Introduction
Medicinal plants and their active compounds have
been reported to exert potent cytotoxic activity against various
types of cancer cell (1). Numerous
plants are consumed as food and are claimed by practitioners of
traditional medicine to promote health (2–4). Northeast
Thai vegetables, such as Cratoxylum formosum (CF) Dyer may
have an affect on human health by exerting antioxidant and
anticancer effects. CF belongs to the Guttiferae family and is a
plant of the tropics, cultivated in many Southeast Asian countries,
including Thailand (5). It is a local
dietary and herbal plant, and its leaves are usually consumed
fresh. CF has been adopted in folk medicine for the treatment of
fever, coughs, stomach ache and peptic ulcers (6,7). CF produces
various secondary metabolites, including phenolic compounds,
triterpenoids, flavonoids (6,8), xanthones, anthraquinones (7), chlorogenic acid, dicaffeoylquinic acids,
and ferulic acid (5). Kukongviriyapan
et al (9) detected potent
antioxidant activity in aqueous extracts of CF leaves. Other
bioactivities demonstrated by CF include anti-inflammatory
(10), antibacterial (11), antimicrobial (12) and anticancer (13). Various parts of the plant have been
found to exert anticancer effects, including the roots, bark and
leaves (14–16).
CF inhibits the proliferation of various types of
cancer cell and induces cancer cell apoptosis. CF root extract has
been reported to display activity against MCF-7 breast cancer
cells, HeLa cervical cancer cells, HT-29 colon cancer cells, and KB
oral cancer cells (6). CF leaf extract
selectively inhibited human U937 leukemia cancer cells when
compared with normal cells based on observations of DNA laddering
and nuclear morphological changes (17). Nonpunya et al (18) and Senggunprai et al (19) showed that ethanolic CF leaf extracts
inhibit cancer cell proliferation and induce apoptosis. CF
selectively increased HepG2 liver cancer cell death when compared
with normal cells by inducing caspase 3, 8 and 9, decreasing the
mitochondrial function and increasing apoptotic body formation
(18). Consistent with the effects of
CF on cholangiocarcinoma (CCA) cells, CF inhibited CCA cell
proliferation, induced cell apoptosis, triggered cell cycle arrest
at the G2/M phase, and downregulated cyclin A and cell
division cycle 25A (CDC25A) protein expression levels (19). Furthermore, CF suppressed nuclear
factor-κB and signal transducer and activator of transcription 3
nuclear translocation and transcriptional activity, and inhibited
cancer progression and metastasis (19).
Problems with increasing drug resistance and drug
toxicity have stimulated investigation into novel anticancer
compounds derived from natural sources, such as plants. As there is
limited information on the effect of CF on liver cancer, the
present study investigated the cytotoxicity and anti-migratory
effects of CF extracts and their mechanisms of action on the human
HepG2 liver cancer cell line. The results demonstrate that CF
exerts potent anticancer activity, and this may provide a novel
approach to liver cancer therapeutic strategies in future.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS) and the other cell culture reagents were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Protease inhibitor cocktail, dihydroethidium (DHE), RIPA
lysis buffer, sulforhodamine B (SRB) and a caspase 3 activity assay
kit were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). JC-1 was purchased from Cayman Chemical Company (Cayman
Chemical Company, Ann Arbor, MI, USA). The primary antibodies
against p21Cip/WAF1 (cat. no. 2947), cyclin D1 (cat. no.
2978), β-actin (cat. no. 4970), and anti-rabbit IgG horseradish
peroxidase (HRP)-linked antibody (cat. no. 7074) antibody were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
iScript reverse transcription Supermix for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
SsoFast EvaGreen Supermix were supplied by Bio-Rad Laboratories,
Inc. (Hercules, CA, USA).
Plant material and extraction
Edible leaves of CF were collected from Udon Thani
Province, Thailand, in May 2014. Identification was performed by
the Pharmaceutical Laboratory Service Center, Faculty of
Pharmaceutical Science, Prince of Songkla University (Hat Yai,
Thailand; specimen no. SKP083030601) was deposited at the Prince of
Songkla University Herbarium. Dried leaves were extracted using 50%
ethanol, filtered, evaporated and lyophilized to obtain the dry
extract. The yield was 12.25% of the starting dry weight of the
leaves. The CF leaf extract was maintained at −20°C until use.
Cell lines and cell culture
The human HepG2 liver cancer cell line was obtained
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and maintained according to the recommendations of the ATCC at 37°C
and 5% CO2 in complete DMEM, supplemented with 100 U/ml
penicillin G, 100 µg/ml streptomycin and 10% FBS. Subsequent to
reaching confluence, the HepG2 cancer cells were detached using
0.25% trypsin-EDTA and 1×106 cells were seeded into the
same complete medium. The DMEM medium was replaced every 3
days.
Cell viability assay
The SRB assay was used to determine the effect of CF
on the viability of HepG2 cancer cells, as previously described
(20). Briefly, cells were cultured in
a 96-well plate for 24 h and fresh medium containing various
concentrations of CF (0–500 µg/ml) were added. Subsequent to 24–48
h, cells were fixed with ice-cold 10% trichloroacetic acid at 4°C,
stained with 0.4% SRB for 30 min at room temperature, and dissolved
with 10 mM Tris base solution. Absorbance was measured at a filter
wavelength of 540 nm using a spectrophotometer (Opsys MR™
Microplate Reader; Dynex Technologies, Chantilly, VA, USA).
