Introduction
Menopause is a major health concern for women, as it
increases susceptibility to various chronic diseases, including
cardiovascular diseases (CVDs), osteoporosis, arthritis,
Alzheimer's disease, obesity, age-related eye diseases and cancer
(1,2).
Menopause is associated with an increase in oxidative stress,
resulting from an imbalance between reactive oxygen species (ROS)
and the antioxidant system (3–5). Previous
studies have demonstrated that oxidative stress is implicated in
the pathogenesis of various chronic diseases (6–9).
Osteoporosis is a skeletal disorder characterized by compromised
bone strength, predisposing patients to increased risk of fracture
(10). Although osteoporosis is a
multifactorial disorder, estrogen deficiency following menopause
serves a critical role in the development of osteoporosis in women
(11). There are multiple mechanisms
underlying the rapid resorption of bone and loss of bone density
due to estrogen deficiency, including direct effects on
osteoblastic and osteoclastic cell lineages and the interaction of
systemic hormones, local cytokines [including tumor necrosis factor
(TNF)-α, interleukin (IL)-1, and IL-6], growth factors, and
transcription factors (11,12). Additionally, increased ROS may also
decrease bone mineral density (BMD) by inducing TNF-α expression
(13).
Hormone replacement therapy (HRT) is established to
prevent bone loss following menopause (14,15).
Previous results have indeed demonstrated that HRT reduces the risk
of fractures, even among women with low fracture risk (16). However, findings from Women's Health
Initiative trials in 2002 indicated that the risks associated with
HRT outweighed the benefits (17,18).
Consequently, various groups have recommended limiting the use of
HRT in postmenopausal women at risk of facture and women younger
than 60 years or within 10 years of menopause (19). Alternatives to estrogen for the
treatment of postmenopausal osteoporosis as recommended by the U.S.
Food and Drug Administration (FDA) include bisphosphonates,
raloxifene, calcitonin and denosumab; however, these drugs have
been reported to exert drug-specific adverse effects (20). In turn, lifestyle modifications have
been undertaken, such as changes in exercise and diet. A number of
epidemiological studies have identified that higher fruit and
vegetable intake was associated with higher BMD (21–25) and a
lower fracture risk (26) in
postmenopausal women. While the mechanisms underlying these
bone-protective effects are yet to be fully elucidated,
antioxidative nutrients and phytochemicals, including vitamin C,
carotenoids and polyphenols, which are contained in fruits and
vegetables, may improve bone health by scavenging ROS (27).
Anthocyanins are a class of natural polyphenol
compounds responsible for the colors of flowers and fruits
(28). The positive health effects of
foods rich in anthocyanins include CVD prevention and anticancer,
anti-inflammatory, antioxidative, anti-obesity, anti-diabetic and
neuroprotective activities (29,30).
Additionally, Welch et al (31) suggested an anti-osteoporotic effect of
anthocyanins in a cohort study of twins, in which the differences
between the highest and lowest fifths of anthocyanin intake were
associated with a 3.4% higher BMD at the spine and a 3.1% higher
BMD at the hip. Berries contain abundant anthocyanins and have been
recognized as valuable sources of natural medicines and dietary
supplements (29,32). Various studies have demonstrated that
berry intake may increase antioxidant status and reduce
inflammatory biomarker levels in vivo (29). Based on these findings, our group
hypothesized that berry consumption may be helpful in alleviating
bone resorption and bone density loss following menopause. However,
a limited number of studies have investigated the impact of berry
intake on bone metabolism in animals and humans (29,32).
Additionally, berries are a natural source of not only anthocyanins
but also vitamin C, a potent antioxidant that may also have a
positive effect on bone (33,34), making it difficult to determine
whether the antioxidant effects of berries are due to anthocyanins,
vitamin C or both. Therefore, in the present study, the effects of
an anthocyanin-rich bilberry (Vaccinium myrtillus) extract (VME) on
bone metabolism were investigated in ovariectomized (Ovx) rats.
Bilberry, a member of the Ericaceous family, is a low-growing shrub
native to Europe and North America and is related to, while
distinct from, varieties of the North American blueberry (V.
corymbosum) (35). The
anthocyanin-rich VME has been utilized in the treatment of various
eye conditions, including cataracts and glaucoma, as well as for
enhancing night vision, due to its proposed anti-inflammatory and
antioxidant effects (35,36). The Ovx rat model is the current
FDA-approved model for the investigation of menopausal bone changes
(37). Ovx has also been demonstrated
to induce oxidative stress and impair antioxidant systems in rat
bone (38).
Materials and methods
Anthocyanin-rich VME
The anthocyanin-rich VME (containing ~39%
anthocyanins) used in the current study was provided in powder form
by Wakasa Seikatsu Co., Ltd. (Kyoto, Japan). VME contains a total
of 15 anthocyanins in all possible combinations of 5 anthocyanidins
(cyanidin, delphinidin, peonidin, petunidin and malvidin)
containing 3 types of sugar moieties (3-O-arabinosides,
3-O-glucosides and 3-O-galactosides) (35). The powder was stored at −20°C until
use and dissolved in distilled water (DW) to a concentration of 50
mg/ml (5%) prior to administration.
