±2.1a,b |
| IR+middle dose
propofol |
81±3.2a,c |
4.33±0.42a,c |
17.12±0.9a,c |
| IR+high dose
propofol |
75±3.5a,b |
5.32±0.38a,b |
19.75±1.2a,b |
Changes in IL-1β, IL-6 and TNF-α in
the serum
Compared with those of the sham group, IL-1β, IL-6
and TNF-α were significantly increased in the IR group rats
(P<0.05). Compared with those of the IR group, IL-1β, IL-6,
TNF-α were significantly reduced in the IR+L, M, H Pro group rats
(P<0.05; Table III).
 | Table III.Serum concentrations of IL-1β, IL-6,
and TNF-α (µmol/l) in the different group (n=10, means ± SD). |
Table III.
Serum concentrations of IL-1β, IL-6,
and TNF-α (µmol/l) in the different group (n=10, means ± SD).
| Group | IL-1β | IL-6 | TNF-α |
|---|
| sham |
173.19±23 |
177.38±15 |
198.21±15 |
| IR |
259.86±18a |
253.48±16a |
562.58±19a |
| IR+low-dose
propofol |
238.68±18a,b |
216.36±19a,b |
385.15±20a,b |
| IR+middle dose
propofol |
194.20±20a,c |
191.13±18a,c |
334.59±21a,c |
| IR+high dose
propofol |
217.58±21a,b |
215.23±18a,b |
362.31±25a,b |
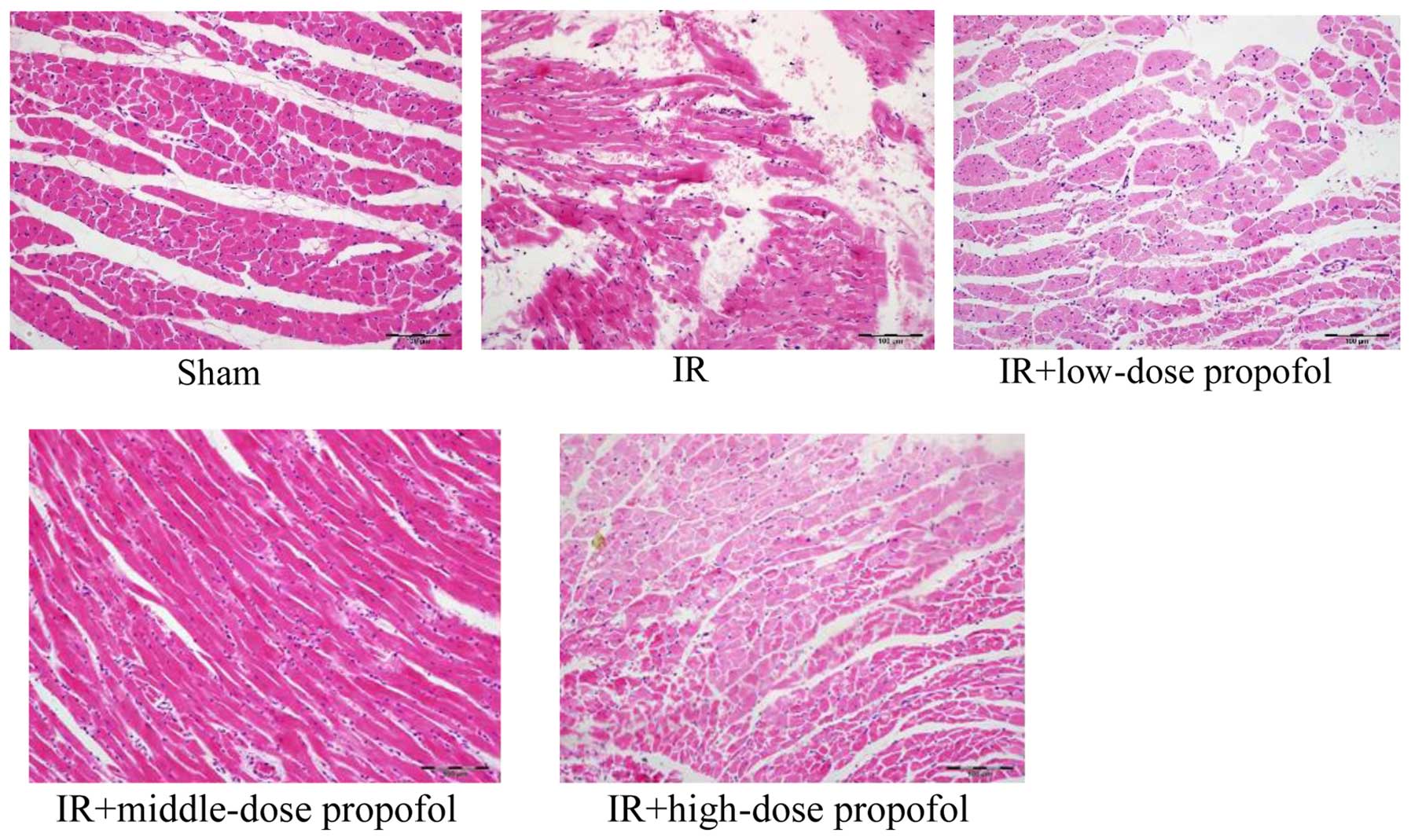
Morphologic changes under light
microscopy
No myocardial fibrosis and normal nuclear morphology
was uniformed in the sham group. Myocardial fibers and stromal
necrosis, eosinophil-enhanced muscle cells, elongated
wavy/fragmented myocardial fibrosis, with most nuclei showing
fragmentation and degeneration were observed in the IR group.