Clonogenic assay
The colony formation assay was used to determine the
effect of CF on the cell regrowth of HepG2 cancer cells, as
previously described (20). Briefly,
500 viable HepG2 cancer cells were seeded in 6-well plates (500
cells/well) for 24 h, treated with various concentrations of CF
(0–200 µg/ml) for 24 h, washed once with phosphate-buffered saline
(PBS) and resuspended in fresh medium. HepG2 cells were grown for
another 24 days. Subsequently, the DMEM medium was discarded, the
cells were washed with PBS buffer three times, fixed with 100%
methanol at −20°C, stained with 0.5% crystal violet in 100%
methanol for 1 h at room temperature, washed with tap water, and
the colonies were viewed and captured using a digital camera (Nikon
D3100). Colonies containing >50 individual cells were counted
using Image-Pro Plus software (Media Cybernetics, L.P., Silver
Spring, MA, USA).
Wound healing assay
Cell migration was assessed using a wound healing
assay, as previously described (20).
Briefly, HepG2 cancer cells were seeded into 24-well plates for 24
h. Cells were scratched using a sterile 0.2-ml pipette tip, certain
cells were untreated and others were treated with different
concentrations of CF (0–100 µg/ml). Images were obtained from 0 to
48 h. The closing of the scratched wound was determined by image
capture of the uncovered area along the scratch. The wound distance
was calculated by dividing the area by the length of the scratch,
and this was compared with the untreated control group. Cell
migration was monitored by phase contrast microscopy (Nikon Eclipse
TS100 inverted microscope; magnification, ×10).
Reactive oxygen species (ROS)
production assay
Intracellular ROS generation was measured using DHE,
the cell-permeable fluorescent probe. Briefly, HepG2 cancer cells
were seeded and cultured in black 96-well plates for 24 h. The
cells were treated with CF (0–250 µg/ml) plus 25 µM DHE in
serum-free medium and maintained at 37°C for 90 min in a 5%
CO2 incubator in the dark. The fluorescence intensity
was measured on a fluorescence microplate reader at 518 nm
(excitation) and 605 nm (emission). Data were expressed as the
percentage of ROS relative to untreated control groups.
Caspase 3 activity assay
Caspase 3 activity was measured using caspase 3
fluorimetric assay kits according to the manufacturer's
instructions. Briefly, HepG2 cancer cells were treated with CF
(0–100 µg/ml) for 24 h, lysed, and the protein concentrations were
measured using Bradford's reagent (Bio-Rad Laboratories, Inc.). The
caspase 3 activity reactions were composed of cell lysates and
buffer containing the caspase 3 substrate, acetyl Asp-Glu-Val-Asp
7-amido-4-methylcoumarin (AMC; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). These mixtures were incubated at 37°C in the dark
for 90 min. The AMC fluorescence intensity was read using a
fluorescence plate reader at 360 nm (excitation) and 460 nm
(emission). AMC served as a standard to calculate the caspase 3
activity.
Measurement of mitochondrial membrane
potentiall (ΔΨm)
JC-1, the lipophilic cationic fluorescent dye, was
used to measure changes in ΔΨm. Briefly, HepG2 cancer cells were
seeded into black 96-well plates for 24 h, treated with CF (0–100
µg/ml) for 24 h, loaded with JC-1, and incubated for 30 min at 37°C
in the dark. Subsequently, the cells were rinsed with PBS buffer
and added 200 µl JC1 assay buffer and then read the fluorescent
intensity. The ΔΨm was measured using a fluorescence plate reader
at 485 nm (excitation) and 535 nm (emission). In normal cells, JC-1
transforms to J-aggregate and in dead cells, JC-1 exists in
monomeric form. The fluorescence intensity ratio of J-aggregates to
JC-1 monomers served as an indicator of the depolarization of
ΔΨm.
Gelatin zymography analysis
The expression levels of matrix metalloproteinase-9
(the MMP-9) in conditioned medium were detected by gelatin
zymography. Briefly, HepG2 cancer cells were seeded in 24-well
culture plates for 24 h. Subsequently, HepG2 cancer cells were
cultured in complete DMEM medium containing different
concentrations of CF (0–100 µg/ml) at 37°C for 48 h. The culture
medium was then collected, centrifuged at 400 × g for 5 min
at 4°C, and the protein concentration was measured using Bradford's
reagent. The protein samples were combined with 2X non-reducing
sample buffer without heating, loaded onto a 10% SDS-polyacrylamide
gel (Bio-Rad Laboratories, Inc.) containing 0.1% (w/v) gelatin and
subjected to electrophoresis at 120 V for 1.5 h. Following
electrophoresis, the gel was washed three times with 2.5% Triton
X-100 to remove the SDS and incubated with developing buffer [50 mM
Tris-HCl buffer (pH 7.45) and 10 mM CaCl2] at 37°C for
12 h. The gels were stained with 0.1% Coomassie Brilliant Blue
R-250 for 1 h at room temperature and washed with destaining
solution until clear bands were observable against an intensely
stained background.
Gene expression assay
Briefly, the HepG2 cancer cells were seeded in
6-well plates for 24 h and treated with CF for 24 h. RNA was
isolated and cDNA was prepared. PCR amplification was performed
using specific primers for ras-related C3 botulinum toxin substrate
1 (rho family, small GTP binding protein Rac1) (RAC1) and cyclin
dependent kinase 6 (CDK6), and ACTB served as an internal control.
The PCR primer sequences are presented in Table I.
 | Table I.Polymerase chain reaction primer
sequences. |
Table I.
Polymerase chain reaction primer
sequences.
|
| Primers |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| RAC1 |
5′ATG-TCC-GTG-CAA-AGT-GGT-ATC3′ |
5′CTC-GGA-TCG-CTT-CGT-CAA-ACA3′ |
| CDK6 |
5′GCT-GAC-CAG-CAG-TAC-GAA-TG3′ |
5′GCA-CAC-ATC-AAA-CAA-CCT-GAC-C3′ |
| ACTB |
5′CAT-GTA-CGT-TGC-TAT-CCA-GGC3′ |
5′CTC-CTT-AAT-GTC-ACG-CAC-GAT3′ |
RT-qPCR was performed in a final reaction volume of
20 µl containing SYBR-Green PCR Master Mix, the target gene and the
internal control, ACTB under the following conditions: Denaturation
at 95°C for 3 min; amplification (40 cycles) at 95°C for 15 sec and
60°C for 30 sec. Expression of each gene was monitored using an
Applied Biosystems® StepOne™ real-time PCR system
(Thermo Fisher Scientific, Inc.). Differences in gene expression
levels were calculated using the 2−ΔΔCq method for
relative quantification, and expressed as the fold change relative
to the untreated control (21).