Animals and diets
The experimental protocol was approved by the Animal
Ethical Committee at Aichi Medical University (Nagakute, Japan;
approval no.: 2013-61). A total of 44 female Sprague-Dawley rats,
at 10 weeks of age, were purchased from Charles River Laboratories
Japan, Inc. (Hino, Shiga, Japan). Upon arrival, they were housed in
a temperature (23±1°C)- and humidity (55±5%)-controlled room under
a 12-h light/dark cycle and provided standard rodent chow (MF;
Oriental Yeast Co., Ltd., Tokyo, Japan) and water via an automatic
watering system ad libitum. After a 2-week acclimation period, the
rats were randomly divided into four groups: Baseline (n=8), Sham
(n=12), Ovx (n=12) and Ovx+VME (n=12). Rats in the Baseline group
were anesthetized by intraperitoneal injection of medetomidine
hydrochloride (Nippon Zenyaku Kogyo Co., Ltd., Koriyama, Japan;
0.15 mg/kg body weight), midazolam (Astellas Pharma, Inc., Tokyo,
Japan; 2 mg/kg body weight) and butorphanol tartrate (Meiji Seika
Pharma Co., Ltd., Tokyo, Japan; 2.5 mg/kg body weight), and were
immediately sacrificed by cardiac puncture. The inclusion of the
Baseline group provided initial values of skeletal measures,
thereby allowing for the determination of changes in skeletal
tissue resulting from surgery and aging (39). Rats in the remaining three groups were
anesthetized and subjected either to sham (Sham) or Ovx (Ovx and
Ovx+VME) surgeries. At 2 days after surgery, rats in the Ovx+VME
group were administered VME by gavage at a dose of 500 mg/kg body
weight (equivalent to 10 ml/kg body weight of 5% VME) daily for 8
weeks. The dose of VME was determined based on previous studies in
rodents (40,41). Rats in the other two groups were given
DW by gavage at a dose of 10 ml/kg body weight daily for 8 weeks. A
total of 3 rats in the Sham group (6.8% of the total) were lost as
a consequence of accidental fatality at 6 days post-surgery. At 8
weeks after surgery, the rats in all three groups were sacrificed.
The rats were administered intraperitoneal injections of the
fluorochrome markers tetracycline-HCl (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany; 20 mg/kg body weight) and calcein
(Sigma-Aldrich; Merck KGaA; 10 mg/kg body weight) at 5 and 2 days
before necropsy, respectively, for the evaluation of bone dynamics
by histomorphometry. At necropsy, the uterus was resected and
weighed to determine whether the Ovx surgery had been successful.
The right femurs from all rats were wrapped in saline-soaked gauze
and stored at −20°C for subsequent densitometry. The right tibiae
were cleaned of soft tissue and fixed in 70% ethanol for bone
histomorphometry.
Bone densitometry
The right femurs were thawed at room temperature.
Bone mineral content (BMC) and BMD were determined by dual-energy
X-ray absorptiometry (DXA; QDR-Discovery A; Hologic, Inc.,
Marlborough, MA, USA) using the QDR-Discovery A high-resolution
scanning software (version 13.3; Hologic, Inc.) designed for the
measurement of small animal bones. Additionally, BMD and BMC in the
proximal, mid-diaphyseal and distal parts of the femur were
determined by dividing the femur into three equal parts according
to length. The coefficients of variation for repeated scans on the
same bone were <1.0%. Prior to measurements, a tissue
calibration scan was performed with the Hologic small animal
phantom.
Peripheral quantitative computed
tomography (pQCT)
Following DXA measurements, pQCT was performed using
an XCT Research SA+ (Stratec Biomedical AG, Birkenfeld, Germany).
The right femurs were placed in a polypropylene tube filled with
saline and were scanned at a 0.46-mm collimation and 0.12-mm voxel
size. The scan line was adjusted using the scout view, and
transverse sections were recorded at the distal femoral metaphysis
[to determine total cross-sectional area (CSA), mm2;
4.0-mm proximal to the distal growth plate) and at the midshaft
(mid-point of the bone length). Analyses were performed using XCT
6.20 software (Stratec Biomedical AG) in contour mode 2 and peel
mode 2 (threshold 464 mg/cm3) for the calculation of
trabecular and total bone parameters at the metaphysis, as well as
in cortical mode 1 (threshold 690 mg/cm3) for the
determination of cortical bone parameters at the diaphysis. At the
femoral metaphysis, trabecular BMC (Tb.BMC; mg), trabecular BMD
(Tb.BMD; mg/cm3) and trabecular cross-sectional area
(Tb.CSA; mm2) were measured. At the midshaft, cortical
BMC (Ct.BMC; mg), cortical BMD (Ct.BMD; mg/cm3),
cortical CSA (Ct.CSA; mm2), cortical thickness (Ct.Th;
mm), periosteal circumference (Peri.C; mm) and endosteal
circumference (Endo.C; mm) were evaluated.
Bone histomorphometry
The right tibiae were trimmed of soft tissue and
fixed at 4°C with 70% ethanol for 14 days. The proximal one-thirds
of the tibiae were stained with Villanueva bone stain (Maruto
Instrument Co., Ltd., Tokyo, Japan) at room temperature for 7 days,
then embedded undecalcified in methyl methacrylate (Wako Pure
Chemical Industries, Ltd., Osaka, Japan) following dehydration in a
graded series of ethanol (70, 95, 95 and 100%). Frontal sections (5
µm) were cut using a microtome (RM2255; Leica Microsystems GmbH,
Wetzlar, Germany) and mounted on slides. Specimens were examined
under a fluorescence microscope (Nikon Corporation, Tokyo, Japan).
Structural and dynamic histomorphometric indices were measured in
the cancellous bone at 0.435–1.7625 mm distal to the epiphyseal
growth plate, which consists of secondary spongiosa, using a
semi-automatic image analysis system (Histometry RT Camera; System
Supply, Co., Ltd., Nagano, Japan) at a magnification of ×250. The
primary indices included tissue volume (TV), bone volume (BV), bone
surface (BS), osteoid volume (OV), osteoid surface (OS), trabecular
thickness (Tb.Th), osteoblast surface (Ob.S), osteoclast surface
(Oc.S), eroded surface (ES), single- and double-labeled surfaces
(sLS and dLS, respectively) and interlabel width. Calculated from
these parameters were the percentages of BV (BV/TV), OV (OV/BV), OS
(OS/BS), Ob.S (Ob.S/BS), Oc.S (Oc.S/BS), ES (ES/BS), sLS and dLS
(sLS/BS and dLS/BS, respectively). Trabecular number (Tb.N) and
mineralizing surface (MS)/BS were calculated as (BV/TV)/Tb.Th and
(sLS/2 + dLS)/BS, respectively. Mineral apposition rate (MAR) was
calculated from the distance between the labels divided by the time
between labels, and was corrected for section obliquity. Bone
formation rate (BFR/BS) was calculated by multiplying the MS/BS by
the MAR, The histomorphometric nomenclature used in the present
study was in accordance with a report of the American Society for
Bone and Mineral Research Histomorphometry Nomenclature Committee
(42).
