Introduction
Osteoporosis is a skeletal disorder characterized by
compromised bone strength that predisposes affected individuals to
an increased risk of fracture. Osteoporosis is more common in women
than in men and greatly reduces the healthy life years expectancy,
defined as the number of years that a person is expected to live in
a healthy condition (1,2). Although osteoporosis is a multifactorial
disorder, estrogen deficiency following menopause is strongly
associated with rapid bone resorption and loss of bone density
(3). Thus, hormone replacement therapy
(HRT) has been regarded as one treatment option for the prevention
of postmenopausal bone loss (4,5), and some
studies have indeed reported that HRT reduces the risk of fractures
(6). However, findings from Women's
Health Initiative trials revealed that the risks associated with
HRT outweighed the benefits, despite the role of HRT in preventing
osteoporotic fracture (7–10). Although related societies recommend HRT
as a first-line option for the prevention and treatment of
osteoporosis in postmenopausal women <60 years, there are still
many concerns about HRT and the potential risks to various aspects
of women's health (11,12).
Alternatives to HRT for the treatment of
postmenopausal osteoporosis include bisphosphonates, selective
estrogen receptor modulators (SERMs), teriparatide and denosumab
(13). However, bisphosphonate
alendronate is only effective in osteoporotic women (T-score
<-2.5) without previous fractures (14), and the SERM raloxifene fails to show
any non-vertebral fracture protection (15). In addition, there is concern about the
side effects of gastroesophageal reflux disease, osteonecrosis of
the jaw and atypical femoral fractures for alendronate and venous
thromboembolism for raloxifene (13).
These unfavorable findings associated with pharmaceutical
alternatives to HRT have heightened interest in diet and lifestyle
changes that can minimize postmenopausal bone loss, and thereby
decrease the necessity for drugs in the treatment of
osteoporosis.
Cocos nucifera Linn. (Arecaceae), also known
as coconut, is widely used in traditional medicine (16,17). Young
coconut juice (YCJ), defined as the juice extracted from the fruit
of a 6-month-old coconut, is consumed by women in Thailand to
alleviate symptoms associated with menopause (18–20), which
suggests that YCJ may have phytoestrogenic effects and may prevent
postmenopausal osteoporosis. The authors demonstrated that
short-term (6-week) supplementation with YCJ in ovariectomized rats
significantly reduced bone loss, which suggests that YCJ
supplementation may mitigate the rapid bone loss observed in women
during early menopause (21). In the
present study, the effects of long-term (12-week) YCJ
supplementation on bone metabolism were examined in ovariectomized
rats to investigate whether such supplementation of YCJ could help
to prevent postmenopausal osteoporosis.
Materials and methods
Preparation of YCJ
The composition of YCJ has been reported by
Pungmatharith (22) previously. Dried
powder of YCJ was prepared as previously described (18,21).
Briefly, YCJ obtained from the fruit of ~6-month-old coconuts was
freeze dried and then reconstituted in distilled water, which
resulted in 5X-concentrated YCJ. The final solutions were stored at
−20°C until use.
Animals and diets
Female Wistar rats (10-weeks-old) were purchased
from Japan SLC, Inc. (Hamamatsu, Japan). Upon arrival, they were
housed in a temperature (23±1°C)- and humidity (55±5%)-controlled
room under a 12 h light-dark cycle and fed standard rodent chow and
water ad libitum. Following acclimatization for 2 weeks, the
rats were randomly divided into four groups (Baseline, n=5 rats;
Sham, Ovx, and Ovx+YCJ groups, n=10 rats per group). The rats in
the baseline group were anesthetized by intraperitoneal injection
of 10% chloral hydrate (400 mg/kg body weight), and immediately
sacrificed by cardiac puncture. This group provided reference
values for skeletal measurements, which enabled the determination
of changes in skeletal tissue resulting from surgery and aging
(23). The rats in the remaining three
groups were anesthetized and subjected either to sham operation
(Sham group) or bilateral ovariectomy (Ovx and Ovx+YCJ groups)
using the dorsal approach. At 2 days following surgery, 5X YCJ was
administered by gavage to rats in the Ovx+YCJ group at a daily dose
of 15 ml/kg body weight (equivalent to 75 ml/kg body weight of 1X
YCJ) for 12 weeks. The dose of YCJ was determined as previously
described (18,21). The rats in the other groups were
administered tap water by gavage daily for 12 weeks. At 12 weeks
following surgery, the rats in all three groups were anesthetized
and sacrificed by cardiac puncture. The rats were given
intraperitoneal injections of the fluorochrome markers calcein
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; 10 mg/kg body
weight) and tetracycline-HCl (Sigma-Aldrich; Merck KGaA; 25 mg/kg
body weight) 5 and 2 days prior to necropsy, respectively, to
evaluate the bone dynamics by histomorphometry. At necropsy, the
uterus was resected and weighed to determine whether the Ovx was
successful. Blood was collected by cardiac puncture; serum was
obtained and stored at −80°C until analysis by ELISA. The right
femurs were wrapped in a saline-soaked gauze and stored at −20°C
for subsequent densitometry. The left femurs were cleaned of soft
tissue and fixed in 70% ethanol for bone histomorphometry. The
present study was conducted in parallel with a previous study of
the authors (24) in order to minimize
the number of control rats that were sacrificed (Baseline, Sham and
Ovx groups). The experimental protocol was approved by the Animal
Ethics Committee of the University of Shizuoka (Shizuoka,
Japan).