Protein extraction and western blot
analysis
Briefly, HepG2 cells were treated with 100 µg/ml CF
for 24 h, lysed with RIPA lysis buffer and centrifuged at 10,000 ×
g at 4°C for 30 min. The supernatant was collected and the
protein concentration was determined. A sample of 20 µg total
protein was then separated by 12% SDS-polyacrylamide gel
electrophoresis (120 V for 1.5 h) and transferred to PVDF membranes
(Immobilon®; EMD Millipore, Billerica, MA, USA). The
membranes were incubated with each primary antibody
(p21Cip/WAF1, cyclin D1 and ACTB), at a dilution of
1:2,500, overnight and with the HRP-conjugated secondary antibody
(dilution, 1:5,000) for 2 h. Bands were detected using an enhanced
Clarity™ Western ECL Substrate (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical comparison of the control and CF groups
was performed using Student's t-test and one-way analysis of
variance, followed by Tukey's post-hoc test. The analyses were
conducted using SigmaStat software version 3.5 (Systat Software
Inc., San Jose, CA, USA) and values are expressed as the mean ±
standard error of the mean of three determinations. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of CF on cytotoxicity and
colony formation efficacy in HepG2 liver cancer cells
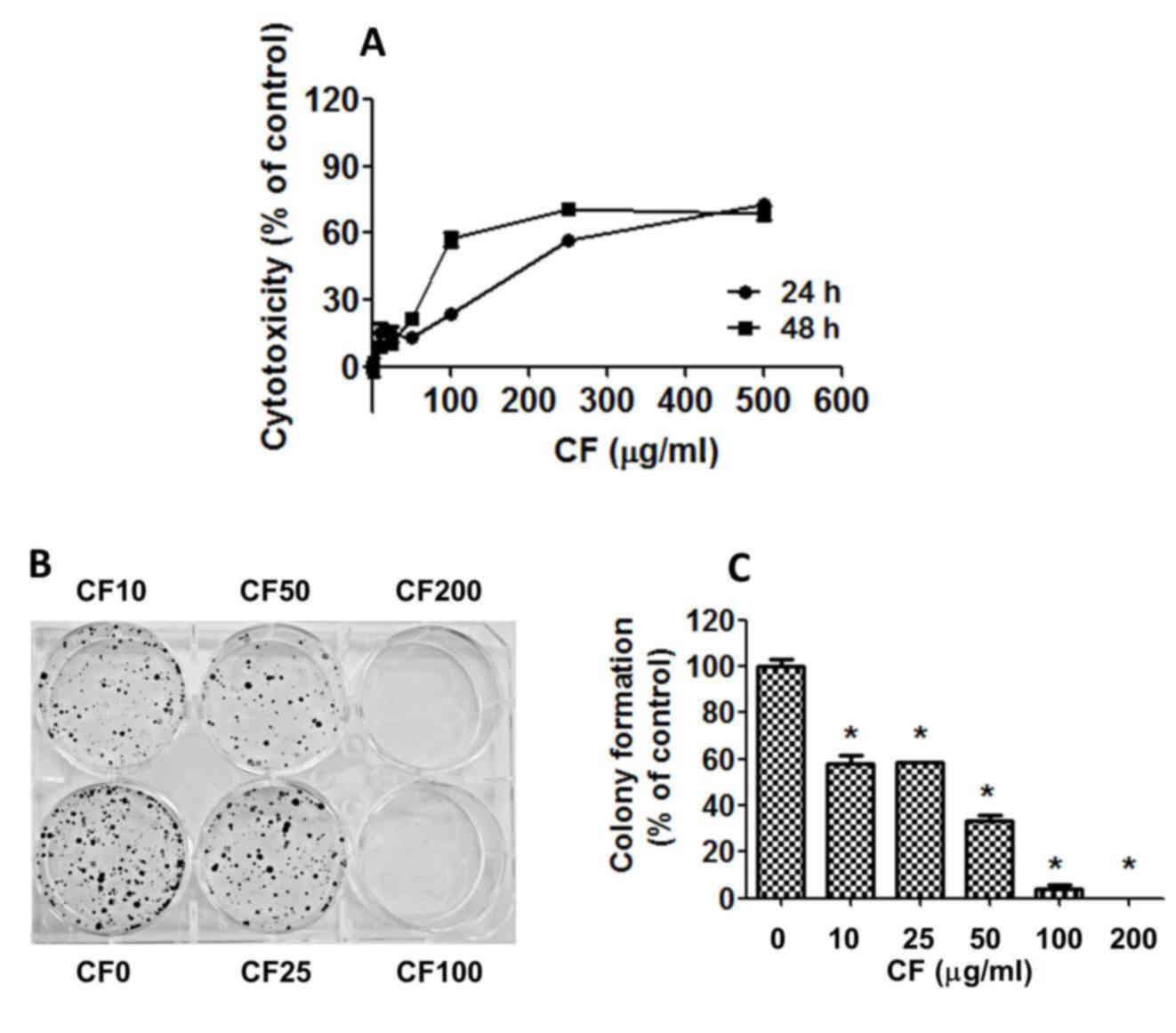
To assess the cytotoxicity of CF on human HepG2
liver cells, the cells were cultured with various concentrations of
CF for 24–48 h and the cytotoxicity was determined using the SRB
assay (Fig. 1A). The results indicate
that cell growth was strongly inhibited in a dose- and
time-dependent manner with half maximal inhibitory concentration
(IC50) values of 219.03±9.96 µg/ml at 24 h and
124.90±6.86 µg/ml at 48 h (Fig.