Statistical analyses
All data are expressed as the mean ± standard error
of the mean (SEM), and all data management and statistical analyses
were performed using JMP 9.0.2 (SAS Institute, Inc., Cary, NC,
USA). The specific effects of Ovx and VME were examined by
comparing values of the Sham, Ovx and Ovx+VME groups with one-way
analysis of variance or analysis of covariance using body weight as
the covariate (43,44), followed by Tukey's honest significant
difference test. Differences were considered significant when
P<0.05.
Results
Effects of VME on the uterus and body
weight of Ovx rats
The uterus weight of rats in the Ovx group was
significantly lower than that of rats in the Baseline and Sham
groups (P<0.01; data not shown), confirming that the Ovx was
successful. There was no significant difference in uterine weight
between the Ovx and Ovx+VME groups, suggesting that VME may lack an
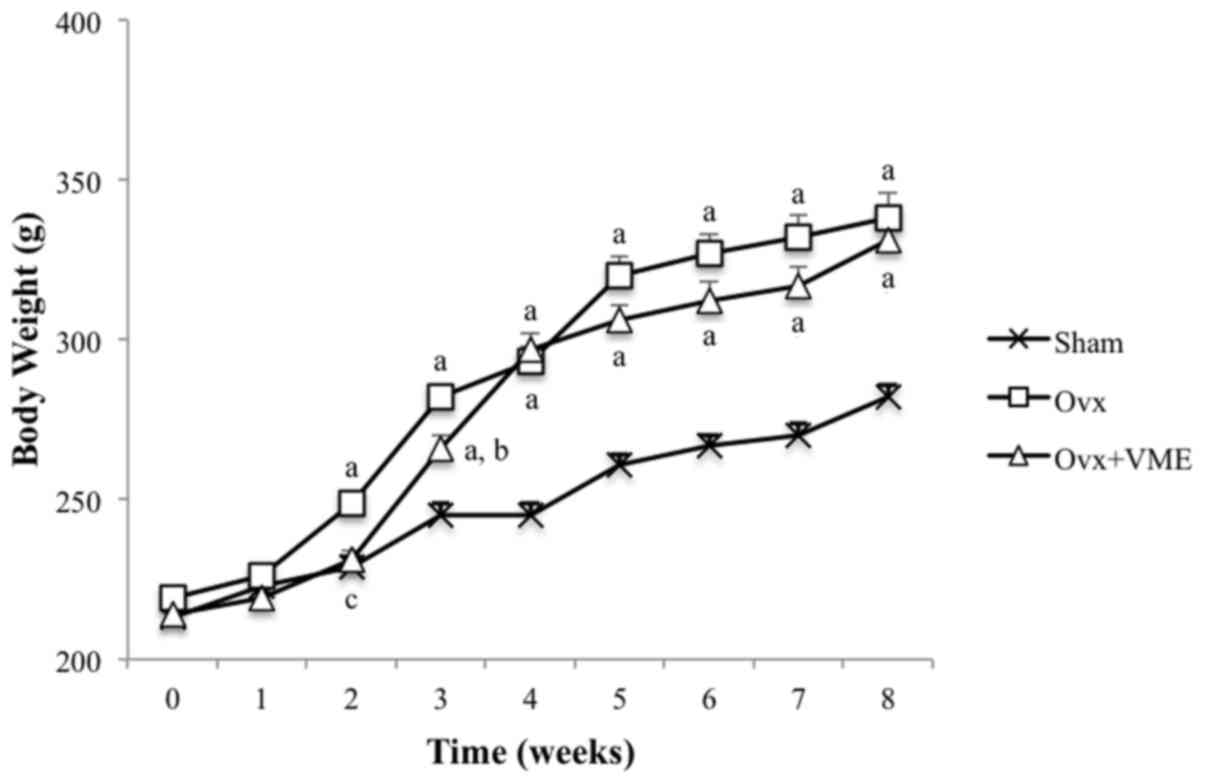
estrogenic property. From 2 weeks post-surgery, the body weights of
rats in the Ovx group were significantly increased compared with
those in the Sham group (P<0.01). Although there was a slight
delay in the increase in body weight of the Ovx+VME group, there
was no significant difference in body weight between the Ovx and
Ovx+VME groups by the end of the experiment (Fig. 1).
DXA of the right femur
The BMD of the right femur of rats in the Ovx group
was significantly lower than that in the Sham group (P<0.01).
Supplementation of VME in the Ovx rats did not result in an
increase in the right femur BMD. When the femur was divided into
three equal segments in length, there were significant decreases in
the BMD of the Ovx group compared with the Sham group (P<0.01)
at the proximal and distal thirds of the femur (sites high in
cancellous bone), though not at the middle third (a site high in
cortical bone). By contrast, right femur BMC values did not differ
significantly between the Sham and Ovx groups. The Ovx rats
supplemented with VME (Ovx+VME group) exhibited no significant
changes in BMC or BMD compared with the rats in the Ovx group,
regardless of the femur site (proximal, diaphyseal or distal;
Table I).
 | Table I.Femur BMC and BMD in the
sham-operated (Sham) rats and ovariectomized rats supplemented with
(Ovx+VME) or without (Ovx) an anthocyanin-rich bilberry
extract. |
Table I.