Bone densitometry
The right femurs were completely thawed at room
temperature, and the bone mineral density (BMD) was measured by
dual-energy X-ray absorptiometry (DXA) and analyzed using small
animal software (version 12.5; QDR-4500A; Hologic, Inc., Bedford,
MA, USA). The BMD was calculated as the bone mineral content (BMC)
divided by the bone area (BA). In addition, the BA, BMC and BMD in
the proximal, mid diaphyseal and distal parts of the femur were
determined by dividing the femur into three equal parts according
to length. The coefficients of variation for the scans and
standards were <1.0%.
Peripheral quantitative computed
tomography (pQCT)
The right femurs were placed in a polypropylene tube
filled with saline solution and scanned using pQCT (XCT Research
SA+; Stratec Medizintechnik GmbH, Pforzheim, Germany) at a voxel
size of 0.12 mm. The scan line was adjusted using scout view, and
transverse sections were recorded at the distal femoral metaphysis
(3 mm proximal to the distal growth plate) and the midshaft (50%
bone length). Images were analyzed using XCT software (version
6.20; Stratec Medizintechnik GmbH) in contour mode 2 and peel mode
2 (threshold 395 mg/cm3) to calculate the trabecular and
total bone parameters at the metaphysis and in cortical mode 1
(threshold 690 mg/cm3) to calculate the cortical bone
parameters at the diaphysis. The following parameters were measured
at the femoral metaphysis: Total BMC (T.BMC; mg), total BMD (T.BMD;
mg/cm3), total cross-sectional area (CSA) (T.CSA;
mm2), trabecular BMC (Tb.BMC; mg), trabecular BMD
(Tb.BMD; mg/cm3), and trabecular CSA (Tb.CSA;
mm2). At the midshaft, the cortical BMC (Ct.BMC; mg),
cortical BMD (Ct BMD; mg/cm3), cortical CSA (Ct.CSA;
mm2), cortical thickness (Ct.Th; mm), periosteal
circumference (Peri.C; mm) and endosteal circumference (Endo.C; mm)
were evaluated.
Bone histomorphometry
The left femurs were stained with Villanueva bone
stain (Maruto Instrument Co., Ltd., Tokyo, Japan) for 7 days prior
to dehydration in a graded series of ethanol and embedded without
decalcification in methyl methacrylate (Wako Pure Chemical
Industries, Ltd., Osaka, Japan). Frontal sections (5 µm) of the
distal femur were cut using a microtome (RM2255; Leica Microsystems
GmbH, Wetzlar, Germany) and mounted on slides. The specimens were
examined under a fluorescence microscope (BX53; Nikon Corporation,
Tokyo, Japan). The structural and dynamic histomorphometric indices
were measured in the cancellous bone at the secondary spongiosa
located 500 µm from the epiphyseal growth plate and 250 µm from the
endocortical surface using a semi-automatic image analysis system
(Histometry RT CAMERA; System Supply Co., Ltd., Ina, Japan) at a
magnification of ×320. The histomorphometric nomenclature used in
the present study is in accordance with the report of the American
Society for Bone and Mineral Research Histomorphometry Nomenclature
Committee (25). Bone section
preparations and histomorphometric measurements were performed at
the Niigata Bone Science Institute (Niigata, Japan).
Serum bone turnover markers
Osteocalcin (OC) and RatLaps (bone-related
degeneration from C-terminal telopeptides of type I collagen) were
determined in order to evaluate bone formation and resorption
status, respectively. OC (Rat-MID Osteocalcin EIA;
Immunodiagnostics Systems Holdings PLC, Tyne & Wear, UK; cat
no. AC-12F1) and RatLaps (RatLaps EIA; Immunodiagnostics Systems
Holdings PLC; cat. no. AC-06F1) was measured using an ELISA
following protocols provided by the manufacturer.