1A).
To determine the effect of CF on the replicative
potential and longer term viability of HepG2 cells, a colony
formation assay was used. CF caused a dose-dependent decline in the
colony forming ability of HepG2 cells with IC50 values
of 20.06±1.52 µg/ml (Fig. 1B and C).
CF inhibited cell regrowth at a lower concentration when compared
with the CF concentration that induces cancer cell death. CF
exhibited cytotoxic and antiproliferative effects against the HepG2
liver cancer cells.
Effects of CF on liver cancer cell
migration
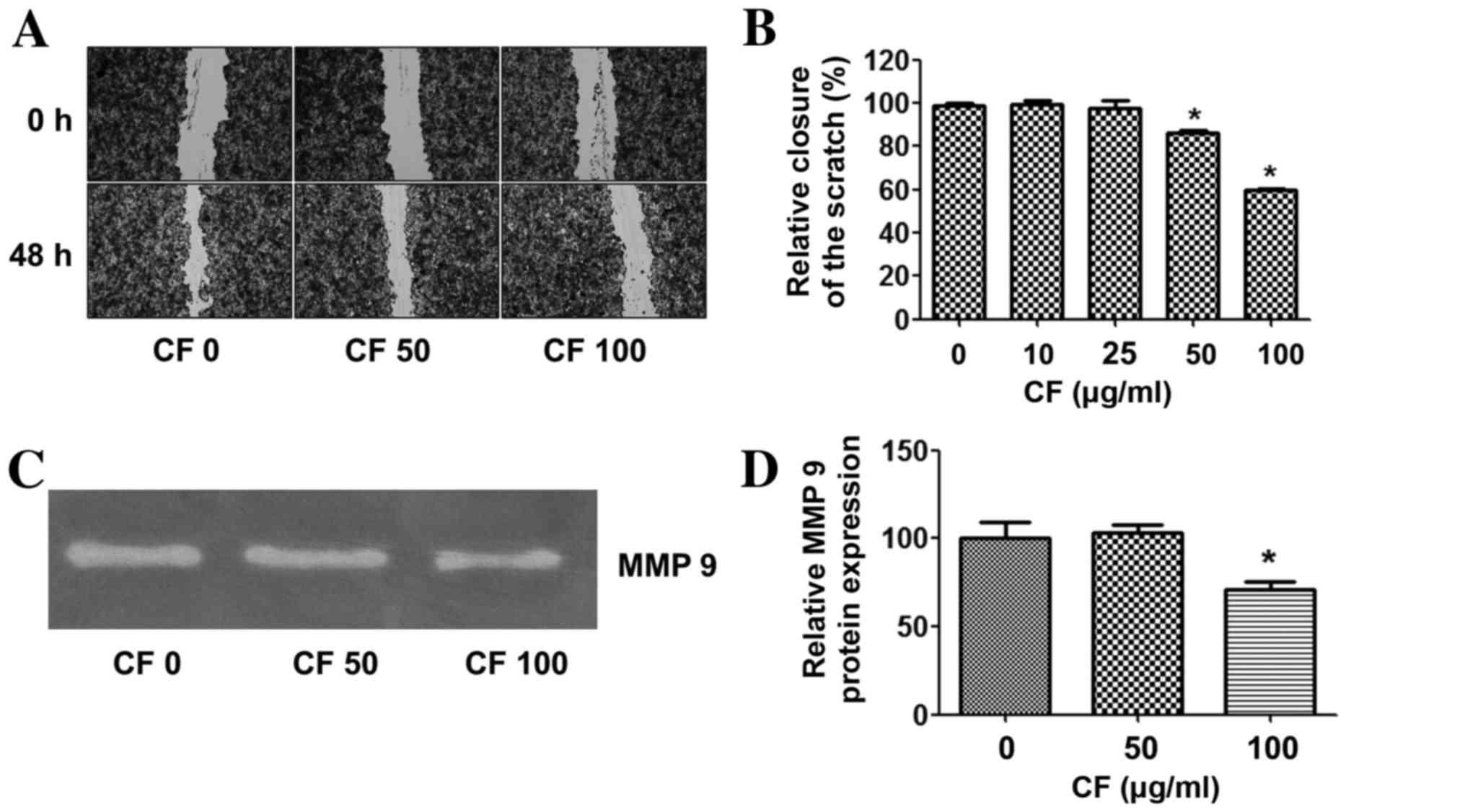
To determine whether CF inhibits cancer cell
migration, a wound healing assay was performed. The results
demonstrate that CF significantly inhibited cancer cell migration
in a dose-dependent manner. At a concentration of 100 µg/ml, CF
inhibited HepG2 cancer cell migration by ~40% when compared with
the untreated control group (Fig. 2A and
B).
The effects of CF on the expression of
invasion-linked matrix metalloproteinase-9 (MMP-9) were then
assessed. The expression level of MMP-9 was relatively high in the
HepG2 cancer cells in the untreated control group. CF treatment
suppressed MMP-9 in a dose-dependent manner, and significantly
decreased MMP-9 at a concentration of 100 µg/ml when compared with
the untreated control group (Fig. 2C and
D).