Eosinophil-enhanced myocardial fibrosis was evident, along with
elongated wavy/fragmented cardiac muscle fibres arranged in an
orderly manner, with most nuclei showing pyknosis and fragmentation
in the IR+low-dose propofol and IR+high-dose propofol groups.
Eosinophil-enhanced myocardial fibrosis, elongation of myocardial
fibers and hyperchromatic nuclei were observed in the
IR+middle-dose propofol group (Fig.
1).
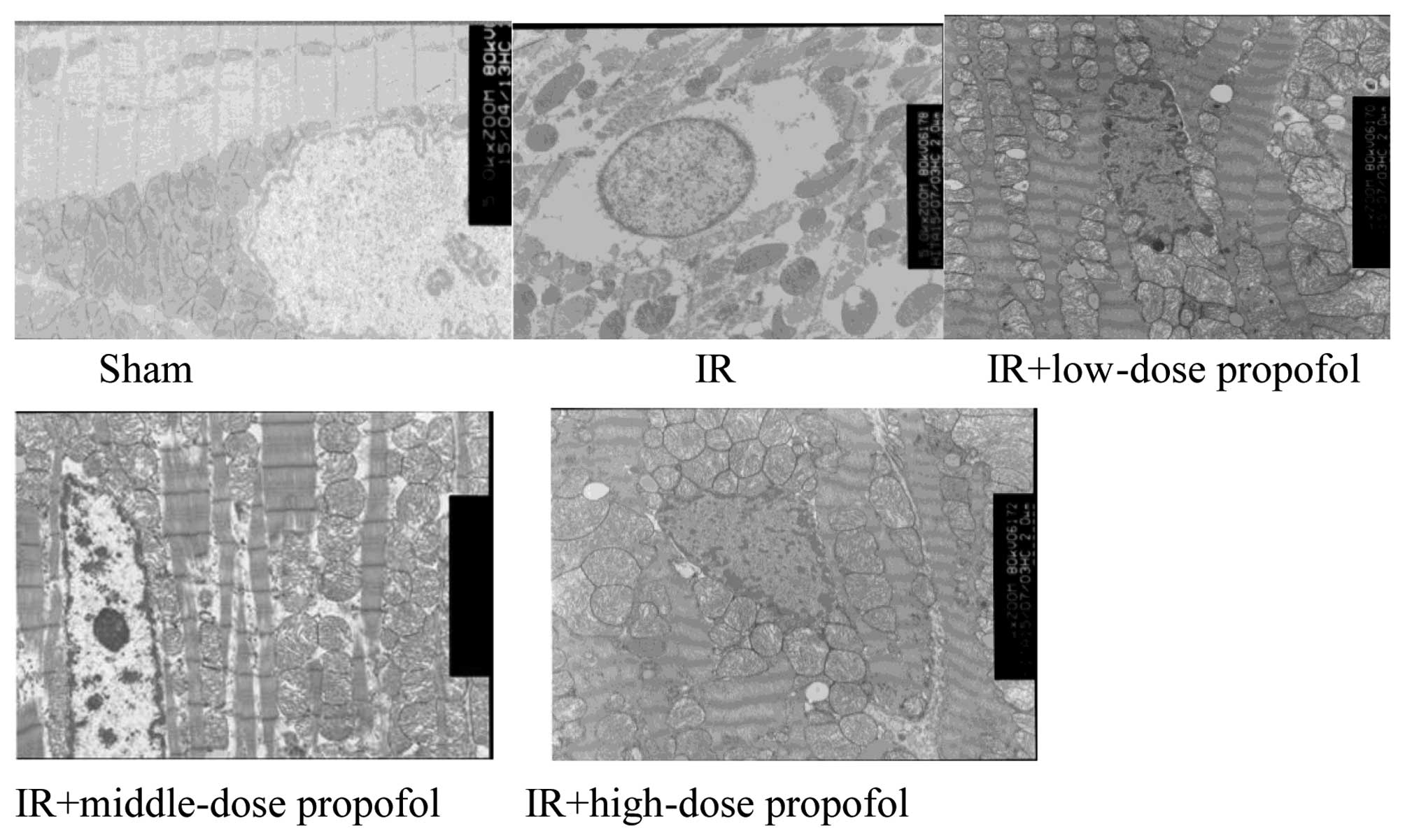
Ultrastrucral changes under electron
microscopy
The integrity of the membrane and cristae of
mitochondria and myocardial fibrosis were clearly visible, and the
integrity of inner and outer nuclear membranes of nuclei was
retained in the sham group rats. In the IR group, swelling of
mitochondria, rupture and disappearance of mitochondrial membranes,
dissolution of edema between mitochondria, absence of chromatin,
disappearance of the outer nuclear envelope, disappearance of some
inner and outer nuclear envelopes, and perinuclear edemawere
observed. Mitochondrial degeneration and necrosis of myocardial
fibers, indistinct sarcomeres, and an absence of muscle-fiber
structure were revealed. In the IR+low-dose propofol and
IR+high-dose propofol groups, mitochondrial edema, mitochondrial
films, and the partial disappearance of mitochondria were observed.
Additionally, high-density chromatin, partial disappearance of the
outer layer of nuclear films (but not of nuclear film within
layers), mild edema between muscle fibers, mild damage to
myocardial fibers, and comparatively neat muscle arrangement, were
observed. In the IR+middle-dose propofol group, some mitochondrial
edema was present, along with chromatin-dense masses but no obvious
perinuclear edema or lighter myocardial fibrosis was observed, and
sarcomeres were aligned (Fig. 2).
Discussion
In the present study, our results demonstrated that