Statistical analyses
The data are presented as the mean ± standard error
of the mean, and data management and statistical analyses were
performed with JMP 9.0.2 (SAS Institute Inc., Cary, NC, USA). The
specific effects of Ovx and YCJ supplementation were examined by
comparing values of the Sham, Ovx and Ovx+YCJ groups using analysis
of variance and analysis of covariance with body weight as the
covariate (26,27), with Tukey's honest significant
difference (HSD) test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of YCJ on body and uterine
weights
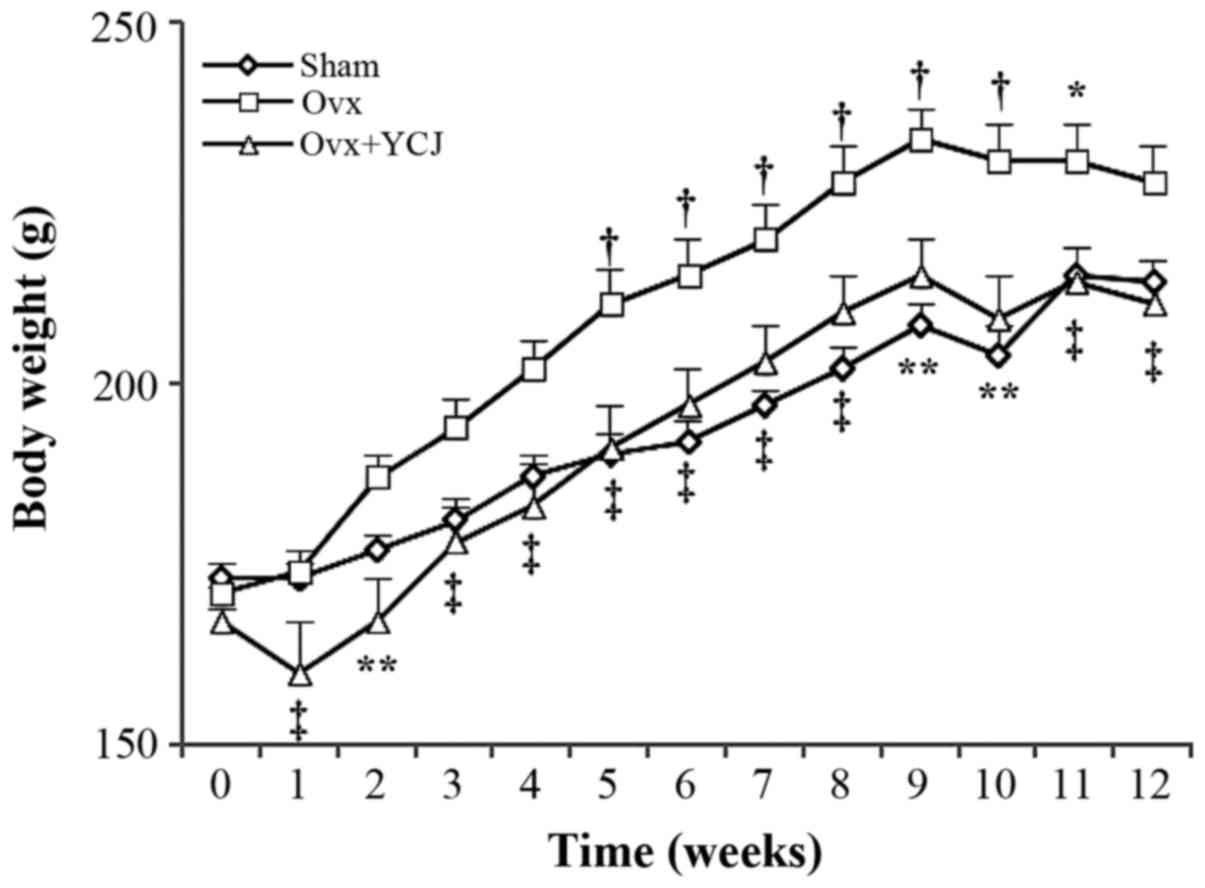
There were early increases in the body weight of
rats in the Ovx group, with significant differences between the Ovx
and Ovx+YCJ groups during the experimental period. At the end of
the study, the Ovx group had a significantly greater body weight
than the Ovx+YCJ group and tended to have a greater body weight
than the Sham group. There was no significant difference in body
weight between the Sham and Ovx+YCJ groups (Fig. 1). The rats in the Ovx group had a
significantly lower mean uterine weight than those in the Sham
group, suggesting that the Ovx procedure was successful. There were
no significant differences in uterine weight between the Ovx and
Ovx+YCJ groups (data not shown).
DXA of the femur
The right femoral BMD of the rats in the Ovx group
was significantly lower than that of the rats in the Sham group.
The BMD of the rats in the Ovx+YCJ group was not significantly
different from that of the rats in the Ovx group. When the femur
was divided into three segments of equal length, there were
significant differences in the BMD and BMC between the Sham and Ovx
groups at the proximal and distal ends of the femur, sites that are
high in cancellous bone, but not at the mid diaphysis, a site that
is high in cortical bone. The rats supplemented with YCJ (Ovx+YCJ
group) presented no significant differences in the BMC or BMD of
the right femur compared with the rats in the Ovx group, regardless
of the femur site (i.e., proximal, diaphyseal and distal sites;
Table I). The BA at the mid diaphysis
was significantly greater in the Ovx group than in the Sham group,
whereas there was no difference in the mid diaphyseal BA between
the Sham and Ovx+YCJ groups.
 | Table I.Femur BMC and BMD in sham-operated
(Sham) and ovariectomized rats supplemented with (Ovx+YCJ) or
without (Ovx) YCJ for 12 weeks. |
Table I.
Femur BMC and BMD in sham-operated
(Sham) and ovariectomized rats supplemented with (Ovx+YCJ) or
without (Ovx) YCJ for 12 weeks.