Effects of CF on ROS formation,
caspase 3 activity and ΔΨm in HepG2 liver cancer cells
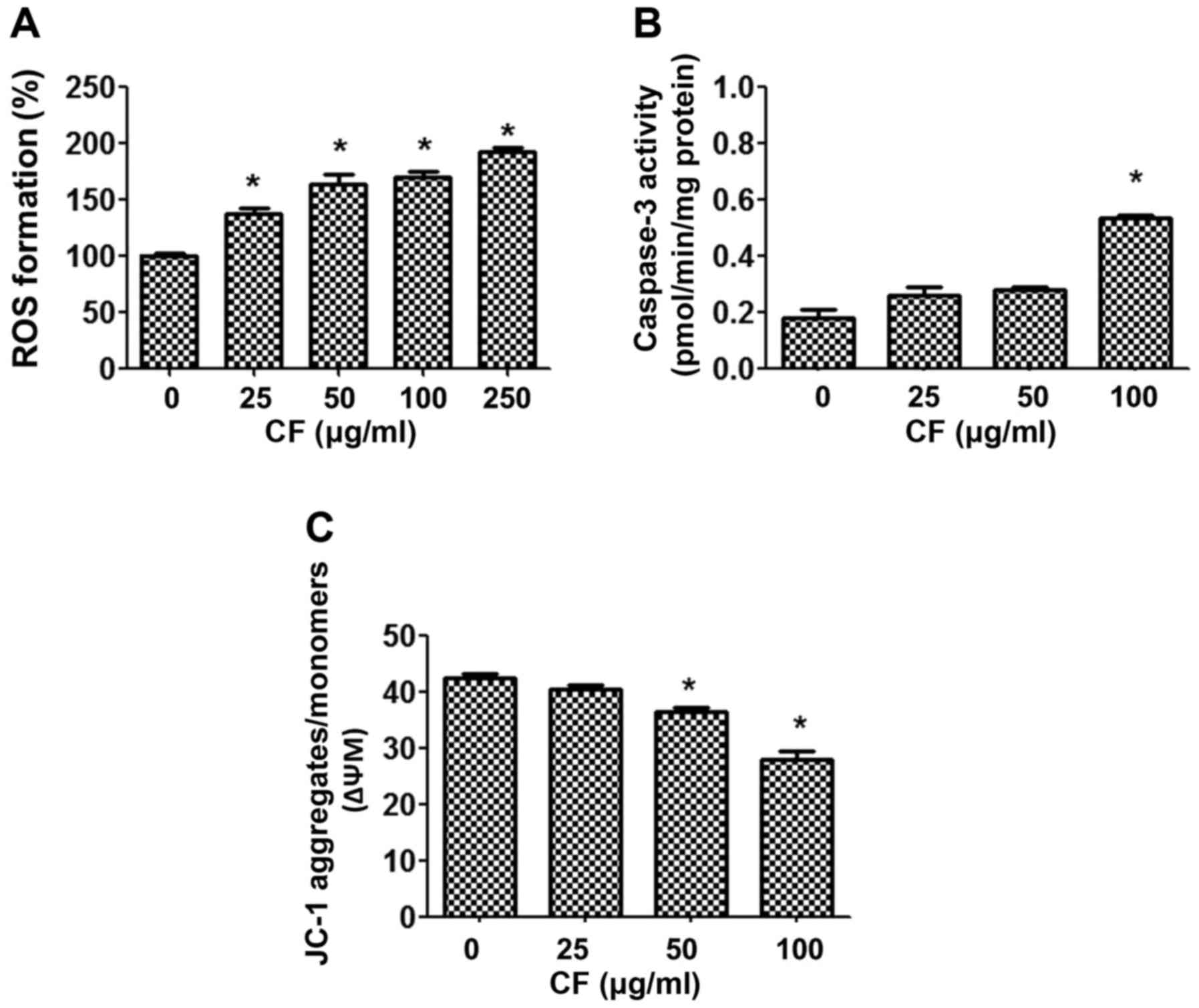
To establish the mechanism by which CF causes cancer
cell death, the intracellular accumulation of ROS was examined
using a DHE-enhanced fluorescent probe. Upon treatment of the HepG2
cancer cells with CF, ROS were released in a dose-dependent manner
(Fig. 3A). CF induced the production
of ROS in a dose-dependent manner when compared with the untreated
control group (P<0.05).
In addition, the involvement of mitochondria in
CF-induced cytotoxicity was determined. The function of
mitochondria was investigated by measuring caspase 3 activities,
and mitochondrial dysfunction using the fluorescent dye JC-1. The
results indicated that CF treatment activates caspase 3 activities
in a dose-dependent manner (Fig. 3B).
Furthermore, CF depolarized the ΔΨm, as shown by the decline in the
JC-1 aggregates/JC-1 monomers ratio. The lowest concentration at
which the decrease in ΔΨm was significant was 50 µg/ml (Fig. 3C).