Femur BMC and BMD in the
sham-operated (Sham) rats and ovariectomized rats supplemented with
(Ovx+VME) or without (Ovx) an anthocyanin-rich bilberry
extract.
| Site | Baseline (n=8) | Sham (n=9) | Ovx (n=12) | Ovx+VME (n=12) |
|---|
| Femur BMC (g) |
|
|
|
|
|
Whole | 0.283±0.006 | 0.357±0.009 | 0.361±0.005 | 0.353±0.008 |
|
Proximal 1/3 | 0.104±0.003 | 0.133±0.003 | 0.133±0.002 |
0.130±0.003a |
| Mid
1/3 | 0.063±0.003 | 0.082±0.002 | 0.089±0.002 | 0.088±0.002 |
| Distal
1/3 | 0.115±0.003 | 0.140±0.003 | 0.141±0.002 |
0.134±0.004a |
| Femur BMD
(g/cm2) |
|
|
|
|
|
Whole | 0.194±0.003 | 0.222±0.003 |
0.208±0.002b |
0.207±0.003b |
|
Proximal 1/3 | 0.200±0.004 | 0.229±0.003 |
0.214±0.002b |
0.213±0.003b |
| Mid
1/3 | 0.157±0.004 | 0.189±0.003 | 0.183±0.002 | 0.185±0.003 |
| Distal
1/3 | 0.215±0.003 | 0.240±0.004 |
0.223±0.002b |
0.219±0.003b |
pQCT of the right femur metaphysis and
diaphysis
The trabecular and cortical parameters of the right
femur determined by pQCT are listed in Table II. There was a significant increase
in the Tb.CSA of the distal metaphysis in the Ovx group compared
with the Sham group (P<0.01), resulting in a significantly lower
Tb.BMD in the Ovx group compared with that in the Sham group
(P<0.01). There were no significant differences in Ct.CSA or
Ct.BMD between the Sham and Ovx groups at the diaphysis. The
administration of VME had no significant effect on any of the
parameters evaluated by pQCT.
 | Table II.Trabecular and cortical parameters
determined by peripheral quantitative computed tomography in the
sham-operated (Sham) and ovariectomized rats supplemented with
(Ovx+VME) or without (Ovx) an anthocyanin-rich bilberry
extract. |
Table II.
Trabecular and cortical parameters
determined by peripheral quantitative computed tomography in the
sham-operated (Sham) and ovariectomized rats supplemented with
(Ovx+VME) or without (Ovx) an anthocyanin-rich bilberry
extract.
| Parameter | Baseline (n=8) | Sham (n=9) | Ovx (n=12) | Ovx+VME (n=12) |
|---|
| Distal
metaphysis |
|
|
|
|
| T.BMC
(mg) | 9.85±0.31 | 10.79±0.46 | 9.46±0.16 |
9.25±0.30a |
| T.BMD
(mg/cm3) | 683±35 | 757±63 | 622±43a | 604±50b |
| T.CSA
(mm2) | 14.4±0.5 | 14.3±0.5 | 15.3±0.5 | 15.3±0.4 |
| Tb.BMC
(mg) | 1.233±0.067 | 0.983±0.139 | 1.414±0.095 | 1.418±0.083 |
| Tb.BMD
(mg/cm3) | 331±12 | 327±16 | 193±6b | 201±8b |
| Tb.CSA
(mm2) | 3.78±0.28 | 3.20±0.52 |
7.29±0.35b |
7.11±0.39b |
| Diaphysis |
|
|
|
|
| Ct.BMC
(mg) | 5.79±0.17 | 7.24±0.14 | 7.80±0.12 | 7.54±0.12 |
| Ct.BMD
(mg/cm3) | 1,270±10 | 1,350±0 | 1,340±0 | 1,340±0 |
| Ct.CSA
(mm2) | 4.57±0.12 | 5.38±0.14 | 5.80±0.07 | 5.63±0.07 |
| Ct.Th
(mm) | 0.557±0.015 | 0.663±0.011 | 0.678±0.07 | 0.668±0.011 |
| Peri.C
(mm) | 9.95±0.12 | 10.19±0.14 | 10.70±0.12 | 10.53±0.11 |
| Endo.C
(mm) | 6.45±0.15 | 6.03±0.12 | 6.44±0.15 | 6.34±0.15 |
Bone histomorphometry
The histomorphometric measurements of the cancellous
bone indices in the proximal tibiae are summarized in Table III. Rats in the Ovx group exhibited
significantly lower structural indices (BV/TV, Tb.Th and Tb.N;
P<0.01, P<0.05 and P<0.01, respectively) and higher bone
formation indices (OV/BV, OS/BS, dLS/BS, MS/BS and BFR/BS; all
P<0.01) compared with those in the Sham group due to estrogen
deficiency. These differences were not significantly affected by
VME administration.
 | Table III.Static and dynamic cancellous bone
indices in the proximal tibiae in the sham-operated (Sham) and
ovariectomized rats supplemented with (Ovx+VME) or without (Ovx) an
anthocyanin-rich bilberry extract. |
Table III.