|
| 0 weeks | 12 weeks |
|---|
|
|
|
|
|---|
| Parameters | Baseline (n=5) | Sham (n =8) | Ovx (n=8) | Ovx+YCJ (n=8) |
|---|
| Femur BMC (g) |
|
|
|
|
|
Whole | 0.215±0.004 | 0.298±0.004 | 0.292±0.009 |
0.276±0.007a |
|
Proximal | 0.081±0.003 | 0.113±0.003 |
0.108±0.004a |
0.103±0.003a |
|
Mid | 0.050±0.003 | 0.070±0.002 | 0.077±0.004 | 0.073±0.003 |
|
Distal | 0.087±0.001 | 0.119±0.002 |
0.112±0.003a |
0.104±0.003b |
| Femur BA
(cm2) |
|
|
|
|
|
Whole | 1.220±0.010 | 1.457±0.017 | 1.535±0.032 | 1.450±0.017 |
|
Proximal | 0.443±0.010 | 0.542±0.009 | 0.563±0.011 | 0.536±0.010 |
|
Mid | 0.340±0.006 | 0.396±0.004 |
0.435±0.012a | 0.409±0.005 |
|
Distal | 0.458±0.004 | 0.542±0.009 | 0.563±0.011 | 0.527±0.011 |
| Femur BMD
(g/cm2) |
|
|
|
|
|
Whole | 0.176±0.002 | 0.204±0.002 |
0.190±0.003b |
0.190±0.003b |
|
Proximal | 0.183±0.002 | 0.209±0.002 |
0.192±0.003b |
0.192±0.004b |
|
Mid | 0.146±0.006 | 0.177±0.007 | 0.176±0.007 | 0.178±0.006 |
|
Distal | 0.190±0.004 | 0.219±0.002 |
0.198±0.003b |
0.198±0.004b |
pQCT of the femur metaphysis and
diaphysis
The Tb.BMC of the distal metaphysis was
significantly higher in the Ovx group than in the Sham group.
However, due to a significant increase in the Tb.CSA, the Tb.BMD in
the Ovx group was significantly lower than that in the Sham group.
There were no significant differences in the parameters between the
Sham and Ovx groups at the diaphysis. YCJ supplementation induced
no significant differences in any parameter evaluated by pQCT at
the diaphyseal and distal sites (Table
II).
 | Table II.Trabecular and cortical parameters
determined by peripheral quantitative computed tomography in
sham-operated (Sham) and ovariectomized rats supplemented with
(Ovx+YCJ) or without (Ovx) YCJ for 12 weeks. |
Table II.
Trabecular and cortical parameters
determined by peripheral quantitative computed tomography in
sham-operated (Sham) and ovariectomized rats supplemented with
(Ovx+YCJ) or without (Ovx) YCJ for 12 weeks.
|
| 0 weeks | 12 weeks |
|---|
|
|
|
|
|---|
| Parameters | Baseline (n=5) | Sham (n=8) | Ovx (n=8) | Ovx+YCJ (n=8) |
|---|
| Distal
metaphysis |
|
|
|
|
| T.BMC
(mg) | 8.72±0.32 | 9.85±0.29 |
8.72±0.22b |
8.37±0.28b |
| T.BMD
(mg/cm3) | 611±14 | 658±11 | 540±8b | 538±11b |
| T.CSA
(mm2) | 14.3±0.3 | 15.0±0.3 | 16.2±0.5 | 15.5±0.3 |
| Tb.BMC
(mg) | 0.774±0.160 | 0.815±0.042 |
1.129±0.046b |
1.050±0.072a |
| Tb.BMD
(mg/cm3) | 304±16 | 253±8 | 177±5b | 167±5b |
| Tb.CSA
(mm2) | 2.65±0.58 | 3.26±0.25 |
6.40±0.27b |
6.30±0.43b |
| Diaphysis |
|
|
|
|
| Ct.BMC
(mg) | 4.55±0.04 | 5.91±0.06 | 6.05±0.13 | 5.82±0.10 |
| Ct.BMD
(mg/cm3) | 1230±5 | 1320±3 | 1310±4 | 1310±4 |
| Ct.CSA
(mm2) | 3.69±0.04 | 4.48±0.04 | 4.63±0.10 | 4.45±0.07 |
| Ct.Th
(mm) | 0.494±0.010 | 0.598±0.004 | 0.594±0.006 | 0.586±0.009 |
| Peri.C
(mm) | 9.04±0.08 | 9.38±0.07 | 9.66±0.15 | 9.44±0.08 |
| Endo.C
(mm) | 5.94±0.13 | 5.63±0.08 | 5.93±0.15 | 5.76±0.10 |
Bone histomorphometry
The histomorphometric indices for distal femoral
metaphyseal cancellous bone are summarized in Table III. The bone volume and trabecular
number were significantly lower in the Ovx group than in the Sham
group, whereas the osteoid volume and osteoid surface were higher
in the Ovx group than in the Sham group. Regarding the dynamic
indices, a larger mineralized surface was found in the Ovx group
than in the Sham group. However, these differences were not
significantly affected by long-term YCJ supplementation.
 | Table III.Dynamic cancellous bone indices in
the distal femoral metaphysis in sham-operated (Sham) and
ovariectomized rats supplemented with (Ovx+YCJ) or without (Ovx)
YCJ for 12 weeks. |
Table III.
Dynamic cancellous bone indices in
the distal femoral metaphysis in sham-operated (Sham) and
ovariectomized rats supplemented with (Ovx+YCJ) or without (Ovx)
YCJ for 12 weeks.