Effects of CF on RAC1 and downstream
gene expression, and protein-associated apoptosis
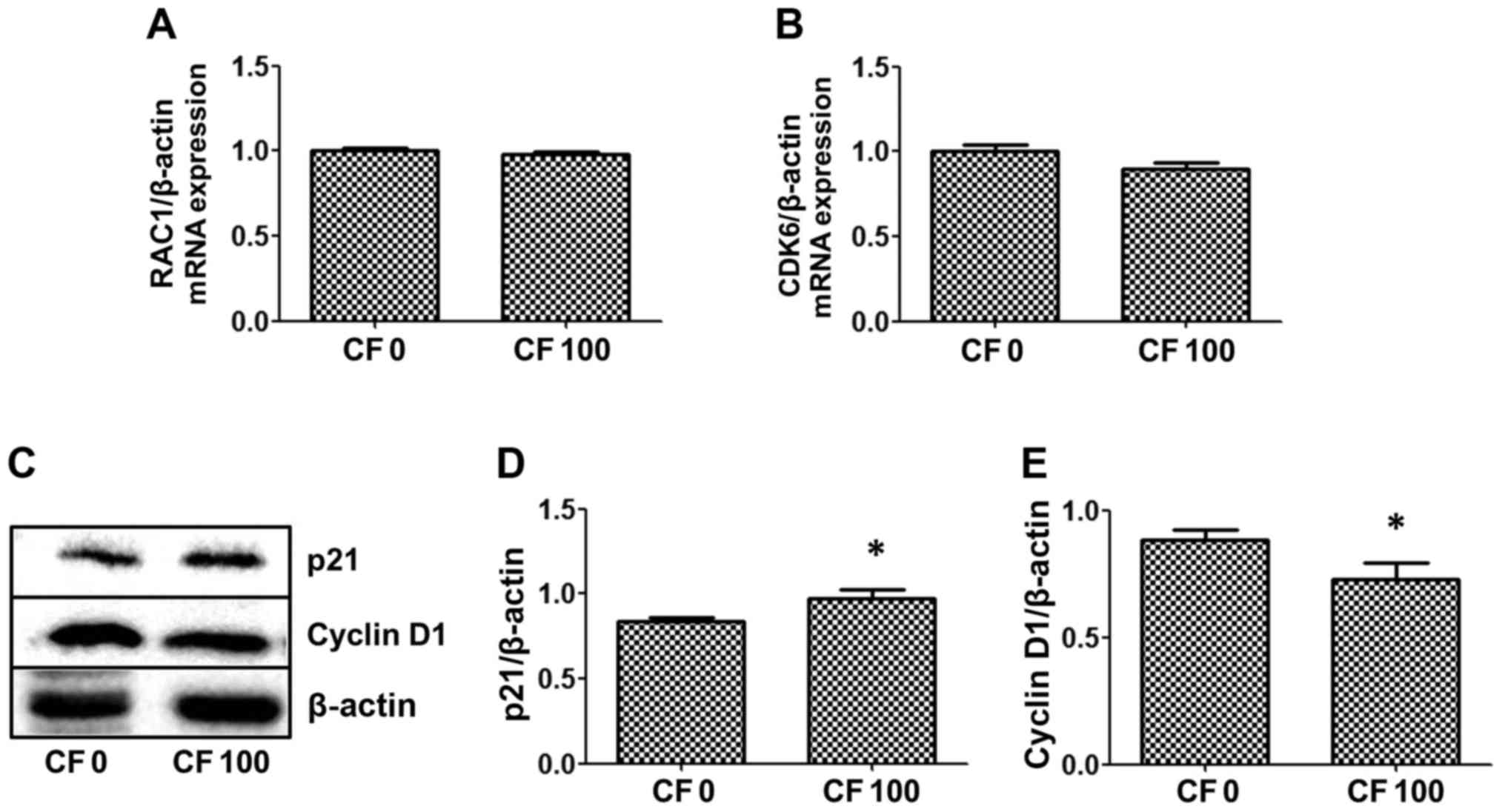
Whether CF inhibits mRNA expression of the cell
cycle regulator, RAC1 and the downstream gene, CDK6 was
investigated. CF did not alter the expression levels of either of
these genes in the HepG2 cells (Fig. 4A
and B). Proteins, p21 and cyclin D1, which are associated with
cell survival, were examined by western blot analysis to determine
whether CF inhibits HepG2 cell proliferation. The results indicated
that CF significantly induces p21 expression to inhibit the cell
cycle, which is correlated with a reduction in cyclin D1 protein
expression in HepG2 cells (Fig. 4C-E).
The present results found that CF causes HepG2 cancer cell death by
stimulating the cell cycle protein inhibitor, p21, and reducing
expression levels of the cell cycle protein, cyclin D1.
Discussion
In the present study, the mechanism by which CF
reduces cell proliferation, decreases cell migration, and enhances
cell death was investigated in the human HepG2 liver cancer cell
line. The results demonstrate that CF-induced cancer cell death may
be due to induction of intracellular ROS, leading to altered
mitochondrial function and increased caspase 3 activity. In
addition, CF increased p21 expression levels and decreased cyclin
D1 protein expression levels. CF also inhibited migration by
decreasing the level of MMP-9 protein expression. This may
represent the mechanism of cancer cell sensitization and killing.
Hence, CF may yield compounds of potential use in the prevention
and treatment of liver cancer.
Cancer is uninhibited cell growth, accelerated
angiogenesis, and stimulated invasion and metastasis (22). Mutations in genes cause cancer by
accelerating cell division or inhibiting normal controls on the
system, such as cell cycle arrest or programmed cell death
(22). Accordingly, two of the
necessary activities of an anticancer compound are to interrupt the
uncontrolled cell proliferation and accelerate cell death of cancer
cells (23). Medicinal herbs and
dietary plants are a popular starting point for anticancer drug
development. In the current study, low concentrations of CF exerted
a marked effect on the induction of liver cancer cell death and
inhibition of colony formation. CF appeared to inhibit the growth
of HepG2 cancer cells. Previous in vitro studies revealed
that CF inhibits growth and induces apoptosis in numerous types of
cancer cell, including liver (18),
oral (24), bile duct (19) and cervical (25). Senggunprai et al (19) demonstrated that CF was potently
cytotoxic against CCA KKU-M156 cells, induced cell apoptosis and
inhibited colony formation.
To elucidate the underlying mechanism of cell growth
inhibition, cell cycle distribution was evaluated using RAC1
and CDK6 gene expression, and cell cycle-associated
proteins, p21 and cyclin D1; their expression levels were measured
in HepG2 cancer cells. The results indicated that CF did not alter
RAC1 or CDK6 expression levels in the HepG2 cells. Conversely, CF
extracts induced p21 expression and downregulated cyclin D1 protein
expression. p21 is a cyclin-dependent kinase inhibitor protein
essential for inhibiting cellular growth and inducing apoptosis
(26). Cyclin D1 binds and activates
CDK6, and thus promotes cell cycle G1/S transitions
(27). The current results indicated
that CF activates cancer cell death or inhibits cancer cell
proliferation by increasing p21 protein expression and inhibiting
cylin D1 protein expression. Similarly, CF extracts have been shown
to induce cell cycle arrest in CCA cells at the G2/M
phase, and downregulate cyclin A and CDC25A protein expression
(19). These results indicate that CF
exerts potent activity against human HepG2 cancer cells.