propofol ameliorated cardiac function, increased serum NO and
decreased ET-1 and inflammatory mediators against MIRI in T2DM
rats.
In 1966, Jennings et al first suggested the
concept of IR injury which involves destruction of the tissue
structure and metabolic disorders (11). For instance, in clinical heart surgery,
infarction after coronary artery ligation is observed after MIRI.
Furthermore, type-2 diabetes is one of the most common endocrine
metabolic diseases, and myocardial injury was increased in type-2
diabetes resulting in diabetic cardiovascular disease having the
highest morbidity and mortality (12,13). In the
present experiment, we used the traditional preparation methods of
a type-2 diabetes model. Fasting glucose ≥14 mol/l was considered
as the successful model. HR, LVSP, and ± dp/dtmax were
significantly reduced after IR was compared with those before
ligation. These results indicate that the MIRI model was
successfully established.
Evidence indicates that the intravenous anesthesic
propofol may inhibit lipid peroxidation, improve mitochondrial
function (14), protect the myocardium
and reduce MIRI in rats (15).
Furthermore, it improves the function of vascular endothelial cells
and promotes the expression of anti-apoptotic proteins, thus
reducing MIRI in T2DM. Several clinical and biochemical indices may
be used for the diagnosis of myocardial injury. cTnT is considered
to be the ‘gold standard’ for the diagnosis of myocardial injury.
In the present study, propofol decreased the cTnT concentration in
serum and ameliorated cardiac function, as reflected by an increase
in HR, LVSP and ± dp/dtmax. In addition, the myocardial
damage degree was significantly decreased after the administration
of propofol. Therefore, propofol has myocardial protection for
type-2 diabetes rat myocardial IR.