|
| 0 weeks | 12 weeks |
|---|
|
|
|
|
|---|
| Parameters | Baseline (n=5) | Sham (n=8) | Ovx (n=8) | Ovx+YCJ (n=8) |
|---|
| Static indices |
|
|
|
|
| Bone
volume (%) | 28.5±1.2 | 28.8±1.3 |
15.0±0.9b |
14.6±2.5b |
|
Trabecular thickness (µm) | 55.4±1.0 | 62.0±1.5 | 64.2±2.2 | 64.9±3.5 |
|
Trabecular number (N/mm) | 5.15±0.20 | 4.63±0.17 |
2.34±0.12b |
2.18±0.23b |
| Osteoid
volume (%) | 4.09±0.44 | 2.03±0.23 |
4.36±0.61a |
4.37±0.46b |
| Osteoid
surface (%) | 26.2±2.4 | 15.7±1.4 |
32.8±2.5b |
33.6±2.4b |
|
Osteoblast surface (%) | 13.39±2.21 | 3.97±0.54 | 7.34±1.23 | 8.54±2.22 |
| Dynamic
indices |
|
|
|
|
|
Mineralizing surface (%) | 33.1±1.5 | 17.7±2.1 |
31.9±2.4b |
25.9±1.8a |
| Mineral
apposition rate (µm/day) | 2.50±0.17 | 1.27±0.05 | 1.45±0.10 | 1.42±0.06 |
| Bone
formation rate (mm3/mm2/year) | 0.302±0.023 | 0.081±0.010 | 0.173±0.023 |
0.135±0.012a |
| Bone
formation rate (%/year) | 1,100±97 | 261±35 | 536±73 | 414±29 |
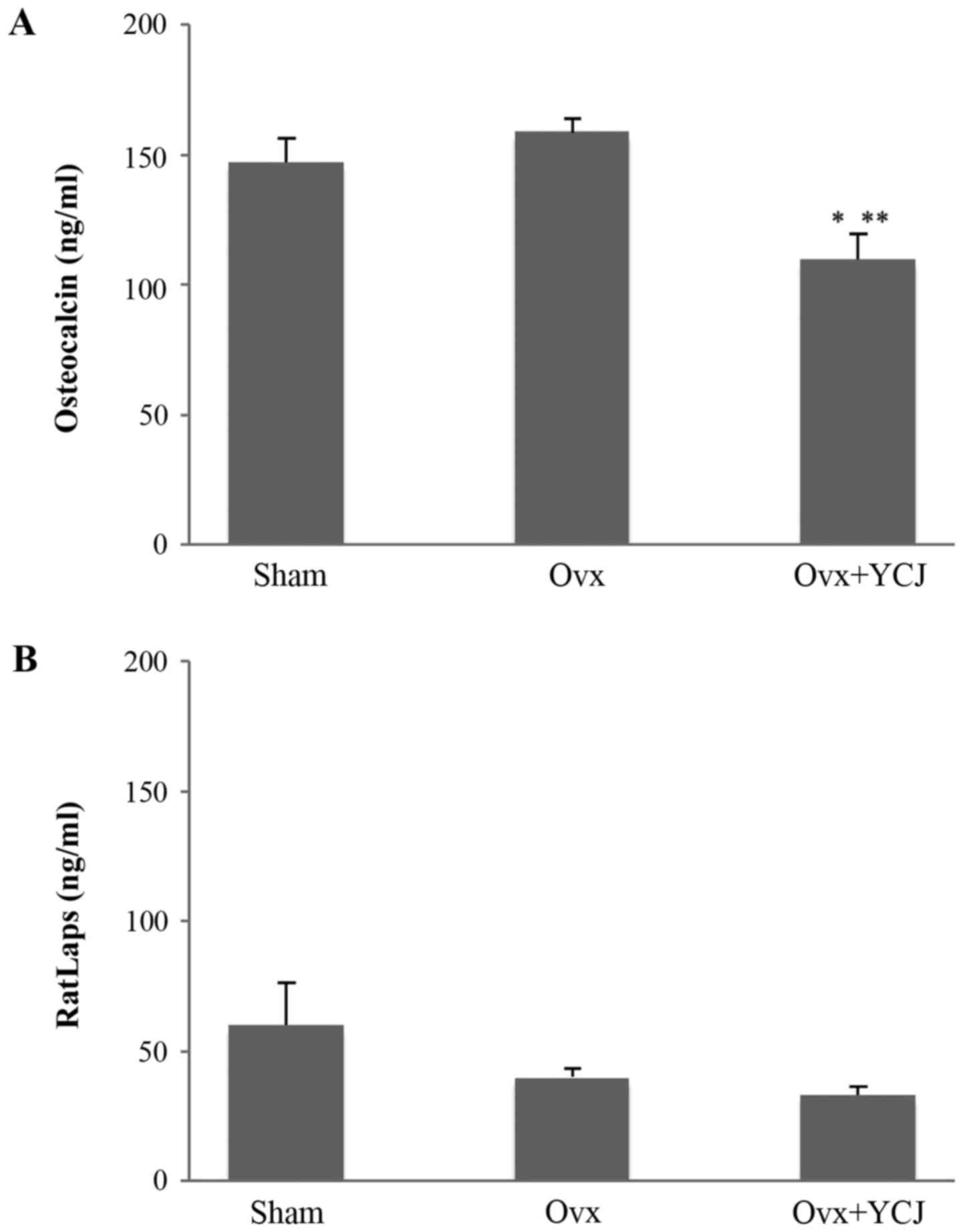
Serum bone turnover markers
The serum OC level was significantly lower in rats
in the Ovx+YCJ group than in those in the Sham and Ovx groups,
which suggests that YCJ supplementation suppressed bone formation.
On the other hand, the serum bone resorption marker RatLaps was not
affected by YCJ supplementation (Fig.
2).