Various studies have screened for compounds that
trigger apoptosis by measuring caspase activity and mitochondrial
membrane potential (28). Caspase
enzymes are proteases that are used to determine whether apoptosis
is being triggered via the intrinsic or extrinsic pathway (29). Caspase 3 activity is the final step in
the two pathways. Results from the present study indicate that CF
induces the late stages of apoptosis because an increase in caspase
3 activity and a decrease in ΔΨm were observed in the HepG2 cancer
cells. The present results demonstrate that CF induces liver cancer
cell apoptosis. ROS are generated by cellular oxidative processes
and trigger different cellular responses, such as cell cycle
arrest, apoptosis or necrosis, depending on the intensity of the
oxidative damage (30). Previous
results have shown that CF exerts antioxidant activity, allowing it
to suppress inflammation and act as a chemopreventive agent. The
current results demonstrate that high concentrations of CF cause
ROS formation in HepG2 cancer cells. Therefore the method by which
CF induces ROS formation in cancer cells was examined in the
current study.
Metastasis is the process by which a tumor cell
leaves the primary tumor, travels to a distant site via the
circulatory system, and establishes a secondary tumor. Such
metastases are the cause of the majority of cancer-associated
mortalities (31,32). The process involves a cascade of
events, including cell adhesion, degradation of the extracellular
matrix, cell movement, cell motility and invasion (33). A compound with the ability to block the
metastasis-associated signaling pathway may be a potential
candidate for chemotherapy. It has been reported that activation of
the Rho family GTPase, RAC1 is a critical event in the integrin-
and growth factor-mediated regulation of cellular migration and
adhesion, implicating the hyperactivation of these proteins in the
progression of metastatic disease (34). The current study found that CF did not
alter RAC1 expression, and may therefore regulate other
genes and proteins. Protein MMP-9 is crucial in tumor invasion and
metastasis. Thus, it was hypothesized that CF may reduce the
ability of HepG2 cancer cells to migrate and invade by decreasing
the expression level of MMP-9. In the present study, treatment with
CF decreased cancer cell migration in HepG2 cancer cells. It should
be noted that the anti-migratory effect of CF was detected at lower
concentrations than the concentrations that inhibited cell growth.
These results indicate that CF suppresses the metastatic potential
of cancer cells, at least in part, by modulating the MMP-9
signaling pathway.
CF accelerates liver cancer cell death and reduces
cancer cell migration, however, the active compounds in this crude
extract have yet to be identified. It has been previously reported
that CF has a high content of phenolic acids and flavonoids. These
compounds are widely distributed in plants, exert antioxidant
effects, and possess the potential to reduce the risk of cancer
(9,13).
Various phenolic acids and flavonoids were identified in the
extracts, with myricetin, syringic acid, and luteolin identified as
the main components (19). Further
studies are required to isolate and identify the compound(s)
contributing to the anticancer properties of the CF extract.
In conclusion, CF leaf extract exerts cytotoxic
activity against the HepG2 liver cancer cell line in in
vitro assays. Furthermore, CF induced apoptosis, in part, by
inhibiting proliferation. CF represents a potentially important
anticancer agent, inhibiting cancer cell proliferation and inducing
cancer cell death by enhancing p21 protein expression levels,
blocking the cell cycle-associated protein, cyclin D1, and inducing
the apoptotic cell death pathway. Additionally, CF inhibits
migration of HepG2 cancer cells by reducing MMP-9 protein
expression levels. Additional studies are required to characterize
the effects of the crude CF extract on other cancer cell lines and
to isolate the phytoconstituents responsible for these effects.
Further in vivo investigation of the mechanism(s) of action
and toxicity are also required before this medicinal plant or its
constituents become a novel option for the treatment of liver
cancer.
Acknowledgements
The present study was financially supported by the
Office of the Higher Education Commission (grant no.
2559A10962004), the Office of Thai Traditional Medical Knowledge
Fund, a Mahasarakham University 2016 Thailand Research Fund (grant
no. TRG5780254), and the National Research Council of Thailand
(grant no. 2559A10902073). The authors thank Dr. Tim Cushnie (MSU
Faculty of Medicine) for language editing of the manuscript. The
authors acknowledge Mahasarakham University Faculty of Science
(Maha Sarakham, Thailand) for equipment support and laboratory
space.
Glossary
Abbreviations
Abbreviations:
|
CF
|
Cratoxy formosum
|
|
CDK6
|
cyclin dependent kinase 6
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
ΔΨm
|
mitochondrial membrane potential
|
|
RAC1
|
ras-related C3 botulinum toxin
substrate 1 (rho family, small GTP binding protein Rac1)
|
References
|
1
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pereira A and Maraschin M: Banana (Musa
spp) from peel to pulp: Ethnopharmacology, source of bioactive
compounds and its relevance for human health. J Ethnopharmacol.
160:149–163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rozza AL and Pellizzon CH: Essential oils
from medicinal and aromatic plants: A review of the
gastroprotective and ulcer-healing activities. Fundam Clin
Pharmacol. 27:51–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sucher NJ and Carles MC: A pharmacological
basis of herbal medicines for epilepsy. Epilepsy Behav. 52:308–318.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maisuthisakul P, Pongsawatmanit R and
Gordon MH: Antioxidant properties of Teaw (Cratoxylum formosum
Dyer) extract in soybean oil and emulsions. J Agric Food Chem.