Since 1980, Furchgott and Zawadzki identified the
endothelial diastolic factor (EDRF) (16). In 1988, Yanagisiawa et al
(17) first extracted ET from swine
aortic endothelial cultures and found that vascular endothelial
cells between blood circulation and vascular smooth muscle cells
play an important role in regulating cardiovascular activity. EDRF
is NO and it has a strong function of diastolic blood vessels and
inhibits vascular smooth muscle cell proliferation and thrombosis
(18). At the time of myocardial
ischemia, the change of NO level was controversial. Previous
findings showed that NO release was increased in coronary artery
myocardial ischemia (19–21). By contrast, other authors found that NO
release was decreased (22,23). The function of endothelium plays an
important role in maintaining stability and normal blood flow
dynamics. The key factor of its function is the NO. Vascular
endothelial often constantly release NO into the vascular smooth
muscle cells so as to maintain vascular tension in a moderate
degree of relaxation state (24). NO
exerts anti-inflammatory effects by inhibiting the neutrophil
adhesion to endothelial cells and decreasing the release of
inflammatory factors. An appropriate amount of NO could protect
cardiomyocytes, reduce damage, inhibit intimal hyperplasia and
ameliorate the heart function following IR injury. As an important
regulator of cardiovascular function, ET plays a significant role
in maintaining vascular tension and cardiovascular system steady
state (25). Endothelial cells
stimulate the synthesis and release of ET-1. ET-1 is responsible
for endothelial dysfunction and inflammation and contributes to
atherosclerotic plaque formation (26). ET-1 induces the left ventricular
afterload increase and participates in the fibrotic process of the
myocardium (27,28). Adrenaline, thromboxane, angiotensin,
insulin, inflammatory factors and hypoxia stimulated the synthesis
of ET-1, and the inhibitors of ET-1 synthesis included NO, PGI2,
atrial natriuretic peptide and heparin. This experiment showed that
propofol could increase the NO level and decrease the serum ET-1
concentration, thus exerting the cardioprotective effects on MIRI
in T2DM rats.
Studies have shown that T2DM and MIRI are associated
with inflammatory mediators (6–8). The
inflammatory factors potentially cause the myocardial damage in
T2DM patients. Thus, inhibition of the release of inflammatory
cytokines is an important strategy to protect heart against
myocardial damage in T2DM patients. As an important inflammatory
cytokine, TNF-α is produced mainly by the activation of
monocytes/macrophages, and participates in certain autoimmune
diseases (29–33). Thus, it could stimulate the NO synthase
(i-NOS) to synthesize and release a large number of NO. NO and the
ultra oxygen anion reaction occurs rapidly, and subsequently the
oxidation ability stronger light free radicals was generated. Which
could make the cell membrane lipid peroxide and the tissue damage
was aggravating (34). IL-1β is
produced mainly by macrophages and it was found that systemic
reactions resulted from injection and secretion of large amounts of
IL-1 (35). IL-6 is mainly generated
by macrophages, T-cells, B-cells and other cell types. IL-6
contributed to the cachexia induced by TNF-α and IL-1, and promotes
glucocorticoid synthesis. IL-6 has an important role against
infection as reflected by increasing the effects of other cytokines
and regulating the immune response, acute-phase response and
hematopoiesis. Inflammation factor occupies an important position
on MIRI. Inflammation caused by myocardial ischemia and hypoxia
promotes the release of large quantities of ILs from monocytes and
macrophages. As neutrophil chemotactic factors with high
specificity, the ILs cause adhesion and gathering of numerous white
blood cells (the obstacles against mini-circulation), an increase
of active oxygen and damage myocardial cells (36–38).
Inflammatory factors resulted in neutrophils releasing
cytotoxicity, aggravating the inflammatory reaction, blocking blood
capillaries, vascular active substances, thereby leading to acute
tissue damage (38). The results of
the present study have shown that propofol decreased the expression
of inflammatory cytokines, such as IL-1β, IL-6, TNF-α in serum.
In conclusion, our data provide strong supportive
evidence that propofol has protective effects on MIRI in T2DM rats
as reflected by ameliorating cardiac function, the increasing serum
NO and decreasing serum ET-1, IL-1β, IL-6 and TNF-α.
References
|
1
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Mattos Matheus AS, Righeti Monteiro
Tannus L, Roberta Arnoldi Cobas R, Palma CC Sousa, Negrato CA and
de Brito Gomes M: Impact of diabetes on cardiovascular disease: An
update (Review). Int J Hypertens. 2013:6537892013.PubMed/NCBI
|
|
3
|
Sun L and Kanwar YS: Relevance of TNF-α in
the context of other inflammatory cytokines in the progression of
diabetic nephropathy. Kidney Int. 88:662–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hashmi S and Al-Salam S: Acute myocardial
infarction and myocardial ischemia-reperfusion injury: A
comparison. Int J Clin Exp Pathol. 8:8786–8796. 2015.PubMed/NCBI
|
|
5
|
Halladin NL: Oxidative and inflammatory
biomarkers of ischemia and reperfusion injuries. Dan Med J.