Static and dynamic cancellous bone
indices in the proximal tibiae in the sham-operated (Sham) and
ovariectomized rats supplemented with (Ovx+VME) or without (Ovx) an
anthocyanin-rich bilberry extract.
| Index | Baseline (n=8) | Sham (n=9) | Ovx (n=12) | Ovx+VME (n=12) |
|---|
| Static indices |
|
|
|
|
| BV/TV
(%) | 23.3±1.2 | 33.5±2.0 |
15.2±0.7b |
14.7±1.0b |
| Tb.Th
(µm) | 64.9±1.9 | 66.8±1.2 |
58.9±1.5a | 61.5±1.2 |
| Tb.N
(/mm) | 3.59±0.12 | 5.02±0.29 |
2.57±0.09b |
2.38±0.15b |
| OV/BV
(%) | 3.20±0.33 | 1.18±0.20 |
4.28±0.30b |
4.67±0.49b |
| OS/BS
(%) | 24.8±2.5 | 11.0±1.7 |
28.7±1.7b |
28.9±2.2b |
| Ob.S/BS
(%) | 1.40±0.28 | 0.72±0.22 | 2.86±0.61 | 4.70±0.97 |
| ES/BS
(%) | 3.06±0.38 | 2.08±0.32 | 5.29±0.46 |
6.22±0.77a |
| Oc.S/BS
(%) | 1.35±0.26 | 0.85±0.22 | 1.99±0.32 | 2.66±0.46 |
| Dynamic
indices |
|
|
|
|
| sLS/BS
(%) | 38.0±0.9 | 33.6±2.0 | 35.3±1.1 | 34.5±1.1 |
| dLS/BS
(%) | 18.9±2.2 | 5.9±1.0 |
18.2±1.2b |
17.1±1.6b |
| MS/BS
(%) | 37.9±2.6 | 22.7±1.5 |
35.9±1.2b |
34.4±1.5b |
| MAR
(µm/day) | 1.69±0.05 | 1.12±0.02 | 1.44±0.03 |
1.52±0.06b |
| BFR/BS
(mm3/mm2/year) | 0.236±0.022 | 0.093±0.007 |
0.188±0.008b |
0.193±0.015b |
Discussion
Estrogen deficiency is associated with an imbalance
in bone metabolism, involving a net increase in bone resorption
over formation, leading to excessive and sustained bone loss
(11). The increase in bone
resorption is the result of increased osteoclastogenesis and
decreased osteoclast apoptosis (11,12). ROS
may promote osteoclast resorption directly, by stimulating
signaling associated with osteoclast differentiation and receptor
activator of nuclear factor (NF)-κB (RANK), or indirectly, by
stimulating osteoblast/osteoclast coupling and subsequent
osteoclast differentiation through RANK ligand (RANKL) (32). A number of studies have demonstrated
that berry extracts may reduce oxidative stress (45–47).
Karlsen et al (48)
demonstrated that anthocyanins isolated from bilberries and black
currants efficiently suppressed LPS-induced activation of NF-κB in
cultured monocytes. Tanabe et al (49) reported that cranberry extract
inhibited RANKL-dependent differentiation of human pre-osteoclasts
and bone resorption activity of osteoclasts. Furthermore, a recent
study by Moriwaki et al (50)
demonstrated that anthocyanin compounds extracted from bilberry and
black currant inhibited osteoclast formation from osteoclast
precursor RAW264.7 cells. Collectively these findings indicate
anthocyanin extracted from berry fruits may alleviate bone
resorption and bone density loss following menopause in women. In
the present study, an anthocyanin-rich bilberry extract was
administered to Ovx rats (Ovx+VME group) for 8 weeks, and BMD was
measured using DXA and pQCT. In the VME-treated rats, there were no
significant changes in BMD or BMC, even in trabecular or cortical
BMD at the metaphyseal and diaphyseal sites, compared with those in
rats in the Ovx group. These results were supported by the bone
histomorphometry results (BV/TV, Tb.Th and Tb.N). Although these
parameters remained stable when the rate of bone resorption was
equal to that of bone formation, bone histomorphometry also
revealed that the anthocyanin-rich bilberry extract did not affect
bone formation (OV/BV, OS/BS, dLS/BS, MS/BS and BFR/BS) or
resorption (Oc.S/BS and ES/BS). These results suggested that the
anthocyanin-rich bilberry extract may not mitigate the bone losses
observed in postmenopausal women.
There have been a limited number of studies that
have investigated the effects of consuming berry fruits or their
extracts on bone metabolism in Ovx-induced bone loss animal models.
Devareddy et al (51)
demonstrated that Ovx rats (6 months old) fed a diet supplemented
with blueberry powder (5% w/w) for 4 months had a higher overall
BMD of the whole body but not at the tibia, femur or fourth lumbar
vertebra compared with rats in an Ovx group. Additionally, they
identified that the supplement treatment down-regulated Ovx-induced
elevation of alkaline phosphatase, collagen and tartrate-resistant
acid phosphatase (TRAP) gene expression, suggesting that the
bone-protective effect of blueberries may be due to the suppression
of bone turnover (51). More
recently, Zheng et al (52)
identified that Ovx mice (14 weeks old) given a diet containing 1%
anthocyanin-rich blackcurrant extract for 12 weeks had
significantly greater femur BMD compared with Ovx control mice.
Notably, they demonstrated that the extract reduced the number of
TRAP-positive osteoclast-like cells and bone resorption activity,
and concluded that the extract may alleviate bone loss by
suppressing osteoclastogenesis and osteoclast function.