Discussion
It was recently reported that ovariectomized rats
supplemented with YCJ for a short term (6 weeks) demonstrated a
significantly higher femoral BMD (as measured by DXA) and higher
bone formation rates (as measured by trabecular bone
histomorphometry) than rats that did not receive YCJ (21), which suggests that YCJ may mitigate the
rapid bone loss observed in women during early menopause. In the
present study, the effects of long-term (12-week) YCJ
supplementation on bone metabolism was examined in ovariectomized
rats to investigate whether YCJ could help prevent the development
of postmenopausal osteoporosis. However, the present study
demonstrated that ovariectomized rats with longer-term (12 weeks)
YCJ supplementation (Ovx+YCJ group) had a BMD similar to that of
rats without YCJ supplementation (Ovx group).
It is unknown why the results of the present
investigation failed to corroborate the findings of the prior study
of the authors, in which trabecular bone histomorphometry revealed
that short-term (6-week) supplementation of YCJ accelerated the
parameters of bone formation without affecting bone resorption
parameters in ovariectomized rats. In addition to its use in
alleviating menopausal symptoms, coconut juice has also been used
as a temporary contraceptive in Thailand and Indonesia. In
contrast, women in these areas are prohibited from drinking coconut
juice because it disrupts their menstrual cycle, and individuals in
Java are afraid to drink coconut milk because it may diminish their
fertility (18–20). It therefore seems that YCJ may exert
its bone protective effects only in the presence of endogenous sex
steroids, suggesting that the positive effect on bone observed 6
weeks following ovariectomy (21) were
no longer apparent at 12 weeks following ovariectomy.
Although we have still not identified the exact
constituents in YCJ that prevented bone loss following Ovx in the
previous study, YCJ contains estrogens (e.g., 17β-estradiol,
estrone and estrone-3-glucuronide), testosterone and various
phytoestrogens (22). All of these
constituents may have positively affected bone parameters in the
authors' previous study. It is important to note that 17β-estradiol
exerts its bone-protective effects by suppressing both bone
formation and resorption, with a greater emphasis on the latter
(28,29). It has been speculated that the effect
of YCJ supplementation on bone may differ from that of estradiol.
Ovariectomy in young, growing rodents decreases BMD of the
trabecular bone and increases radial growth of the cortical bone,
and both of these effects can be reversed by estrogen (30). Estrogen exerts many of its effects via
two nuclear estrogen receptors (ERs), ERα and ERβ. Although both
receptors have been detected in all skeletal cell types, for
example chondrocytes, bone marrow stromal cells, osteoblasts,
osteocytes and osteoclasts and their progenitor cells, the vast
majority of the estrogen bone-sparing effects are mediated by ERα
in both the cortical and cancellous bone compartments (31,32). By
contrast, ERβ may not serve a major role in bone, with the possible
exception that it may exert an inhibitory effect on periosteal
apposition (30,31). In the present study, DXA measurements
demonstrated that the BA at the mid diaphysis, a site that is high
in cortical bone, was significantly greater in the Ovx group than
in the Sham group, whereas there was no difference in the mid
diaphyseal BA between the Sham and Ovx+YCJ groups. YCJ contains
various phytoestrogens, such as β-sitosterol (58% of the total
composition of 1 ml YCJ), stigmastatrienol, stigmasterol,
fucosterol and α-spinasterol (22).
Phytoestrogens are plant derivatives that behave differently from
estrogen but similarly to SERMs with a higher affinity for ERβ
(33). The serum bone formation marker
OC was significantly lower in rats in the Ovx+YCJ group than in
those in the Ovx group. However, bone histomorphometry indicated
that trabecular bone formation was not affected by YCJ
supplementation. Thus, the authors speculate that long-term
supplementation with YCJ may prevent the periosteal apposition of
cortical bone, possibly via ERβ.
Unexpectedly, long-term supplementation with YCJ
(Ovx+YCJ) resulted in a significantly lower body weight compared to
no supplementation. Ovariectomized animals present body weight gain
and excessive feeding. The treatment of ovariectomized animals with
estrogen prevents these changes, and these effects are mediated, in
large part, through ERα (34,35). The present study demonstrated that the
uterine weight of the rats that were administered YCJ at a dose
equivalent to 75 ml/kg/day was not significantly different from
that of the rats that were not administered YCJ. Punghmatharith
(22) reported that 17β-estradiol was
present in YCJ at 2.45 pg/ml and that the subcutaneous injection of
an ethereal extract of YCJ at a dose equivalent to 7,500 ml/kg/day
for 3 consecutive days significantly increased the uterine wet
weight of immature rats; however, this effect was not seen when YCJ
was injected at 4,000 ml/kg/day (22).
Thus, the dose of 17β-estradiol in YCJ is small, suggesting that
YCJ may alleviate body weight gain following Ovx in rats through
mechanisms different from those of ERα. However, some recent
transgenic studies have indicated that ERβ may serve a key role in
preventing obesity and obesity-related metabolic disease (36,37).
Radenahmad et al (20)
demonstrated that both ERα and ERβ were highly expressed in a
cutaneous wound of ovariectomized rats supplemented with YCJ,
although ERα is predominantly expressed in normal skin. These
findings may endorse the authors' speculation that the effects of
YCJ may be exerted through ERβ.
In conclusion, long-term supplementation with YCJ
did not improve indices of bone mass or bone histomorphometry in
ovariectomized rats, arguing against the benefits of YCJ in
preventing osteoporosis in postmenopausal women. Although
accumulated evidence suggests that consuming a phytoestrogen-rich
diet alleviates symptoms associated with menopause, studies into
the direct effects of dietary phytoestrogens on bone in
postmenopausal women are extremely limited (38). Thus, physicians should not hesitate to
initiate drug treatment in women at risk of osteoporosis and
fracture, even if patients prefer diet and lifestyle alterations.