62:B50542015.PubMed/NCBI
|
|
6
|
Marfella R, Di Filippo C, Portoghese M,
Siniscalchi M, Martis S, Ferraraccio F, Guastafierro S, Nicoletti
G, Barbieri M, Coppola A, et al: The ubiquitin-proteasome system
contributes to the inflammatory injury in ischemic diabetic
myocardium: The role of glycemic control. Cardiovasc Pathol.
18:332–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao X, Li Y, Tao M, Wang S, Zhang L, Lin
J, Xia Z and Liu HM: Effects of glucose concentration on propofol
cardioprotection against myocardial ischemia reperfusion injury in
isolated rat hearts. J Diabetes Res. 2015:5920282015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu F, Chen MR, Liu J, Zou Y, Wang TY, Zuo
YX and Wang TH: Propofol administration improves neurological
function associated with inhibition of pro-inflammatory cytokines
in adult rats after traumatic brain injury. Neuropeptides. 58:1–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Markovic-Bozic J, Karpe B, Potocnik I,
Jerin A, Vranic A and Novak-Jankovic V: Effect of propofol and
sevoflurane on the inflammatory response of patients undergoing
craniotomy. BMC Anesthesiol. 16:182016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Samir A, Gandreti N, Madhere M, Khan A,
Brown M and Loomba V: Anti-inflammatory effects of propofol during
cardiopulmonary bypass: A pilot study. Ann Card Anaesth.
18:495–501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jennings MA, Brock LG and Florey L: A
comparison of connective tissue lining aortic grafts with
extravascular connective tissue. Proc R Soc Lond B Biol Sci.
165:206–223. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cha SA, Yun JS, Lim TS, Hwang S, Yim EJ,
Song KH, Yoo KD, Park YM, Ahn YB and Ko SH: Severe hypoglycemia and
cardiovascular or all-cause mortality in patients with type 2
diabetes. Diabetes Metab J. 40:202–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee E, Oh HJ, Park JT, Han SH, Ryu DR,
Kang SW and Yoo TH: The incidence of cardiovascular events is
comparable between normoalbuminuric and albuminuric diabetic
patients with chronic kidney disease. Medicine (Baltimore).
95:e31752016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shao H, Li J, Zhou Y, Ge Z, Fan J, Shao Z
and Zeng Y: Dose-dependent protective effect of propofol against
mitochondrial dysfunction in ischaemic/reperfused rat heart: Role
of cardiolipin. Br J Pharmacol. 153:1641–1649. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Noh HS, Shin IW, Ha JH, Hah Y-S, Baek SM
and Kim DR: Propofol protects the autophagic cell death induced by
the ischemia/reperfusion injury in rats. Mol Cells. 30:455–460.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Furchgott RF and Zawadzki JV: The
obligatory role of endothelial cells in the relaxation of arterial
smooth muscle by acetylcholine. Nature. 288:373–376. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yanagisawa M, Kurihara H, Kimura S, Tomobe
Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K and Masaki T: A novel
potent vasoconstrictor peptide produced by vascular endothelial
cells. Nature. 332:411–415. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moncada S, Palmer RM and Higgs EA: Nitric
oxide: Physiology, pathophysiology, and pharmacology. Pharmacol
Rev. 43:109–142. 1991.PubMed/NCBI
|
|
19
|
Kilbourn RG, Jubran A, Gross SS, Griffith
OW, Levi R, Adams J and Lodato RF: Reversal of endotoxin-mediated
shock by NG-methyl-L-arginine, an inhibitor of nitric oxide
synthesis. Biochem Biophys Res Commun. 172:1132–1138. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wildhirt SM, Dudek RR, Suzuki H, Pinto V,
Narayan KS and Bing RJ: Immunohistochemistry in the identification
of nitric oxide synthase isoenzymes in myocardial infarction.