The reasons underlying the inconsistent results
between the present study and previous studies are unknown. A
previous randomized prospective study designed to examine whether
the consumption of freeze-dried blackberries or blueberries (45 g
daily) could prevent smoking-induced bone loss in postmenopausal
women demonstrated that the loss of total body BMD was significant
in women who consumed blueberries but not blackberries for 9
months, despite the higher content of anthocyanins in blueberries
compared with blackberries (652.2 vs. 284.1 mg per 45 g
freeze-dried berry) (53). Notably,
87% (247.1 mg) of the anthocyanins in the freeze-dried blackberries
was cyanidin-3-glucoside, which was a greater percentage than the
1% (6.6 mg) in the freeze-dried blueberries. These findings
suggested that the profile or ingredients, and not the total amount
of anthocyanins, may be responsible for the discrepancies between
previous studies (53), although the
active ingredients in the anthocyanins that may prevent bone loss
are yet to be identified. Kaume et al (54) fed 5% and 10% (w/w) blackberry diets
rich in cyanidin-3-glucoside for 100 days to Ovx rats (9 months
old) and demonstrated that the 5% (but not 10%) blackberry diet
prevented a loss of BMD at the tibia, femur and fourth lumbar
vertebra. However, they failed to identify any significant changes
in bone formation and resorption markers following diet
supplementation, and thus were unable to conclude whether the
results were due to the suppression of bone resorption or
acceleration of bone formation. Moriwaki et al (50) demonstrated that delphinidin, one of
the aglycone nuclei of anthocyanins, prevented bone loss in Ovx
mice (7 weeks old). They also observed a significant decrease in
osteoclast number in delphinidin-treated, soluble RANKL-induced
osteoporotic mice, and assumed that delphinidin may prevent bone
loss by suppressing bone resorption. Although cyanidin and
delphinidin are major anthocyanidins retained in bilberry (55), the current study identified no
substantial effects on bone mass, bone formation or bone resorption
parameters on examination by bone histomorphometry. Further studies
are required to determine which active ingredients have
bone-protective effects in berry-extracted anthocyanins.
Differences in the experimental conditions may have
also resulted in discrepancies with previous studies. Zhang et
al (56) fed a diet supplemented
with 10% freeze-dried blueberry powder to pre-pubertal rats between
postnatal day 20 (PND20) and PND34, after which the diets were
either continued (long-term feeding) or switched to a control
casein diet (short-term feeding). Rats were then Ovx on PND60 and
sacrificed 1 or 3 weeks thereafter, and bone parameters were
investigated. The results indicated that the short- or long-term
blueberry diet prevented Ovx-induced bone loss at the tibia; bone
histomorphometry revealed that the rats fed either short- or
long-term blueberry diet had a higher BV/TV, osteoblast number and
BFR/BS compared with those in rats in control groups. They also
observed that the bone-protective effect of the blueberry diet was
exerted through the suppression of osteoblastic cell senescence
associated with acute loss of myosin expression following Ovx. The
results from their study were in contrast with those from previous
in vitro and in vivo studies (29,32). These
other studies suggested that the effects of berry fruits or their
extract on bone metabolism were more likely to be exerted through
the suppression of bone resorption. Although previous in
vivo studies (51,52) used mature Ovx rats, the rats used in
the study by Zhang et al (56)
were young Ovx rats in pre-pubertal growth stage. The ages of rats
and the time after Ovx are important factors that may influence
bone response following Ovx (39). In
the present study, 3-month-old Ovx rat models were used to induce
bone loss. Although 3-month-old rats are regarded as mature, their
bone growth slows but has not stopped (39). Therefore, a favorable effect of VME on
bone, if any, may have been overwhelmed by the age of the rats
and/or substantial changes associated with Ovx.
In conclusion, supplementation of Ovx rats with an
anthocyanin-rich bilberry extract did not prevent Ovx-induced bone
loss, at least under the experimental conditions of the present
study. As there has been discrepancies between results from
previous studies on the effects of berry fruits or their extracts
on bone metabolism, further investigations are warranted to
determine whether the consumption of berry fruits or anthocyanins
extracted from berry fruits may be beneficial in mitigating bone
loss in postmenopausal women.
References
|
1
|
Greendale GA, Lee NP and Arriola ER: The
menopause. Lancet. 353:571–580. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lobo RA, Davis SR, De Villiers TJ, Gompel
A, Henderson VW, Hodis HN, Lumsden MA, Mack WJ, Shapiro S and Baber
RJ: Prevention of diseases after menopause. Climacteric.
17:540–556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sánchez-Rodríguez MA, Zacarías-Flores M,
Arronte-Rosales A, Correa-Muñoz E and Mendoza-Núñez VM: Menopause
as risk factor for oxidative stress. Menopause. 19:361–367. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolesnikova L, Semenova N, Madaeva I,
Suturina L, Solodova E, Grebenkina L and Darenskaya M: Antioxidant
status in peri- and postmenopausal women. Maturitas. 81:83–87.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taleb-Belkadi O, Chaib H, Zemour L, Fatah
A, Chafi B and Mekki K: Lipid profile, inflammation, and oxidative
status in peri- and postmenopausal women. Gynecol Endocrinol.
32:982–985. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Horke S and Förstermann U: Vascular
oxidative stress, nitric oxide and atherosclerosis.
Atherosclerosis. 237:208–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang YT, Chang WN, Tsai NW, Huang CC,
Kung CT, Su YJ, Lin WC, Cheng BC, Su CM, Chiang YF, et al: The
roles of biomarkers of oxidative stress and antioxidant in
Alzheimer's disease: A systematic review. BioMed Res Int.
2014:1823032014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zetterberg M: Age-related eye disease and
gender. Maturitas. 83:19–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
NIH Consensus Development Panel on
Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis
prevention, diagnosis, and therapy. JAMA. 285:785–795. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raisz LG: Pathogenesis of osteoporosis:
Concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reid IR: MenopausePrimer on the metabolic
bone diseases and disorders of mineral metabolism. John Wiley &
Sons, Inc.; pp. 165–170. 2013, View Article : Google Scholar
|
|
13
|
Lean JM, Jagger CJ, Kirstein B, Fuller K
and Chambers TJ: Hydrogen peroxide is essential for
estrogen-deficiency bone loss and osteoclast formation.