Nevertheless, considering the results of our previous study
(21), YCJ may be beneficial in
mitigating bone loss in women during early menopause, which may
delay the start and reduce the total duration of anti-osteoporotic
drug therapy. In addition, YCJ consumption may help to prevent body
weight gain following menopause.
Acknowledgements
The present study was supported, in part, by a JMS
Bayer Schering Pharma Grant from the Japan Menopause Society (to
H.M.) and a Scientific Research Grant from the Amano Foundation of
Industrial Technology (to A.M.).
References
|
1
|
Daan NM and Fauser BC: Menopause
prediction and potential implications. Maturitas. 82:257–265. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Salomon JA, Wang H, Freeman MK, Vos T,
Flaxman AD, Lopez AD and Murray CJ: Healthy life expectancy for 187
countries, 1990-2010: A systematic analysis for the Global Burden
Disease Study 2010. Lancet. 380:2144–2162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raisz LG: Pathogenesis of osteoporosis:
Concepts, conflicts, and prospects. J Clin Invest. 115:3318–3325.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wells G, Tugwell P, Shea B, Guyatt G,
Peterson J, Zytaruk N, Robinson V, Henry D, O'Connell D and Cranney
A; Osteoporosis Methodology Group and The Osteoporosis Research
Advisory Group, : Meta-analyses of therapies for postmenopausal
osteoporosis. V. Meta-analysis of the efficacy of hormone
replacement therapy in treating and preventing osteoporosis in
postmenopausal women. Endocr Rev. 23:529–539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dören M, Nilsson JA and Johnell O: Effects
of specific post-menopausal hormone therapies on bone mineral
density in post-menopausal women: A meta-analysis. Hum Reprod.
18:1737–1746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Torgerson DJ and Bell-Syer SE: Hormone
replacement therapy and prevention of nonvertebral fractures: A
meta-analysis of randomized trials. JAMA. 285:2891–2897. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rossouw JE, Anderson GL, Prentice RL,
LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA,
Howard BV, Johnson KC, et al: Writing Group for the Women's Health
Initiative Investigators: Risks and benefits of estrogen plus
progestin in healthy postmenopausal women: Principal results From
the Women's Health Initiative randomized controlled trial. JAMA.
288:321–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cauley JA, Robbins J, Chen Z, Cummings SR,
Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, et
al: Women's Health Initiative Investigators: Effects of estrogen
plus progestin on risk of fracture and bone mineral density: The
Women's Health Initiative randomized trial. JAMA. 290:1729–1738.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson GL, Limacher M, Assaf AR,
Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R,
Caan B, et al: Women's Health Initiative Steering Committee:
Effects of conjugated equine estrogen in postmenopausal women with
hysterectomy: The Women's Health Initiative randomized controlled
trial. JAMA. 291:1701–1712. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jackson RD, Wactawski-Wende J, LaCroix AZ,
Pettinger M, Yood RA, Watts NB, Robbins JA, Lewis CE, Beresford SA,
Ko MG, et al: Women's Health Initiative Investigators: Effects of
conjugated equine estrogen on risk of fractures and BMD in
postmenopausal women with hysterectomy: Results from the women's
health initiative randomized trial. J Bone Miner Res. 21:817–828.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Brien J, Jackson JW, Grodstein F,
Blacker D and Weuve J: Postmenopausal hormone therapy is not
associated with risk of all-cause dementia and Alzheimer's disease.
Epidemiol Rev. 36:83–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beral V, Gaitskell K, Hermon C, Moser K,
Reeves G and Peto R; Collaborative Group On Epidemiological Studies
Of Ovarian Cancer, : Menopausal hormone use and ovarian cancer
risk: Individual participant meta-analysis of 52 epidemiological
studies. Lancet. 385:1835–1842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Connor KM: Evaluation and treatment of
osteoporosis. Med Clin North Am. 100:807–826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cummings SR, Black DM, Thompson DE,
Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R,
Rubin SM, Scott JC, et al: Effect of alendronate on risk of
fracture in women with low bone density but without vertebral
fractures: Results from the Fracture Intervention Trial. JAMA.
280:2077–2082. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ettinger B, Black DM, Mitlak BH,
Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas
PD, Zanchetta JR, Stakkestad J, et al: Multiple Outcomes of
Raloxifene Evaluation (MORE) Investigators: Reduction of vertebral
fracture risk in postmenopausal women with osteoporosis treated
with raloxifene: Results from a 3-year randomized clinical trial.
JAMA. 282:637–645. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DebMandal M and Mandal S: Coconut (Cocos
nucifera L.: Arecaceae): in health promotion and disease
prevention. Asian Pac J Trop Med. 4:241–247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dua K, Sheshala R, Ling TY, Hui Ling S and
Gorajana A: Anti-inflammatory, antibacterial and analgesic
potential of cocos nucifera linn.: A review. Antiinflamm
Antiallergy Agents Med Chem. 12:158–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Radenahmad N, Vongvatcharanon U,
Withyachumnarnkul B and Connor RJ: Serum levels of 17β-estradiol in
ovariectomized rats fed young-coconut juice and its effect on wound
healing. Songklanakarin J Sci Technol. 28:897–910. 2006.
|
|
19
|
Radenahmad N, Saleh F, Sawangjaroen K,
Rundorn W, Withyachumnarnkul B and Connor JR: Young coconut juice
significantly reduces histopathological changes in the brain that
are induced by hormonal imbalance: A possible implication to
postmenopausal women. Histol Histopathol. 24:667–674.