Cardiovasc Res. 29:526–531. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hoshida S, Yamashita N, Igarashi J,
Nishida M, Hori M, Kamada T, Kuzuya T and Tada M: Nitric oxide
synthase protects the heart against ischemia-reperfusion injury in
rabbits. J Pharmacol Exp Ther. 274:413–418. 1995.PubMed/NCBI
|
|
22
|
Yao SK, Akhtar S, Scott-Burden T, Ober JC,
Golino P, Buja LM, Casscells W and Willerson JT: Endogenous and
exogenous nitric oxide protect against intracoronary thrombosis and
reocclusion after thrombolysis. Circulation. 92:1005–1010. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zweier JL, Wang P and Kuppusamy P: Direct
measurement of nitric oxide generation in the ischemic heart using
electron paramagnetic resonance spectroscopy. J Biol Chem.
270:304–307. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Node K, Kitakaze M, Kosaka H, Komamura K,
Minamino T, Inoue M, Tada M, Hori M and Kamada T: Increased release
of NO during ischemia reduces myocardial contractility and improves
metabolic dysfunction. Circulation. 93:356–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mayyas F, Al-Jarrah M, Ibrahim K, Mfady D
and Van Wagoner DR: The significance of circulating endothelin-1 as
a predictor of coronary artery disease status and clinical outcomes
following coronary artery catheterization. Cardiovasc Pathol.
24:19–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lewicki L, Siebert J, Marek-Trzonkowska N,
Masiewicz E, Kolinski T, Reiwer-Gostomska M, Targonski R and
Trzonkowski P: Elevated serum tryptase and endothelin in patients
with ST segment elevation myocardial infarction: Preliminary
report. Mediators Inflamm. 2015:3951732015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kolettis TM, Barton M, Langleben D and
Matsumura Y: Endothelin in coronary artery disease and myocardial
infarction. Cardiol Rev. 21:249–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen J, Chen MH, Guo YL, Zhu CG, Xu RX,
Dong Q and Li JJ: Plasma big endothelin-1 level and the severity of
new-onset stable coronary artery disease. J Atheroscler Thromb.
22:126–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carswell EA, Old LJ, Kassel RL, Green S,
Fiore N and Williamson B: An endotoxin-induced serum factor that
causes necrosis of tumors. Proc Natl Acad Sci USA. 72:3666–3670.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang X, Marciano DL, Leeman SE and Amar S:
LPS induces the interaction of a transcription factor, LPS-induced
TNF-alpha factor, and STAT6(B) with effects on multiple cytokines.
Proc Natl Acad Sci USA. 102:5132–5137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kleine TO, Zwerenz P, Zöfel P and
Shiratori K: New and old diagnostic markers of meningitis in
cerebrospinal fluid (CSF). Brain Res Bull. 61:287–297. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Huang Q, Ong CN, Yang XF and Shen
HM: Chrysin sensitizes tumor necrosis factor-alpha-induced
apoptosis in human tumor cells via suppression of nuclear
factor-kappaB. Cancer Lett. 293:109–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh U, Kumar A, Sinha R, Manral S, Arora
S, Ram S, Mishra RK, Gupta P, Bansal SK, Prasad AK, et al:
Calreticulin transacetylase catalyzed modification of the TNF-alpha
mediated pathway in the human peripheral blood mononuclear cells by
polyphenolic acetates. Chem Biol Interact. 185:263–70. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suzuki Y, Deitch EA, Mishima S, Lu Q and
Xu D: Inducible nitric oxide synthase gene knockout mice have
increased resistance to gut injury and bacterial translocation
after an intestinal ischemia-reperfusion injury. Crit Care Med.
28:3692–3696. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oleszycka E, Moran HB, Tynan GA, Hearnden
CH, Coutts G, Campbell M, Allan SM, Scott CJ and Lavelle EC: IL-1α
and inflammasome-independent IL-1β promote neutrophil infiltration
following alum vaccination. FEBS J. 283:9–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma J, Qiao Z and Xu B: Effects of ischemic
preconditioning on myocardium Caspase-3, SOCS-1, SOCS-3, TNF-α and
IL-6 mRNA expression levels in myocardium IR rats. Mol Biol Rep.
40:5741–5748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishihara Y, Sekine M, Nakazawa M and
Shimamoto N: Suppression of myocardial ischemia-reperfusion injury
by inhibitors of cytochrome P450 in rats. Eur J Pharmacol.
611:64–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hadi NR, Al-Amran F, Yousif M and Zamil
ST: Antiapoptotic effect of simvastatin ameliorates myocardial
ischemia/reperfusion injury. ISRN Pharmacol. 2013:8150942013.
View Article : Google Scholar : PubMed/NCBI
|