54:2719–2725. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
N KC Boonnak, Chantrapromma S,
Ponglimanont C, Fun HK, Kanjana and Opas ALS: Bioactive prenylated
xanthones and anthraquinones from Cratoxylum formosum ssp.
Pruniflorum. Tetrahedron. 64:102006.
|
|
7
|
Duan YH, Dai Y, Wang GH, Zhang X, Chen HF,
Chen JB, Yao XS and Zhang XK: Bioactive xanthones from the stems of
Cratoxylum formosum ssp. Pruniflorum. J Nat Prod. 73:1283–1287.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maisuthisakul P and Gordon MH:
Characterization and storage stability of the extract of Thai mango
(Mangifera indica Linn. Cultivar Chok-Anan) seed kernels. J Food
Sci Technol. 51:1453–1462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kukongviriyapan U, Luangaram S,
Leekhaosoong K, Kukongviriyapan V and Preeprame S: Antioxidant and
vascular protective activities of Cratoxylum formosum, Syzygium
gratum and Limnophila aromatica. Biol Pharm Bull. 30:661–666. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sripanidkulchai K, Teepsawang S and
Sripanidkulchai B: Protective effect of Cratoxylum formosum extract
against acid/alcohol-induced gastric mucosal damage in rats. J Med
Food. 13:1097–1103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raksat A, Laphookhieo S, Cheenpracha S,
Ritthiwigrom T and Maneerat W: Antibacterial compounds from the
roots of Cratoxylum formosum spp. Pruniflorum. Nat Prod Commun.
9:1487–1489. 2014.PubMed/NCBI
|
|
12
|
Suddhasthira T, Thaweboon S, Dendoung N,
Thaweboon B and Dechkunakorn S: Antimicrobial activity of
Cratoxylum formosum on Streptococcus mutans. Southeast Asian J Trop
Med Public Health. 37:1156–1159. 2006.PubMed/NCBI
|
|
13
|
Waiyaput W, Payungporn S, Issara-Amphorn J
and Panjaworayan NT: Inhibitory effects of crude extracts from some
edible Thai plants against replication of hepatitis B virus and
human liver cancer cells. BMC Complement Altern Med. 12:2462012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kowsalya R, Kaliaperumal J, Vaishnavi M
and Namasivayam E: Anticancer activity of Cynodon dactylon L. Root
extract against diethyl nitrosamine induced hepatic carcinoma.
South Asian J Cancer. 4:83–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moore J, Yousef M and Tsiani E: Anticancer
effects of rosemary (Rosmarinus officinalis L.) Extract and
rosemary extract polyphenols. Nutrients. 8:pii:E7312016. View Article : Google Scholar
|
|
16
|
Nair SV, Hettihewa M and Rupasinghe HP:
Apoptotic and inhibitory effects on cell proliferation of
hepatocellular carcinoma HepG2 cells by methanol leaf extract of
Costus speciosus. Biomed Res Int. 2014:6370982014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Machana S, Weerapreeyakul N, Barusrux S,
Thumanu K and Tanthanuch W: FTIR microspectroscopy discriminates
anticancer action on human leukemic cells by extracts of Pinus
kesiya; Cratoxylum formosum ssp. Pruniflorum and melphalan.
Talanta. 93:371–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nonpunya A, Weerapreeyakul N and Barusrux
S: Cratoxylum formosum (Jack) Dyer ssp. Pruniflorum (Kurz) Gogel.
(Hóng yá mù) extract induces apoptosis in human hepatocellular
carcinoma HepG2 cells through caspase-dependent pathways. Chin Med.
9:122014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Senggunprai L, Thammaniwit W,
Kukongviriyapan V, Prawan A, Kaewseejan N and Siriamornpun S:
Cratoxylum formosum extracts inhibit growth and metastasis of
cholangiocarcinoma cells by modulating the NF-kB and STAT3
pathways. Nutr Cancer. 68:328–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buranrat B, Senggunprai L, Prawan A and
Kukongviriyapan V: Simvastatin and atorvastatin as inhibitors of
proliferation and inducers of apoptosis in human cholangiocarcinoma
cells. Life Sci. 153:41–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schwartz GK and Shah MA: Targeting the
cell cycle: A new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Promraksa B, Daduang J, Chaiyarit P,
Tavichakorntrakool R, Khampitak T, Rattanata N, Tangrassameeprasert
R and Boonsiri P: Cytotoxicity of cratoxylum formosum subsp.
Pruniflorum Gogel extracts in oral cancer cell lines. Asian Pac J
Cancer Prev. 16:7155–7159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Promraksa B, Daduang J, Khampitak T,
Tavichakorntrakool R, Koraneekit A, Palasap A, Tangrassameeprasert
R and Boonsiri P: Anticancer potential of cratoxylum formosum
subsp. Pruniflorum (Kurz.) Gogel extracts against cervical cancer
cell lines. Asian Pac J Cancer Prev. 16:6117–6121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koceva-Chyła A, Jedrzejczak M, Skierski J,
Kania K and Jóźwiak Z: Mechanisms of induction of apoptosis by
anthraquinone anticancer drugs aclarubicin and mitoxantrone in
comparison with doxorubicin: Relation to drug cytotoxicity and
caspase-3 activation. Apoptosis. 10:1497–1514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu MJ, Wang Z, Li HX, Wu RC, Liu YZ and
Wu QY: Mitochondrial dysfunction as an early event in the process
of apoptosis induced by woodfordin I in human leukemia K562 cells.
Toxicol Appl Pharmacol. 194:141–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barzilai A and Yamamoto K: DNA damage
responses to oxidative stress. DNA Repair (Amst). 3:1109–1115.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morrissey MA, Hagedorn EJ and Sherwood DR:
Cell invasion through basement membrane: The netrin receptor DCC
guides the way. Worm. 2:e261692013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: Integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|