2009.PubMed/NCBI
|
|
20
|
Radenahmad N, Saleh F, Sayoh I,
Sawangjaroen K, Subhadhirasakul P, Boonyoung P, Rundorn W and
Mitranun W: Young coconut juice can accelerate the healing process
of cutaneous wounds. BMC Complement Altern Med. 12:2522012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morii Y, Matsushita H, Minami A, Kanazawa
H, Suzuki T, Subhadhirasakul S, Watanabe K and Wakatsuki A: Young
coconut juice supplementation results in greater bone mass and bone
formation indices in ovariectomized rats: A preliminary study.
Phytother Res. 29:1950–1955. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pungmatharith B: Sex hormone-like
substances in young coconut juice and their effects on uterine
growth in rats. Songklanakarin J Sci Technol. 10:221–226. 1988.
|
|
23
|
Kalu DN: The ovariectomized rat model of
postmenopausal bone loss. Bone Miner. 15:175–191. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohyama Y, Matsushita H, Minami A, Kanazawa
H, Suzuki T, Watanabe K and Wakatsuki A: Effect of the ethanol
extract of Pleurotus eryngii on bone metabolism in ovariectomized
rats. Climacteric. 17:492–499. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dempster DW, Compston JE, Drezner MK,
Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR
and Parfitt AM: Standardized nomenclature, symbols, and units for
bone histomorphometry: A 2012 update of the report of the ASBMR
Histomorphometry Nomenclature Committee. J Bone Miner Res. 28:2–17.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lane MA, Black A, Handy AM, Shapses SA,
Tilmont EM, Kiefer TL, Ingram DK and Roth GS: Energy restriction
does not alter bone mineral metabolism or reproductive cycling and
hormones in female rhesus monkeys. J Nutr. 131:820–827.
2001.PubMed/NCBI
|
|
27
|
Mardon J, Trzeciakiewicz A, Habauzit V,
Davicco MJ, Lebecque P, Mercier S, Tressol JC, Horcajada MN,
Demigné C and Coxam V: Dietary protein supplementation increases
peak bone mass acquisition in energy-restricted growing rats.
Pediatr Res. 66:513–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wronski TJ, Cintrón M, Doherty AL and Dann
LM: Estrogen treatment prevents osteopenia and depresses bone
turnover in ovariectomized rats. Endocrinology. 123:681–686. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coxam V, Bowman BM, Mecham M, Roth CM,
Miller MA and Miller SC: Effects of dihydrotestosterone alone and
combined with estrogen on bone mineral density, bone growth, and
formation rates in ovariectomized rats. Bone. 19:107–114. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Windahl SH, Vidal O, Andersson G,
Gustafsson JA and Ohlsson C: Increased cortical bone mineral
content but unchanged trabecular bone mineral density in female
ERbeta(−/−) mice. J Clin Invest. 104:895–901. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manolagas SC, Almeida M and Jilka RL:
Gonadal steroidsPrimer on the Metabolic Bone Diseases and disorders
of Mineral Metabolism. John Wiley & Sons, Inc.; Hoboken, NJ:
pp. 195–207. 2013, View Article : Google Scholar
|
|
32
|
Vinel A, Hay E, Valera MC, Buscato M,
Adlanmerini M, Guillaume M, Cohen-Solal M, Ohlsson C, Lenfant F,
Arnal JF, et al: Role of ERα in the effect of estradiol on
cancellous and cortical femoral bone in growing female mice.
Endocrinology. 157:2533–2544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oseni T, Patel R, Pyle J and Jordan VC:
Selective estrogen receptor modulators and phytoestrogens. Planta
Med. 74:1656–1665. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Geary N, Asarian L, Korach KS, Pfaff DW
and Ogawa S: Deficits in E2-dependent control of feeding, weight
gain, and cholecystokinin satiation in ER-alpha null mice.
Endocrinology. 142:4751–4757. 2001. View Article : Google Scholar
|
|
35
|
Van Pelt RE, Gavin KM and Kohrt WM:
Regulation of body composition and bioenergetics by estrogens.
Endocrinol Metab Clin North Am. 44:663–676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yepuru M, Eswaraka J, Kearbey JD, Barrett
CM, Raghow S, Veverka KA, Miller DD, Dalton JT and Narayanan R:
Estrogen receptor-{β}-selective ligands alleviate high-fat diet-
and ovariectomy-induced obesity in mice. J Biol Chem.
285:31292–31303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weigt C, Hertrampf T, Zoth N, Fritzemeier
KH and Diel P: Impact of estradiol, ER subtype specific agonists
and genistein on energy homeostasis in a rat model of nutrition
induced obesity. Mol Cell Endocrinol. 351:227–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chiechi LM, Secreto G, D'Amore M, Fanelli
M, Venturelli E, Cantatore F, Valerio T, Laselva G and Loizzi P:
Efficacy of a soy rich diet in preventing postmenopausal
osteoporosis: The Menfis randomized trial. Maturitas. 42:295–300.
2002. View Article : Google Scholar : PubMed/NCBI
|