Endocrinology. 146:728–735. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wells G, Tugwell P, Shea B, Guyatt G,
Peterson J, Zytaruk N, Robinson V, Henry D, O'Connell D and Cranney
A: Osteoporosis Methodology Group and The Osteoporosis Research
Advisory Group: Meta-analyses of therapies for postmenopausal
osteoporosis. V. Meta-analysis of the efficacy of hormone
replacement therapy in treating and preventing osteoporosis in
postmenopausal women. Endocr Rev. 23:529–539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dören M, Nilsson JA and Johnell O: Effects
of specific post-menopausal hormone therapies on bone mineral
density in post-menopausal women: A meta-analysis. Hum Reprod.
18:1737–1746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Torgerson DJ and Bell-Syer SE: Hormone
replacement therapy and prevention of nonvertebral fractures: A
meta-analysis of randomized trials. JAMA. 285:2891–2897. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rossouw JE, Anderson GL, Prentice RL,
LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA,
Howard BV, Johnson KC, et al: Writing Group for the Women's Health
Initiative Investigators: Risks and benefits of estrogen plus
progestin in healthy postmenopausal women: Principal results From
the Women's Health Initiative randomized controlled trial. JAMA.
288:321–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cauley JA, Robbins J, Chen Z, Cummings SR,
Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, et
al: Women's Health Initiative Investigators: Effects of estrogen
plus progestin on risk of fracture and bone mineral density: The
Women's Health Initiative randomized trial. JAMA. 290:1729–1738.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baber RJ, Panay N, Fenton A and Group
IMSW: IMS Writing Group: 2016 IMS Recommendations on women's
midlife health and menopause hormone therapy. Climacteric.
19:109–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Camacho PM, Petak SM, Binkley N, Clarke
BL, Harris ST, Hurley DL, Kleerekoper M, Lewiecki EM, Miller PD,
Narula HS, et al: American association of clinical endocrinologists
and american college of endocrinology clinical practice guidelines
for the diagnosis and treatment of postmenopausal
osteoporosis-2016. Endocr Pract. 22 Suppl 4:1–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tucker KL, Hannan MT, Chen H, Cupples LA,
Wilson PW and Kiel DP: Potassium, magnesium, and fruit and
vegetable intakes are associated with greater bone mineral density
in elderly men and women. Am J Clin Nutr. 69:727–736.
1999.PubMed/NCBI
|
|
22
|
Chen YM, Ho SC and Woo JL: Greater fruit
and vegetable intake is associated with increased bone mass among
postmenopausal Chinese women. Br J Nutr. 96:745–751.
2006.PubMed/NCBI
|
|
23
|
Prynne CJ, Mishra GD, O'Connell MA, Muniz
G, Laskey MA, Yan L, Prentice A and Ginty F: Fruit and vegetable
intakes and bone mineral status: A cross sectional study in 5 age
and sex cohorts. Am J Clin Nutr. 83:1420–1428. 2006.PubMed/NCBI
|
|
24
|
Zalloua PA, Hsu YH, Terwedow H, Zang T, Wu
D, Tang G, Li Z, Hong X, Azar ST, Wang B, et al: Impact of seafood
and fruit consumption on bone mineral density. Maturitas. 56:1–11.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li JJ, Huang ZW, Wang RQ, Ma XM, Zhang ZQ,
Liu Z, Chen YM and Su YX: Fruit and vegetable intake and bone mass
in Chinese adolescents, young and postmenopausal women. Public
Health Nutr. 16:78–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu L, Dibley M, D'Este C, Phillips M,
Porteous J and Attia J: Food groups and risk of forearm fractures
in postmenopausal women in Chengdu, China. Climacteric. 12:222–229.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiu R, Cao WT, Tian HY, He J, Chen GD and
Chen YM: Greater intake of fruit and vegetables is associated with
greater bone mineral density and lower osteoporosis risk in
middle-aged and elderly adults. PLoS One. 12:e01689062017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen L, Xin X, Yuan Q, Su D and Liu W:
Phytochemical properties and antioxidant capacities of various
colored berries. J Sci Food Agric. 94:180–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hubert PA, Lee SG, Lee SK and Chun OK:
Dietary polyphenols, berries, and age-related bone loss: A review
based on human, animal, and cell studies. Antioxidants. 3:144–158.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li D, Wang P, Luo Y, Zhao M and Chen F:
Health benefits of anthocyanins and molecular mechanisms: Update
from recent decade. Crit Rev Food Sci Nutr. 57:1729–1741. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Welch A, MacGregor A, Jennings A,
Fairweather-Tait S, Spector T and Cassidy A: Habitual flavonoid
intakes are positively associated with bone mineral density in
women. J Bone Miner Res. 27:1872–1878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Đudarić L, Fužinac-Smojver A, Muhvić D and
Giacometti J: The role of polyphenols on bone metabolism in
osteoporosis. Food Res Int. 77:290–298. 2015. View Article : Google Scholar
|
|
33
|
Morton DJ, Barrett-Connor EL and Schneider
DL: Vitamin C supplement use and bone mineral density in
postmenopausal women. J Bone Miner Res. 16:135–140. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sahni S, Hannan MT, Gagnon D, Blumberg J,
Cupples LA, Kiel DP and Tucker KL: Protective effect of total and
supplemental vitamin C intake on the risk of hip fracture - a
17-year follow-up from the Framingham Osteoporosis Study.
Osteoporos Int. 20:1853–1861. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Canter PH and Ernst E: Anthocyanosides of
Vaccinium myrtillus (bilberry) for night vision - a systematic
review of placebo-controlled trials. Surv Ophthalmol. 49:38–50.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Head KA: Natural therapies for ocular
disorders, part two: Cataracts and glaucoma. Altern Med Rev.
6:141–166. 2001.PubMed/NCBI
|
|
37
|
Calciolari E, Donos N and Mardas N:
Osteoporotic Animal Models of Bone Healing: Advantages and
Pitfalls. J Invest Surg. 30:342–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Muthusami S, Ramachandran I, Muthusamy B,
Vasudevan G, Prabhu V, Subramaniam V, Jagadeesan A and Narasimhan
S: Ovariectomy induces oxidative stress and impairs bone
antioxidant system in adult rats. Clinica chimica acta;
international journal of clinical chemistry. 360:81–86. 2005.
View Article : Google Scholar
|
|
39
|
Kalu DN: The ovariectomized rat model of
postmenopausal bone loss. Bone Miner. 15:175–191. 1991. View Article : Google Scholar
|
|
40
|
Sakakibara H, Ogawa T, Koyanagi A,
Kobayashi S, Goda T, Kumazawa S, Kobayashi H and Shimoi K:
Distribution and excretion of bilberry anthocyanins [corrected] in
mice. J Agric Food Chem. 57:7681–7686. 2009. View Article : Google Scholar
|
|
41
|
Miyake S, Takahashi N, Sasaki M, Kobayashi
S, Tsubota K and Ozawa Y: Vision preservation during retinal
inflammation by anthocyanin-rich bilberry extract: Cellular and
molecular mechanism. Lab Invest. 92:102–109. 2012. View Article : Google Scholar
|
|
42
|
Dempster DW, Compston JE, Drezner MK,
Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR
and Parfitt AM: Standardized nomenclature, symbols, and units for
bone histomorphometry: A 2012 update of the report of the ASBMR
Histomorphometry Nomenclature Committee. J Bone Miner Res. 28:2–17.
2013. View Article : Google Scholar
|
|
43
|
Lane MA, Black A, Handy AM, Shapses SA,
Tilmont EM, Kiefer TL, Ingram DK and Roth GS: Energy restriction
does not alter bone mineral metabolism or reproductive cycling and
hormones in female rhesus monkeys. J Nutr. 131:820–827. 2001.
|
|
44
|
Matsushita H, Minami A, Kanazawa H, Suzuki
T, Subhadhirasakul S, Watanabe K and Wakatsuki A: Long-term
supplementation with young coconut juice does not prevent bone loss
but rather alleviates body weight gain in ovariectomized rats.
Biomed Rep. 6:585–591. 2017. View Article : Google Scholar
|
|
45
|
Jakesevic M, Aaby K, Borge GI, Jeppsson B,
Ahrné S and Molin G: Antioxidative protection of dietary bilberry,
chokeberry and Lactobacillus plantarum HEAL19 in mice subjected to
intestinal oxidative stress by ischemia-reperfusion. BMC Complement
Altern Med. 11:82011. View Article : Google Scholar
|
|
46
|
Mane C, Loonis M, Juhel C, Dufour C and
Malien-Aubert C: Food grade lingonberry extract: Polyphenolic
composition and in vivo protective effect against oxidative
stress. J Agric Food Chem. 59:3330–3339. 2011. View Article : Google Scholar
|
|
47
|
Kim B, Ku CS, Pham TX, Park Y, Martin DA,
Xie L, Taheri R, Lee J and Bolling BW: Aronia melanocarpa
(chokeberry) polyphenol-rich extract improves antioxidant function
and reduces total plasma cholesterol in apolipoprotein E knockout
mice. Nutr Res. 33:406–413. 2013. View Article : Google Scholar
|
|
48
|
Karlsen A, Retterstøl L, Laake P, Paur I,
Bøhn SK, Sandvik L and Blomhoff R: Anthocyanins inhibit nuclear
factor-kappaB activation in monocytes and reduce plasma
concentrations of pro-inflammatory mediators in healthy adults. J
Nutr. 137:1951–1954. 2007.
|
|
49
|
Tanabe S, Santos J, La VD, Howell AB and
Grenier D: A-type cranberry proanthocyanidins inhibit the
RANKL-dependent differentiation and function of human osteoclasts.
Molecules. 16:2365–2374. 2011. View Article : Google Scholar
|
|
50
|
Moriwaki S, Suzuki K, Muramatsu M, Nomura
A, Inoue F, Into T, Yoshiko Y and Niida S: Delphinidin, one of the
major anthocyanidins, prevents bone loss through the inhibition of
excessive osteoclastogenesis in osteoporosis model mice. PLoS One.
9:e971772014. View Article : Google Scholar
|
|
51
|
Devareddy L, Hooshmand S, Collins JK,
Lucas EA, Chai SC and Arjmandi BH: Blueberry prevents bone loss in
ovariectomized rat model of postmenopausal osteoporosis. J Nutr
Biochem. 19:694–699. 2008. View Article : Google Scholar
|
|
52
|
Zheng X, Mun S, Lee SG, Vance TM, Hubert
P, Koo SI, Lee SK and Chun OK: Anthocyanin-rich blackcurrant
extract attenuates ovariectomy-induced bone loss in mice. J Med
Food. 19:390–397. 2016. View Article : Google Scholar
|
|
53
|
Kaume L, Gbur EE, DiBrezzo R, Howard LR
and Devareddy L: Antioxidant-rich berries exert modest bone
protective effects in postmenopausal smokers without improving
biomarkers of bone metabolism. J Funct Foods. 9:202–210. 2014.
View Article : Google Scholar
|
|
54
|
Kaume L, Gilbert W, Smith BJ and Devareddy
L: Cyanidin 3-o-beta-d-glucoside improves bone indices. J Med Food.
18:690–697. 2015. View Article : Google Scholar
|
|
55
|
Joseph SV, Edirisinghe I and
Burton-Freeman BM: Berries: Anti-inflammatory effects in humans. J
Agric Food Chem. 62:3886–3903. 2014. View Article : Google Scholar
|
|
56
|
Zhang J, Lazarenko OP, Blackburn ML,
Shankar K, Badger TM, Ronis MJ and Chen JR: Feeding blueberry diets
in early life prevent senescence of osteoblasts and bone loss in
ovariectomized adult female rats. PLoS One. 6:e244862011.
View Article : Google Scholar
|